A Rare Case Of Dedifferentiated Chondrosarcoma: Discrepancy Between Imaging And Histopathology Examination
Gede EWI, Satrio Adiwardhana GKI, Santosa C*
Faculty of Medicine, Department of Orthopaedic and Traumatology, Udayana University -Sanglah General Hospital, Bali, Indonesia.
*Corresponding Author
Claudia Santosa,
Department of Orthopaedic and Traumatology,
Faculty of Medicine, Udayana University -Sanglah General Hospital,
Bali, 80113, Indonesia.
E-mail: csantosa.md@gmail.com
Received: April 12, 2018; Accepted: April 30, 2018; Published: April 30, 2018
Citation: Gede EWI, Satrio Adiwardhana GKI, Santosa C. A Rare Case Of Dedifferentiated Chondrosarcoma: Discrepancy Between Imaging And Histopathology Examination. Int J Surg Res. 2018;5(2):94-99. doi: dx.doi.org/10.19070/2379-156X-1800021
Copyright: Santosa C© 2018. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Chondrosarcoma is a malignant tumor that produces cartilage matrix occurring in the third to sixth decades and has a male to female ratio of 2:1. Dedifferentiated chondrosarcoma is the most malignant form of chondrosarcoma and rare in its occurrence, accounting for approximately 10% of all chondrosarcomas, and is resistant to chemotherapy and radiotherapy. We reported a case of a 50-year-old woman having persistent and severe pain over her left distal thigh accompanied by progressive swelling in the thigh since 5 months ago. Laboratory tests were within normal limits. Imaging examination revealed a large soft tissue mass with moth-eaten pattern lytic lesion, wide zone transition, cortex destruction, with hair-on-end periosteal reaction, but there was no sign of abrupt transition between lytic lesion and cartilaginous tumor, which is indicative of dedifferentiated chondrosarcomas. Confirmation with histopathology study is consistent with the diagnosis of dedifferentiated chondrosarcoma. The correlation between clinical, radiographic findings, and histopathology study is essential for accurate diagnosis and optimal clinical management of dedifferentiated chondrosarcomas.
2.Introduction
3.Case Report
4.Discussion
5.Conclusion
6.References
Keywords
Dedifferentiated Chondrosarcoma; Diagnosis; Histopathology.
Introduction
Chondrosarcoma is a type of malignant tumor which originated from cartilage cells. Chondrosarcoma is the second most common primary malignant bone tumor, occurring more commonly in men that in women with a ratio of 2:1. It most frequently occurs in the third to sixth decades of life [1]. The most common sites are the femur, humerus, and pelvis. Chondrosarcoma is most commonly separated into three histological grades: low (grade 1), medium (grade 2), and high (grade 3). The higher the grade, the more likely it is to metastasize. A type of chondrosarcoma that has a higher risk of metastasizing than a grade 3 is the uncommon, so called dedifferentiated chondrosarcoma (DDCS) which is also the most malignant type of the chondrosarcomas. Metastases occur early and frequently involve the lungs, lymph nodes, and viscera. Regardless of the treatment, the prognosis is ominous with 90% of patients dying with distant metastases within 2 years. The median survival is 13 months with a five-year survival rate of 24%. Dedifferentiated chondrosarcoma is a fatal malignancy despite aggressive wide resection, or even amputation. Adjuvant chemotherapy or radiotherapy doesnot produce a statistically significant benefit.
Case Report
A 50 years old female presented to our hospital complaining of persistent and severe pain over the left lower thigh which started 3 months ago and has progressively worsen 2 days before admission. She also presented with gradually increasing swelling of left lower thigh for the last one month.She also experienced weight loss. No history of trauma and similar illness within the family were reported.
Physical examination revealed swelling, hyperemia, mild joint tenderness medial to the right knee, and limited range of motion of the hip and knee. Distal vascular was found to be patent, which included the dorsalispedis artery, posterior tibial artery, andpopliteal artery.
Routine laboratory investigations such as complete blood count, biochemical parameters, and tumor markers were within normal limit during initial evaluation. The result is as shown: Leucocytes 5.99.103μL (4.1-11.0); Hemoglobin 11, 3 g/dl (12-16); Hematrocit 36, 4% (36-46); Platelet 361.103μL (140-440); AST 14.2 U/L (11- 27), ALT 9,1 U/L (11-34); Albumin 3,94 g/dL (3.4 - 4.8); Glucose 112 mg/dL (70-140); BUN 7 mg/dL (8-23); Creatinine 0.39 mg/ dL(0.5-0.9); Sodium 133mmol/L (136-145); Potassium 4.42 mmol/L (3.5-5.1); LDH 255 U/L (80-240); ALP 91 mg/dL (42- 96); CRP 2.5 mg/L (0-5); CEA 0.59 μg/ml (<5); AFP <0.5μg/ml (<0.8); CA125 <4 (<35).
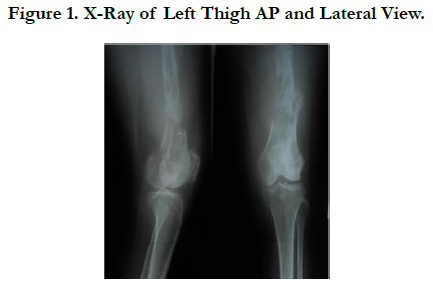
Plain radiographs of the left thighshowed moth-eaten pattern lytic lesion, wide zone transition, cortex destruction, with hairon- end periosteal reaction (Figure 1 and 2).
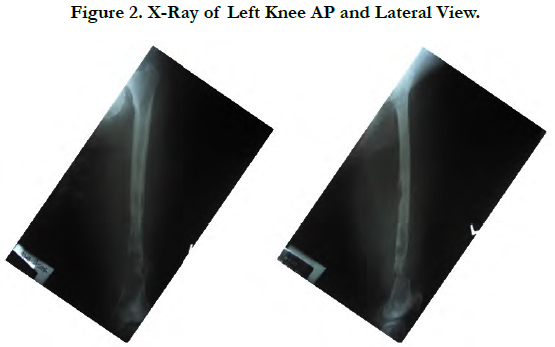
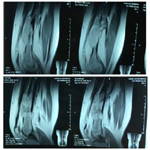
T2-weighted MRI of the left thigh demonstrated an irregular solid mass with well-defined margin, large destruction and intraosseous lesion with internal calcification located eccentrically within the distal third of femur (Figure 3).
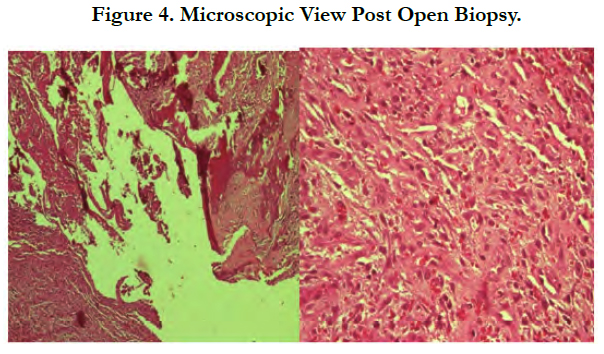
Open biopsy showed tissue specimen containing tumor mass composed of proliferating neoplastic cells partially infiltrative in between the hyaline cartilage and partly forming a solid structure. Some of the neoplastic cells showed appearance of an oval-like round shape with increasing N/C ratio, mild nuclei pleomorphic, and vesicular chromatin with prominent nucleoli. Majority of the neoplastic cells showed a spindle shape appearance with eosinophilic cytoplasm, partly vesicular chromatin within the nuclei and prominent nucleoli. Mitotic count number is 5/10 highpower fields. The tissue is surrounded by what appeared to be trabecular bone with high distribution of osteoclastic giant cells. Necrotic area is present with dense infiltration of inflammatory polymorphonuclear cells and lymphocytes with scant number of plasma cells and macrophages (Figure 4).
The tissue specimen consists of trabecular bone and bone marrow composed of hematopoietic cells. There is no increase in the number of plasma cells. The conclusion was dedifferentiated chondrosarcoma.
The histopathological examination after wide excision contained two pieces of tissue specimens. The size of tissue specimen I is 20x9x4 cm and tissue specimen II is 9x4x1.5 cm covered with skin in the size of 6.5x 1.2x 0.5 cm. The tumor mass is 18.5x9x4 cm in size, irregular in shape, white and brownish grey in color with rubbery consistency on specimen I. The distance between the tumor mass and the resection border is 1 cm proximal and 0.5 cm distal. There is an infiltrative tumor mass surrounding the bone tissue. Tissue specimen II consist of muscle tissue, fat and connective tissue covered with skin and no tumor mass.
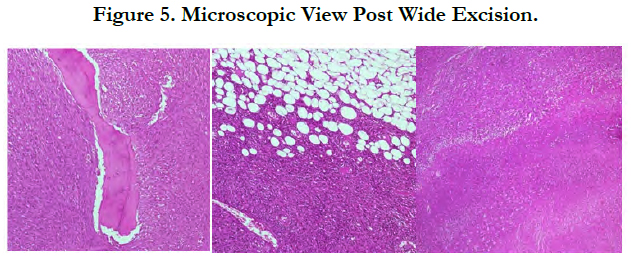
The tissue specimen containing the tumor mass is composed of the proliferation of neoplastic infiltrative cells embedded in between the connective tissue, fat, bone trabecular and skeletal muscle surrounding the mass to form a solid structure. Fasciculus, storiform and the rest formed a cartwheel structure. There are necrosis areas (+25%-50% of tumors) with infiltration of inflammatory polymorphonuclear cell, lymphocytes and a small number of macrophages. Extravasation of erythrocytes could also be seen (Figure 5).
Neoplastic cellsshowed spindle morphology, eosinophilic cytoplasm, spindle nuclei, increasing N/C ratio, moderate to severe nuclei pleomorphic, partly hyperchromatic, partly vesicular chromatin with prominent nucleoli. Mitotic count number is 10 to 40/10 high-power fields. Intravassa invasion is not seen. There are mild infiltration of lymphocytes and distribution of osteoclast giant cells around the tumor mass.
The tissue specimen containing the tumor mass is composed of proliferation of chondroblastic infiltrating neoplastic cells embedded in between the surrounding cartilage matrix. The outer section of neoplastic-like cells formed a solid structure, fasciculus, storiform and the other cells are forming an infiltrative cartwheel structure among the surrounding connective tissue.
Chondroblastic neoplastic cellsshowed oval round morphology with basophilic cytoplasm, round oval nuclei, increasing N/C ratio, mild nuclei pleomorphic, hyperchromatic, some of which appeared with prominent nucleoli. Mitosis was difficult to find.
Neoplastic cellsshowed spindle morphology with eosinophilic cytoplasm, spindle nuclei, increasing N/C ratio, severe nuclei pleomorphic, some hyperchromatic and some vesicular chromatin with prominent nucleoli.
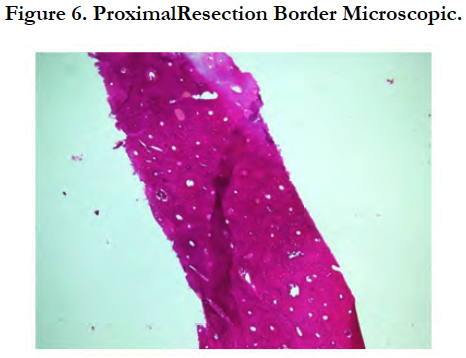
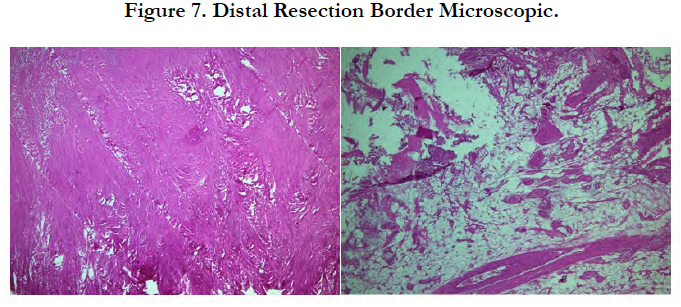
The tissue specimen consisting of bone trabecule (Figure 6) revealed no infiltration with neoplastic cells. Similar findings were found on the tissue specimen consisting of cartilage tissue, connective tissue, mature fat tissue and blood vessels (Figure 7).
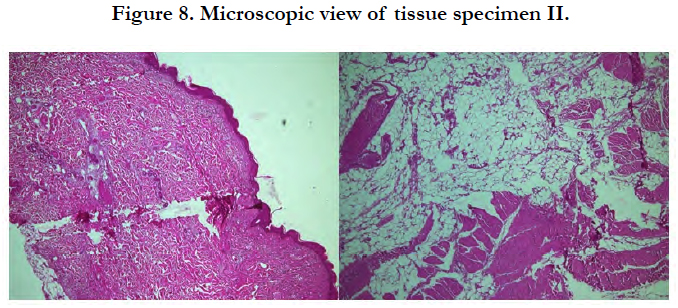
Microscopic view fromtissue specimen II (Figure 8) showed the tissue was covered with skin and consisted of fibrocollagen connective tissue, skeletal muscle, mature fat tissue and blood vessels. There are multinucleated giant cells and scant number of lymphocytes cells. No infiltration of neoplastic cell was found.
The tissue specimen from the trabecular bone as well as the bone marrow tissue showed infiltration of neoplastic cells and formed a solid structure. The neoplastic cells showed similar morphology to the tumor mass found in the bone.
The result from the tissue biopsy post wide excision concluded that the left distal femur sample showed dedifferentiated chondrosarcoma (DDCS), pT2 pNxpMx. The proximal and distal resection border were free from neoplastic cells and there’s no infiltration of neoplastic cell in tissue sample II. There is evidence of neoplastic cells infiltration in the specimen taken from the medulla of proximal femur.
The tumor had undergone surgical wide excision with safe margin. Wide excision was done from left femoral shaft until distal femur followed by placement of Kuntscher nail and bone spacer until proximal of left tibia.
Discussion
Chondrosarcoma is the second most common primary malignant bone tumor which originates from cartilage cells. Chondrosarcoma is occurring more commonly in men that in women with ratio 2:1. It is most frequently in the fourth to sixth decades. The most common localizations are at the femur, humerus, and pelvis. The most frequently reported symptoms are pain, soft tissue masses, and pathologic fractures [2]. Correlation of the clinical, radiographic, and histopathology data is essential for accurate diagnosis and evaluation of the aggressiveness and metastatic potential of a tumor. Radiographs are important not only for detection but also to decide on follow-up analysis with MRI or histology. MRI is used to depict the extent of intraosseous and soft tissue involvement. Radiographic findings often suggest the diagnosis of chondrosarcoma because of identification of typical "ring-and-arc" chondroid matrix mineralization (representing the enchondral ossification) and aggressive features of deep endosteal scalloping and soft-tissue extension. Chondrosarcomas grow with lobular type architecture, and these hyaline cartilage nodules demonstrate high water content and peripheral enchondral ossification [3].
There are numerous types of primary chondrosarcomas, including conventional intramedullary, clear cell, juxtacortical, myxoid, mesenchymal, extraskeletal, and dedifferentiated. The term dedifferentiated chondrosarcoma (DDCS) was introduced by Dahlin and Beabout in 1971, which is the most malignant type of the chondrosarcoma. Dedifferentiated chondrosarcomas account for approximately 10% of chondrosarcomas. They are most frequent in 50 and 60 years and the age range 29 to 85 years [2]. An essential histological feature of DDCS is an abrupt transition between the cartilaginous, either an enchodroma or a low grade chondrosarcoma and high grade non cartilaginous sarcoma components. Staals et al., in 2007, classified DDCS into central lesions (those arising in enchondromas) and peripheral lesions (those arising in osteochondromas); they found nodifference in the survival between both classes of lesions [4]. At least three mechanisms for the origin of dedifferentiated chondrosarcoma have been hypothesized. The most frequent theory is that the high-grade noncartilaginous component arises in a longstanding lower-grade chondrosarcoma. A second hypothesis is that the noncartilaginous sarcoma results from malignant transformation in an inflamed fibrous pseudocapsule of a lower-grade chondrosarcoma. Finally, the third theory is the possibility that malignant cell lines arise simultaneously in a chondrosarcoma with differing ability to differentiate [3, 5, 6]. The definitive diagnosis of dedifferentiated chondrosarcoma always is based on the histology which is divided in a variety of ways, including by histological grade and by whether it is primary or secondary or peripheral or central.
The clinical result of current case are woman, 50-year-old, having persistent and severe pain over her left distal thigh since 5 months ago. From literature, most commonly located in the pelvis, femur and humerus [5]. The most commonly involved sites are bones of the pelvis (the ilium is the most frequently involved bone), followed by the proximal femur, proximal humerus, distal femur, and ribs. In the long bones, it occurs in the metaphysis or diaphysis. The most frequently reported symptoms are pain, soft tissue masses, and pathologic fractures [2, 4, 7].
DDCS may have a wide range of radiographic appearances, depending on the proportion of conventional chondrosarcoma that has transformed to a non-cartilaginous high-grade malignancy. When the proportion of high-grade lesion is small, the lesion may appear identical to a low-grade chondrosarcoma with an elongated multilobulated osteolytic lesion, cortical thickening, and endosteal scalloping containing “rings and arcs” of chondroid. As the high-grade non-cartilaginous focus increases in size, there is a progression of aggressive bone lysis and a decrease in matrix mineralization. Associated soft-tissue masses, often large, are almost invariably seen. Radiologically, there was lytic lesion with presence of heavily calcified centralarea surrounded by less denser periphery with splotchy calcification and extension of lesion to the soft tissue as seen in other studies [5, 8, 9]. The abrupt transition between lytic lesion and cartilaginous tumor that usually is evident on X-ray inclassical dedifferentiated chondrosarcomas [10]. This cartilaginous malignancy is characterized by conventional lowgrade chondrosarcoma with abrupt transition to foci that have dedifferentiated into a higher-grade, more aggressive component. The non-cartilaginous component may be either small or extensive and is most frequently malignant fibrous histiocytoma, osteosarcoma, or fibrosarcoma [3]. In our case, plain radiographs of the left thigh showed lytic lesion with a moth-eaten pattern, wide zone oftransition, cortex destruction with hair-on-end periosteal reaction and large soft tissue mass.
Magnetic Resonance Imaging (MRI) can be helpful in differentiating between benign and malignant lesions in several ways. Greater than 90% medullary involvement can be suggestive of chondrosarcoma, while the absence of 90% medullary involvement of non-contiguous areasof cartilage within the bone can suggest the presence of an enchondroma [1]. Many surgeons consider MRI critical for surgical planning because it can illustrate the extent of tumor involvement in bone and soft tissues [3, 11]. In our case, the patient had a tumor developed on the left distal surface of the femur with irregular solid mass, well defined margin, large destruction and intraosseous lesion with internal calcificationlocated eccentrically.
The definitive diagnosis of dedifferentiated chondrosarcoma is based on the histology which characteristically shows the presence of a well-differentiated chondrosarcoma juxtaposed to an anaplastic, non-chondroid sarcoma. The differentiated portion of the tumor consists most often of malignant fibrous histiocytoma or fibrosarcoma. Less commonly, however, osteosarcomatous or rhabdomyosarcomatous elements may also be present. There is abrupt demarcation between the two components and binucleation frequently seen. Permeation of cortical bone is an important characteristic. From this case, both open biopsy and post wide excision showed proliferation of spindle-shaped neoplastic cells, eosinophilic cytoplasm, nuclei partly vesicular chromatin with prominent nucleoli. Some of the neoplastic cells showed a picture like oval round shape, increasing N/C ratio, mild nuclei pleomorphic, vesicular chromatin with prominent nucleoli. Around the tissue seems trabecular bone, distribution of osteoclast giant cells, necrotic area with dense infiltration of inflammatory cells PMN, lymphocytes and a little bit of plasma cells and macrophages. Both of proximal and distal resection showed no infiltration of neoplastic cell was found.It summarized the dedifferentiated chondrosarcoma (DDCS). As reported in earlier study, Dedifferentiated areas may resemble malignantfibrous histiocytoma (MFH), osteosarcoma andfibrosarcoma. In this case, areas of osteosarcoma, fibrosarcoma and MFH were present with predominance of MFH [11]. Other study showed the abrupt transition between low-grade chondrosarcomas and fibrosarcoma [3].
Wide or radical surgical margins are mandatory in patients with dedifferentiated central chondrosarcomas according to established oncologic principles of sarcoma surgery. Chemotherapy usually is added to the treatment whenever the dedifferentiated component is sensitive to chemotherapy and the patient is in good general condition. Histologic review indicated that the tumor response to preoperative chemotherapy was very poor, which is consistent with previous reports by others. In a recent retrospective study from the Mayo Clinic on dedifferentiated chondrosarcomas, Dickey et al., reported no statistically significant difference in survival between 15 patients who underwent surgery alone and 22 patients who were managed with a combination of surgery and chemotherapy. In addition, those authors observed that patients had poor tumor responses after they received preoperative chemotherapy, and they reported that the role of chemotherapy in dedifferentiated chondrosarcomas should be reconsidered. Current study showed no evidence that chemotherapy improves the outcome of patients with dedifferentiated central chondrosarcoma. First, tumor responses were poor in all patients who received preoperative chemotherapy. Second, if all patients with non-metastatic disease who both underwent surgery and received neoadjuvant chemotherapy were compared with patients who underwent surgery alone, then there was no difference in overall survival.
Conclusion
The correlation between clinical, radiographic findings, and histopathology study is essential for accurate diagnosis and optimal clinical management of dedifferentiated chondrosarcomas.
References
- Kenneth H, Randall R, Chondrosarcoma in: Oncol. Basic Sci. 7th ed. Lippincott Williams & Wilkins; 2008. p. 191–202.
- Milchgrub S, Hogendoorn P. Dedifferentiated chondrosarcoma. World health organization classification of tumours Pathology and genetics Tumours of soft tissue and bone. 2002.
- Murphey MD, Walker EA, Wilson AJ, Kransdorf MJ, Temple HT, Gannon FH. From the archives of the AFIP: imaging of primary chondrosarcoma: radiologic-pathologic correlation. Radiographics. 2003 Sep;23(5):1245-78. PubMed PMID: 12975513.
- Staals EL, Bacchini P, Bertoni F. Dedifferentiated central chondrosarcoma. Cancer. 2006 Jun 15;106(12):2682-91. PubMed PMID: 16691621.
- Littrell LA, Wenger DE, Wold LE, Bertoni F, Unni KK, White LM, Kandel R, Sundaram M. Radiographic, CT, and MR imaging features of dedifferentiated chondrosarcomas: a retrospective review of 174 de novo cases. Radiographics. 2004 Sep;24(5):1397-409. PubMed PMID: 15371616.
- Mercuri M, Campanacci L. Dedifferentiated chondrosarcoma. Skeletal Radiol. 1995 Aug 1;24(6):409-16.
- Swarts SJ, Neff JR, Johansson SL, Bridge JA. Cytogenetic analysis of dedifferentiated chondrosarcoma. Cancer Genet Cytogenet. 1996 Jul 1;89(1):49- 51.
- Rosai J. Bone and joints. Rosai and Ackerman's Surg Pathol. 2004.
- Bansal R, Pai RR, Nayak R, Raghuveer CV. Metastatic chondrosarcoma in soft tissue diagnosed by fine needle aspiration (FNA) cytology. Cytopathology. 1996 Feb;7(1):70-2. PubMed PMID: 8833878.
- Şişu AM, Tatu FR, Stana LG, Petrescu CI, Tatu C, Motoc A. Chondrosarcoma of the upper end of the femur. Rom J Morphol Embryol. 2011;52(2):709-13. PubMed PMID: 21655665.
- Badge SA, Gangane NM, Shivkumar VB, Sharma SM. Dedifferentiated chondrosarcoma of right proximal femur. Med. J. Dr. D.Y. Patil Univ. 2016 Mar 1;9(2):261.