Investigation of The Malondialdehyde, Antioxidant Enzymes, Antioxidant Substance and Some Biochemical Parameter Levels in Cattle with Actinomycosis
Apaydin Yildirim B1*, Ertekin A2, Yildirim F3, Kirbas A4, Tutuncu M5, Saglam K6
1 Faculty of Veterinary Medicine, Department of Biochemistry, Ataturk University, Erzurum, Turkey.
2 Faculty of Veterinary Medicine, Department of Biochemistry, Ondokuz Mayıs University, Samsun.
3 Faculty of Veterinary Medicine, Department of Zootechnics, Atatürk University, Erzurum, Turkey.
4 Faculty of Veterinary Medicine, Department of Internal Diseases, Atatürk University, Erzurum, Turkey.
5 Faculty of Veterinary Medicine, Department of Internal Diseases, Ondokuz Mayıs University, Samsun, Turkey.
6 Faculty of Veterinary Medicine, Department of Surgery, Ondokuz Mayıs University, Samsun, Turkey.
*Corresponding Author
Betul Apaydin Yildirim
Associate Proffesor,
Ataturk University Veterinary Faculty,
Department of Biochemistry, 25240 Erzurum, Turkey.
Tel: +90 4422317090
Fax: +90 442315563
E-mail: betul_apaydin@hotmail.com
Received: October 28, 2016; Accepted: November 14, 2016; Published: November 16, 2016
Citation: Apaydin Yildirim B, Ertekin A, Yildirim F, Kirbas A, Tutuncu M, et al., (2016) Investigation of The Malondialdehyde, Antioxidant Enzymes, Antioxidant Substance and Some Biochemical Parameter Levels in Cattle with Actinomycosis. Int J Vet Health Sci Res. 4(7), 151-154. DOI : dx.doi.org/10.19070/2332-2748-1600031
Copyright: Apaydin Yildirim B© 2016. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Actinomycosis is commonly known as “lumpy jaw” that is a specific disease characterized with mandibular osteomyelitis in cattle. In our study, malondialdehyde, superoxide dismutase and some biochemical parameter levels in cattles with Actinomycosis were investigated. Thirty cattle were used in this study. 15 number of these were used as a control group of healthy individuals. Out of fifteen were Actinomycosis group. Statistical analysis showed that, concentrations of malondialdehyde were higher (P<0.001) and superoxide dismutase concentrations were lower (P<0.001), catalase, glutathione peroxidase and glutathione levels were significantly lower (P<0.01) in the cattles with Actinomycosis than in healthy ones. Glucose, cholesterol and LDL levels were significantly increased (P<0.001), HDL levels were significantly decreased (P<0.001) in the Actinomycosis group according to control group. Triglyceride concentrations were markedly increased (P<0.01) and albumin concentration was decreased (P<0.01). The enzyme activities of ALT were significantly increased (P<0.05) and as to AST enzyme’s activities were decreased (P<0.05). There was no statistically significant differences for the total protein levels in the Actinomycosis Group.
2.Introduction
3.Materials and Methods
3.1. Animals
3.2. Biochemical Analysis
3.3. Statistical Analysis
4.Results
5.Discussion
6.References
Keywords
Actinomycosis; Cattle; Malondialdehyde; Oxidative Stress.
Introduction
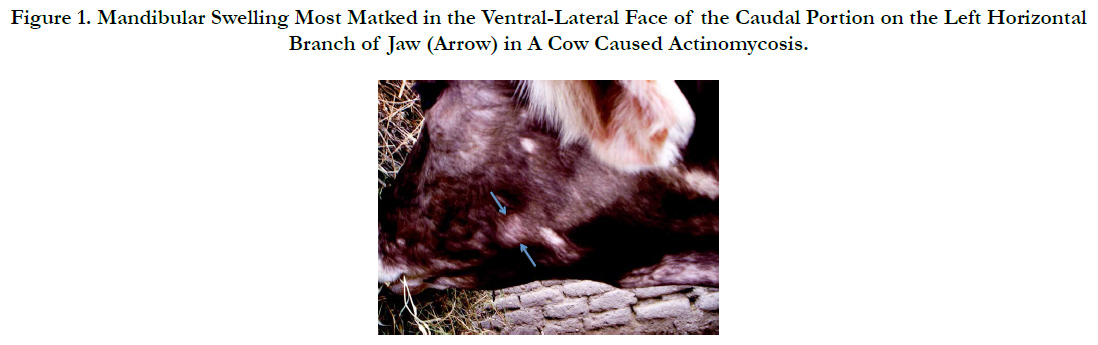
Actinomycosis is commonly known as “lumpy jaw” that is a specific disease characterized with mandibular osteomyelitis in cattle [11] [Figure 1]. According to literature, Actinomyces was first reported in human in 1857 [1]. Actinomyces bovis is the primary etiologic agent of actinomycosis or lumpy jaw in cattle and is an important cause of economic losses in livestock because of its widespread occurrence and poor response to the routine clinical treatment [6, 13]. The losses occur directly from the debilitation of affected cattle and indirectly from the slaughter of animals [6]. The basic lesion in actinomycosis is represented by granulation tissue having small abscesses, sulfa granules, and occasionally draining sinus tracts. Involvement of adjacent bone frequently results in facial distortion, loose teeth, and dyspnea due to swelling in the nasal cavity [11].
Figure 1. Mandibular Swelling Most Marked in the Ventral-Lateral Face of the Caudal Portion on the Left Horizontal Branch of Jaw (Arrow) in A Cow Caused Actinomycosis.
Oxidative stress is defined as an imbalance between production and regulation of reactive oxygen species. Reactive oxygen species mainly free radicals are directly involved in oxidative damage of cellular macromolecules such as lipids, proteins and nucleic acids in tissues. They can produce a variety of pathological changes through lipid peroxidation and DNA damage. Malondialdehyde (MDA) is the breakdown product of the major chain reactions leading to the oxidation of polyunsaturated fatty acids and thus causing oxidative stress. There are also antioxidant defense systems against different oxidants in the organism. These systems such as antioxidant vitamins, superoxide dismutase (SOD), catalase (CAT), glutathione (GSH) and glutathione peroxidase (GPx) protect the cells against lipid peroxidation [22]. The reactive oxygen species are reported to oxidise biomolecules and cause extensive lipid peroxidation in biological membranes, which lead to cell death and tissue injury. The antioxidant systems protect the cellular biomolecules against damage caused by free radicals. They involve enzymes such as SOD, GPx and CAT and non enzyme factors such as GSH and vitamins. Changes in circulating levels of the antioxidants are indications of the occurrence of oxidative stress [7].
Thirty Native Black Cattle were used in the study. The animals were obtained from Erzurum in the eastern part of Turkey, then divided into two groups of 15 in each group. Control group contained normal healthy animals (15 animal) and the other group consisted of actinomycosis animals (15 animal). The animals with Actinomycosis were determined based on clinical symptoms. The observed clinical symptoms; thickening of one or both lips, that is this thickening it is extended to the jaw from cheeks, due to the abscess tongue, lips, pharynx and upper jaw swelling of the lymph nodes. These animals had salivation, swallowing and chewing difficulty. Also observed in these animals as clinical symptoms, malnutrition and weakness because of the teeth loosening and falling. This study was approved by the Ethics Committee of Ataturk University (Date: 19.10.2015, Ethics Approval Protocol number: 36643897-200).
Blood samples were taken according to the procedures from V. Jugularis. Whole blood was collected into heparinized tubes. Plasma was obtained from these whole blood samples by centrifugation (3000 rpm for 10 min) and used for the determination of the biochemical parameters. The levels of malondialdehyde were determined by Yoshioka et al., [23] in serum using reacts with tiobarbituric acid (TBA) reagent under acidic conditions to generate a pink colored product which determined at 532 nm as mmol MDA/L, was used as a standart Tetramethoxypropane.
SOD activity was determined using Sun et al., procedure [19] xanthine-xanthine oxidase system was used as superoxide generator, and nitroblue tetrazolium (NBT) was used as an indicator. SOD activity was then measured by the degree of inhibition of the reaction unit of enzyme provides 50% inhibition of NBT reduction. Results are determined as U/mL. CAT activities was based on spectrophotometric assay of Hydrogen peroxide and colorimetric method with Ammonium Molybdate. Results are determined as KU/ L [8]; GPx levels were determined at 37°C and 412 nm according to methods of Matkovics et al., Results are determined as U/m L [12]; GSH levels was measured by continuous reduction of 5,5'-dithiobis, 2-nitrobenzoic acid (DTNB) in the presence of glutathione reductase (GR), oxidized glutathione (GSSG) and NADPH, at 412nm according to method described by Tietze [20] with Biotek ELISA Reader (Bio Tek μQuant MQX200 Elisa reader/USA). The activities of plasma ALT, AST and measures of glucose, albumin, cholesterol, triglyseride, HDL, LDL and total protein were determined using commercial diagnostics kits with Mindray Perfect Plus 400 Autoanalyser.
Independent t test was used for statistical analysis (SPSS, 20.0 version). Datas are expressed as mean ± standard deviation.
Results
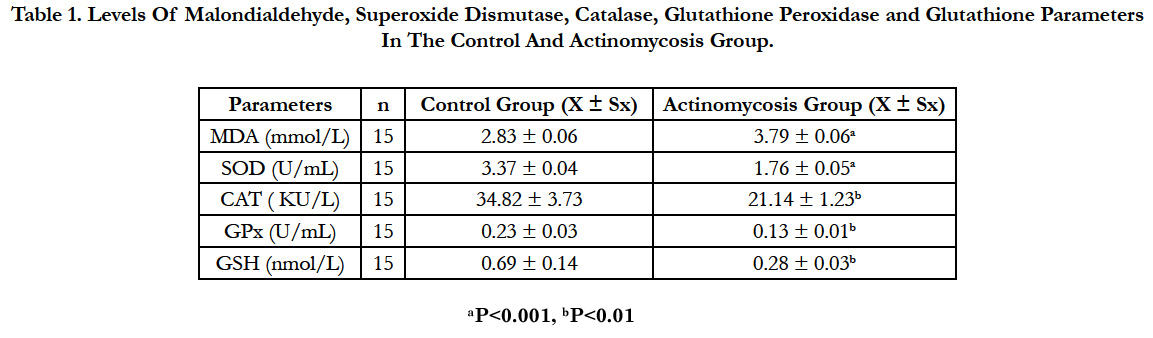
Presented in Table 1 are concentrations of lipid peroxidation product (MDA) and SOD, CAT, GPx and GSH in the Control and Actinomycosis groups (mean ± SD).
Table 1. Levels Of Malondialdehyde, Superoxide Dismutase, Catalase, Glutathione Peroxidase and Glutathione Parameters In The Control And Actinomycosis Group.
Statistical analysis showed that, concentrations of malondialdehyde were higher (P<0.001) and superoxide dismutase concentrations were lower (P<0.001), catalase, glutathione peroxidase and glutathione levels were significantly lower (P<0.01) in the cattles with Actinomycosis than in healthy ones.
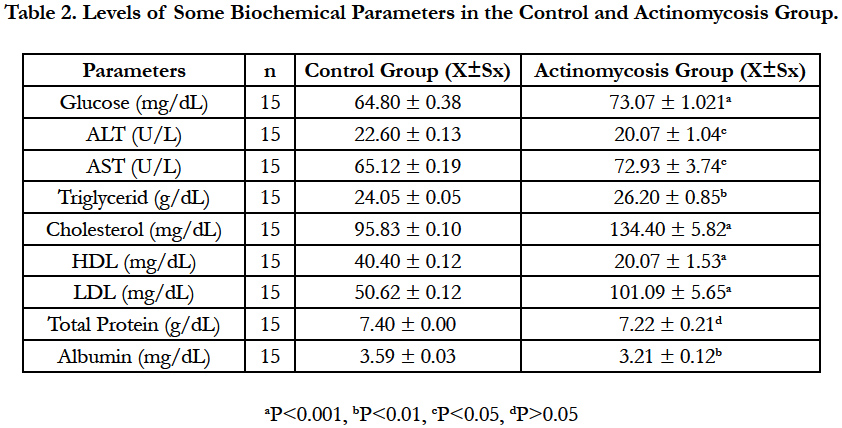
Glucose, ALT, AST, Triglycerides, Cholesterol, HDL, LDL, Total Protein and Albumin levels of Actinomycosis and Control groups are shown in Table 2.
Glucose, cholesterol and LDL levels were significantly increased (P<0.001), HDL levels were significantly decreased (P<0.001) in the Actinomycosis group according to control group. Triglyceride concentrations were markedly increased (P<0.01) and Albumin concentration was decreased (P<0.01). The enzyme activities of ALT was significantly increased (P<0.05) and as for AST enzyme’s activities were decreased (P<0.05). There was no statistically significant differences for the total protein levels in the Actinomycosis Group.
Discussion
Actinomyces bovis is a symbiotic inhabitant of oral mucosa that gains access through the abrading and penetrating injury to the buccal mucosa and dental alveoli. Involvement of adjacent bone frequently results in facial distortion, loose teeth and dyspnea due to swelling in the nasal cavity. The most common manifestation of this disease in cattle is a rarefying osteomyelitis of the bones of the head, particularly mandible and maxilla, though the rare cases may involve soft tissues, particularly the alimentary tract [6].
Stoichiometric studies have shown that every disease process is capable of causing oxidative stress to the host, through the initiation of free radical mechanism. This oxidative stress brings about a misbalance in the antioxidant/pro-oxidant balance, such that the pro-oxidant state is favoured [15]. MDA is the main oxidative product of polyunsaturated fatty acids in lipid peroxidation. It can disrupt the structure of lipid membrane causing a serious effect on normal cell function. The changes in its content can reflect cell injury caused by reactive oxygene species (ROS) [9]. Disturbances of the oxidant/antioxidant balance resulting from the increased production of ROS are causative factors in the oxidative damage of cellular structures and molecules, such as lipids, proteins and nucleic acids. In particular, biological membranes that are rich in unsaturated fatty acids are cellular structures susceptible to free radical attack. Greater extent of lipid peroxidation in co-exposed group indicates a higher degree of free radical insult to the cellular membranes possibly due to an additive or synergistic effect of the two compounds on genera of reactive oxygen species [14]. Therefore, increased blood MDA levels in this study might be due to the higher rate of oxidative metabolic activity, and higher concentration of readily oxidizable membrane polyunsaturated fatty acids.
Antioxidant agents protect cells from oxidative stress damage. SOD, a chain breaking antioxidant enzyme, play an important role in protection against the deleterious effect of lipid peroxidation. SOD can prevent oxidative stress though catalyzing the dismutation reaction of ROS into O2 and H2O2 in living organisms [4, 21]. Superoxide radicals are produced in mitochondria and endoplasmic reticulum as a consequence of auto-oxidation of electron transport chain components. SOD converts superoxide into hydrogen peroxide and oxygen [5]. Stress conditions, in which free radical generation occurs, result in the decrease in antioxidant enzyme activity, owing to their excessive utilisation [10]. The observed decrease in SOD activity in the Actinomycosis group could result from inactivation by H2O2. Decreased SOD activity in the present study is suggestive of free radical generation resulting in the depletion of this enzyme owing to its excessive utilisation.
CAT is considered by many scientists as an important and sensitive biomarker of oxidative stress, better than SOD. CAT also are the main enzymes of the enzymatic antioxidant defense system responsible for protection against an increase in ROS production. H2O2, formed by the catalytic reaction of SOD, is both a reactive form of oxygen and a normal cellular metabolite, and it is further detoxified by GPx and CAT. The catabolism of H2O2 leads to the formation of the superoxide radical anion [16, 17]. The data of the present study showed that CAT activity was significantly decreased in the group with Actinomycosis. The reduced activities of CAT could be due to their depletion or inhibition as a result of the increased production of free radicals. Glutathione is important for the detoxification of toxicants, thus measurement of its activity is considered as a good indicator of antioxidant status or oxidative stress. GSH is a major endogenous antioxidant that participates in detoxification reactions and counterbalances free radical mediated damage by eliminating the compounds responsible for lipid peroxidation. There is an inverse relationship between oxidative stress and glutathione levels due to increase in the utilisation [7]. The decline in glutathione levels in this study could be due to increased utilisation of this intracellular antioxidant by GPx or Glutathione-s-transferase. In addition, this declination can also be justified either due to the inhibited synthesis of GSH or increased utilisation of GSH for etoxification of toxicant-induced free radicals [3, 18].
GPx is the general name of an enzyme family with peroxidase activity whose main biological role is to protect the organism from oxidative damage. The biological function of GPx is to reduce lipid hydroperoxides conversion to their corresponding alcohols and to reduce free hydrogen peroxide reaction with [2]. Declines in circulating levels of the GPx could have been resulted from the occurrence of oxidative stress.
Glucose, cholesterol and LDL levels were significantly increased (P<0.001), HDL levels were significantly decreased (P<0.001) in the Actinomycosis group according to control group. Triglyceride concentrations were markedly increased (P<0.01) and Albumin concentration was decreased (P<0.01). The enzyme activities of ALT were significantly increased (P<0.05) and as to ASTenzyme’s activities were decreased (P<0.05). There was no statistically significant differences for the total protein levels in the Actinomycosis Group.
As a result, the concentrations of malondialdehyde were higher (P<0.001) and superoxide dismutase concentrations were lower (P<0.001), catalase, glutathione peroxidase and glutathione levels were significantly lower (P<0.01) in the cattles with Actinomycosis than in healthy ones. Glucose, cholesterol and LDL levels were significantly increased (P<0.001), HDL levels were significantly decreased (P<0.001) in the Actinomycosis group according to control group. Triglyceride concentrations were markedly increased (P<0.01) and albumin concentration was decreased (P<0.01). The enzyme activities of ALT were significantly increased (P<0.05) and as for AST enzyme’s activities ere decreased (P<0.05). There was no statistically significant differences for the total protein levels in the Actinomycosis Group.
References
- Akhtara M, Zadeb MP, Shahaneb PL, Bangdea AP, Soıtkar SM (2015) Scalp actinomycosis presenting as soft tissue tumour: A case reportwith literature review. Int J Surg Case Rep. 16: 99–101.
- Aly N, Gendy K, Mahmoud F, El-Sebae A (2010) Protective effect of vitamin C against chlorpyrifos oxidative stress in male mice. FAO. 97: 7–12.
- Beutler E (1989) Nutritional and metabolic aspects of glutathione. Annu Rev Nutr. 9: 287–302.
- Chakraborty SP, Roy S, Pramanık P (2016) In vitro dose and duration dependent approaches for the assessment of ameliorative effects of nanoconjugated vancomycin against Staphylococcus aureus infection induced oxidative stress in murine peritoneal macrophages. Microb Pathog. 91: 74-84.
- Dubey N, Raina R, Khan AM (2012) Toxic effects of deltamethrin and fluoride on antioxidant parameters in rats. Fluoride. 45(3): 242–246.
- Farooq U, Qayyum A, Samad HA, Chaudhry HR, Ahmad N (2010) Field Surgical Intervention of Bovine Actinomycosis. Pak Vet J. 30 (4): 249-250.
- Gill KK, Sandhu HS, Kaur R (2015) Evaluation of lipid peroxidation and antioxidant status on fenvalerate, nitrate and their co-exposure in Bubalus bubalis. Pestic Biochem Physiol. 123: 19–23.
- Goth L (1991) A simple method for determenation of serum catalase activity and revision of serum catalase activity and revision of reference range. Clin Chim Acta. 196(2-3): 143–152.
- Guo-Di L, Sheng Z, Wang YF, Han YL, Zhou Y, et al., (2015) Glutathione peroxidase 1 expression, malondialdehyde levels and histological alterations in the liver of Acrossocheilus fasciatus exposed to cadmium chloride. Gene 57(2): 210-218.
- Hertwig B, Steb P, Feierabend J (1992) Light dependence of catalase synthesis and degradation in leaves and the influence of interfering stres conditions. Plant Physiol. 100(3): 1547–1553.
- Masand A, Kumar N, Patial V (2015) Actinomycosis (lumpy jaw) in cow: a case report. Comp Clin Pathol. 24(3): 541–543.
- Matkovics B, Szabo L, Varga IS (1988) Determination of enzyme activities in lipid peroxidation and glutathione pathways. Laboratoriumi Diagnosztika. 15: 248–249.
- Mehta HK, Mehesave SS, Pradhan S (2012) Actinomycosis in a Bull- A Case Report. Intas Polivet. 13(1): 60-61.
- Meral I, Mert H, Mert N, Deger Y, Yoruk I, et al., (2007) Effects of 900- MHz electromagnetic field emitted from cellular phone on brain oxidative stress and some vitamin levels of guinea pigs. Brain Res. 1169: 120–124.
- Niki E (1996) Free radical- induced oxidative damage and nutritional oxidants. Proc of 2nd W.H.O. Symposium on Health Issues for 21st Century. 105-108.
- Regoli F, Gorbi S, Frenzilli G, Nigro M, Corsi I, et al., (2002) Oxidative stress in ecotoxicology: from the analysis of individual antioxidants to a more integrated approach. Mar Environ Res. 54(3-5): 419–423.
- Sinclair AJ (1993) Free radical mechanisms and vascular complications of diabetes mellitus. Diabetes Rev. 2: 7-10.
- Singh SP, Coronella JA, Benes H, Cochrane BJ, Zimniak P (2001) Catalytic function of Drosophila melanogaster glutathione S-transferase DmGSTS1-1 (GST2) in conjugation of lipid peroxidation end products. Eur J Biochem. 268(10): 2912–2923.
- Sun Y, Oberley LW, Li Y (1988) A simple method for clinical assay of superoxide dismutase. Clin Chem. 34(3): 497-500.
- Tietze F (1969) Enzymic method for quantitavite determination of nanogram amounts of total and oxidized glutathione. Anal Biochem. 27(3): 502- 522.
- Xiaolu W, Wang L, Ren Q, Yin S, Liang F, et al., (2016) Two superoxide dismutases (SODs) respond to bacterial challenge identified in the marbled eel Anguilla marmorata. Aquaculture. 451: 316–325.
- Yang L, Tan G, Fu Y, Feng J, Zhang M (2010) Effects of acute heat stres and subsequent stress removal on function of hepatic mitochondrial respiration, ROS production and lipid peroxidation in broiler chickens. Comp Biochem Physiol. 151: 204–208.
- Yoshioka T, Kawada K, Shımada T, Mori M (1979) Lipid peroxidation in maternal and cordblood and protective mechanism against activated- oxygen toxicity in the blood. Am J Obstet Gynecol. 135(3): 372-376.