Design and Experimental Analysis of a Cetirizine Hydrochloride Drug Sensor
Ren LJ*, Xu G
College of Safety Engineering, Chongqing University of Science and Technology, Chongqing, China.
*Corresponding Author
Lingyan J Ren,
College of Safety Engineering,
Chongqing University of Science and Technology,
Chongqing, 401331, China.
E-mail: lingyanr@yahoo.com
Received: February 22, 2018; Accepted: May 16, 2018; Published: May 17, 2018
Citation: Ren LJ, Xu G. Design and Experimental Analysis of a Cetirizine Hydrochloride Drug Sensor. Int J Nano Stud Technol. 2018;7(1):128-131. DOI : dx.doi.org/10.19070/2167-8685-1800023
Copyright: Ren LJ© 2018. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
A cetirizine hydrochloride PVC membrane drug sensor was developed by using the ion association complex produced with sodium tetra phenyl borate and cetirizine hydrochloride as electroactive substance. In sodium dihydrogen phosphate-sodium hydroxide buffer solution of pH 5.0, the linear range of the sensor is 1.58×10-6~1.0×10-1 mol/L, the slope is 46mV/pC (20°C) and the detection limit is 6.31×10-7 mol/L. The sensor has fast response and good reproducibility. Using the sensor for the determination of cetirizine hydrochloride in drugs, the relative standard deviation is less than 3% and the results are consistent with the labeled content in preparations.
2.Introduction
3.Experiment
3.1 Main Instruments and Reagents
3.2 Preparation of Electroactive Substance
3.3 Preparation of PVC Membrane
3.4 Sensor Assembly and Electric Potential Determination
4.Experimental Results and Discussion
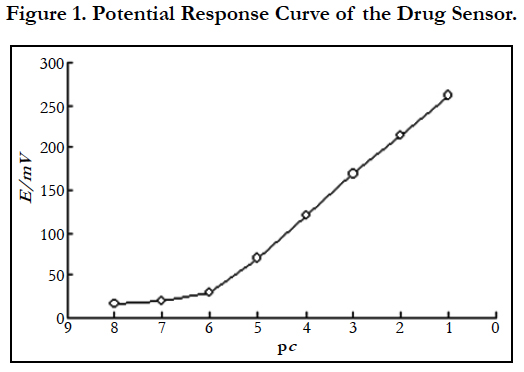
4.1 Potential Response Curve of the Sensor
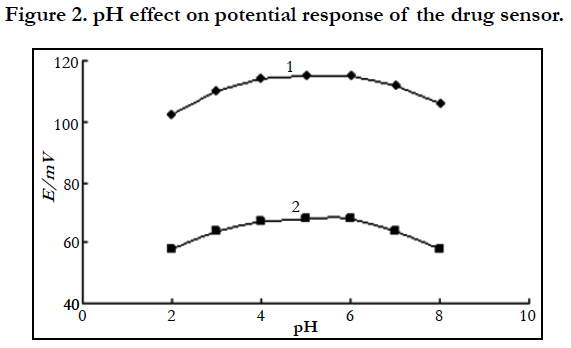
4.2 Influence of pH Value on the Potential Response Performance of the Sensor
4.3 Composition Optimization of Sensor Membrane
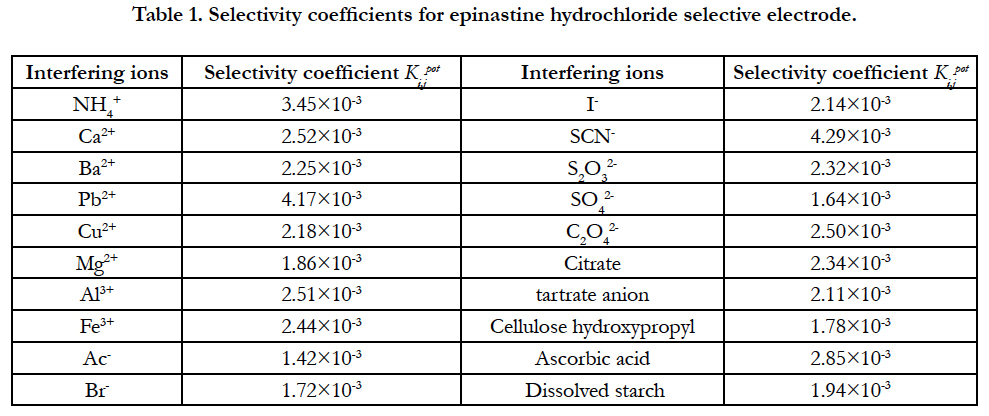
4.4 Selectivity of the Sensor
4.5 Response Mechanism of the Sensor
4.6 Application of the Sensor
5.Conclusion
6.References
Key Words
Cetirizine Hydrochloride; Drug Sensor; Development; Application.
Introduction
The chemical name of cetirizine hydrochloride is (±)-2-[2-[4-(4-chlorophenyl) phenylmethyl]-1-piperazinyl] ethoxy acetic acid dihydrochloride (C21H25ClN2O3•2HCl). Cetirizine hydrochloride is an antihistamine with selective antagonism to H1 receptors. Characterized by rapid onset, long duration of action and good safety, it is now a clinical medicine of autonomous production commonly used in China for treating allergic rhinitis, urticarial, allergic skin pruritus and other allergic diseases. According to literature, methods for the determination of cetirizine hydrochloride include high performance liquid chromatography [1], high performance thin layer chromatography [2], liquid chromatography-mass spectrometry [3], UV spectrophotometry [4], capillary electrophoresis [5], capillary electrophoresis electrochemiluminescence [6], potentiometric titration [7], perchloric acid titration [7], linear sweep polarography [8], electrochemiluminescence [9] and fluorescence quenching method [10], etc. These methods are cumbersome and have high requirements for instruments and equipment’s. Though they have shown that the system can be miniaturized by a handheld system [11-13], this is yet to be done. In this paper, a cetirizine hydrochloride PVC membrane drug sensor was developed by using the ion association complex produced with sodium tetraphenylborate and cetirizine hydrochloride as electroactive substance. Using the sensor for the determination of cetirizine hydrochloride in drugs, the relative standard deviation is less than 3%. The sensor has high response speed, simple operation, high sensitivity and high determination accuracy.
Here is the list of the instruments and the reagents used for this experiment. PHS-3B precision pH meter (Shanghai Precision Instruments Co., Ltd.); TU-1901 UV-visible spectrophotometer (Beijing Purkinje General Instrument Co., Ltd.), 232-saturated calomel electrode (Shanghai LIDA Instrument Factory).Onitro phenyl octyl ether (O-NPOE) (synthesized according to [14]). Phosphate buffer solution (pH 5.0): take 500mL sodium dihydrogen phosphate solution of 0.2mol/L and adjust the pH value to 5.0 with sodium hydroxide solution of 0.2mol/L.
PVC adhesive: weigh PVC powder of appropriate amount, dissolve it with tetrahydrofuran and prepare PVC adhesive with mass fraction of 8% and 5% respectively; Cetirizine hydrochloride standard solution (1.0mol/L) and deionized water.
Drip 50mL sodium tetraphenylborate solution of 0.1mol/L into 50mL cetirizine hydrochloride solution of 0.1mol/L while stirring. A white precipitate is produced that contains the chlorine ions, the precipitated part is washed with deionized water until without Cl- ions removed and dry it under vacuum at 60°C to obtain electroactive substance. To ensure that the Chlorine ion has been removed, a testing with AgNo3 was performed.
9.2mg of electroactive substance, 248.2mg plasticizer and 1.32g PVC adhesive of 8% were added into a small beaker successively. The solution was stirred quickly and poured on a clean small sheet glass (try to remove bubbles) and let it air dry. Doing this we were able to obtain PVC membrane.
The PVC membrane obtained was cut and stuck on a 1 cm diameter PVC pipe with PVC adhesive of 5%, 0.1mol•L-1 potassium chloride solution. The adhesive was then left to air dry and an Ag/AgCl electrode was inserted to form a PVC membrane drug sensor. Soak the sensor in 1.0×10-3mol•L-1 cetirizine hydrochloride standard solution for 30min before use, and determine the electric potential of the sensor according to the following formula: Ag/AgCl |0.1mol•L-1 KCl | PVC membrane | test solution‖KC1 (saturated), Hg2C12, Hg.
Experimental Results and Discussion
Dilute with phosphate buffer solution of pH 5.0, prepare 50mL cetirizine hydrochloride standard solutions of 10-1mol/L~10-8mol/L respectively, use the self-made cetirizine hydrochloride drug sensor as indicator electrode and saturated calomel electrode as reference electrode to measure the electrode potential of each solution and draw the potential response curve of the sensor.
From Figure 1, it can be seen that the sensor has a good selectivity for cetirizine hydrochloride. The linear response range of the sensor is 1.58×10-6~1.0×10-1mol/L, the slope is 46mV/pC (20°C), the detection limit is 6.31×10-7mol/L and the response time is t95% < 10s. Using the sensor to measure cetirizine hydrochloride solutions of 1.0×10-2mol/L and 1.0×10-4mol/L 20 times alternately (20°C), the relative standard deviation of potential readings is less than 5%. After 2 months of continuous use, the potential response performance has not declined.
According to the potential response curve test method of the sensor, the influence of hydrochloric acid-sodium hydroxide, acetic acid-sodium acetate, acetic acid-ammonium acetate and sodium dihydrogen phosphate-sodium hydroxide buffer solutions on the performance of the cetirizine hydrochloride drug sensor was tested respectively.
The results showed (Figure 2) that, when sodium dihydrogen phosphate and sodium hydroxide solution is used to adjust the acidity and the pH value of the solution is between 4~6, the sensor has optimal performance and the potential reading is stable. Therefore, sodium dihydrogen phosphate-sodium hydroxide buffer solution of pH 5.0 was used to control the acidity in the experiment.
In sodium dihydrogen phosphate-sodium hydroxide buffer solution, the influence of different types and different amounts of plasticizer (o-nitrophenyl octyl ether, dimethyl phthalate, dioctyl succinate and dioctyl phthalate, etc.), different amounts of electroactive substance and different amounts of PVC on the potential response performance of the sensor were tested. The results showed that, when dioctyl phthalate is used as plasticizer and electroactive substance: PVC: dioctyl phthalate = 2.5: 29.1: 68.4 (w/w), the potential response performance measured is ptimal and the potential reading is most stable.
The selectivity coefficient of the sensor was measured under interference from some common ions and some organic matters by using the different solution and iso-activity method. Among them, Cl-, Na+, K+, Zn2+ and glucose do not interfere with the measurement. Table 1 shows the selectivity coefficient for other interfering substances.
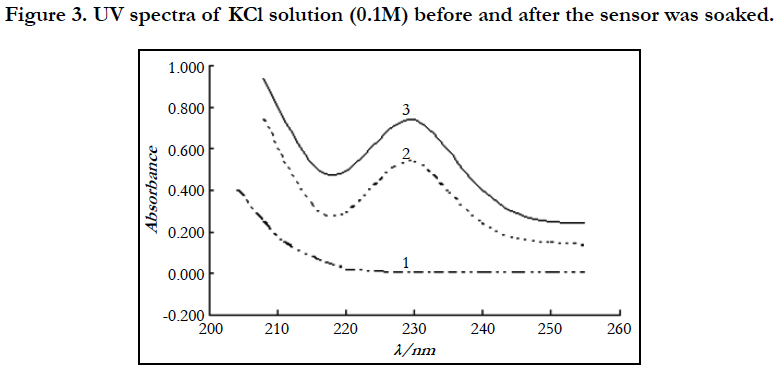
The response mechanism of drug sensors is seldom studied. Someone once used AC impedance to study the response mechanism of drug sensors [15], stating that electroactive substances (contained ions) in the membrane are mainly ionic substance and deeming that the response mechanism of drug sensors might have something to do with the migration and diffusion of solution contained ions (drug ions) and membrane contained ions. In order to further confirm this conclusion, the ultraviolet absorption spectra of KCl solution in a pipe were tested before and after the sensor was soaked in cetirizine hydrochloride solution of 10-3 mol/L.
The results showed (Figure 3) that, before the sensor is soaked, the KCl solution basically has no ultraviolet absorption. After the sensor was soaked in cetirizine hydrochloride solution of 10-3 mol/L for 2h, it was taken out to immediately measure the ultraviolet absorption spectrum of the KCl solution, and the spectrum was compared with the ultraviolet absorption peak of cetirizine hydrochloride solution. The two spectra are basically the same, with obvious absorption peak at 231nm. It indicates that cetirizine hydrochloride drug ions in the test solution migrate and diffuse with membrane contained ions through the membrane channels and enter the KCl solution and thus the response mechanism of the drug sensor mainly depends on the migration and diffusion of drug ions.
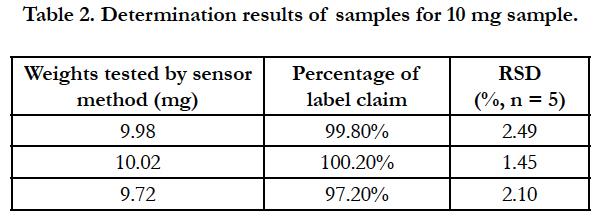
Accurately weigh 10 cetirizine hydrochloride tablets (manufactured by Lunan Beite Pharmaceutical Co., Ltd.) and grind them, weigh about 0.1g and put it in a small beaker, dissolve with deionized water and filter, adjust the pH value to 5.0 with sodium dihydrogen phosphate-sodium hydroxide solution, set the volume to a 50mL volumetric flask, and measure the content with the cetirizine hydrochloride drug sensor by using the standard curve method (Table 2).
Conclusion
The cetirizine hydrochloride drug sensor developed in this paper is characterized by fast response, high sensitivity, good selectivity, simple operation and economical efficiency of instruments required, etc. Using the sensor for content determination of cetirizine hydrochloride in tablets, the relative standard deviation is less than 3% and the results are consistent with the labeled content in preparations.
References
- Fang K, Ruijing LI. Determination of Cetirizine hydrochloride Dipersible Tablets by HPLC. Qilu Pharma. Affairs. 2009;25(5):282-283.
- Makhija SN, Vavia PR. Stability indicating HPTLC method for the simultaneous determination of pseudoephedrine and cetirizine in pharmaceutical formulations. J Pharm Biomed Anal. 2001 Jun 1;25(3-4):663-7.
- Yu lG, Wen YN, Song Z, et al. HPLC-MS determination of cetrizine hydrochloride in human plasma. Chin. J Anal Lab. 2007;26(4):73-76.
- Li Jing, Yu Jun, Hou FY. Using UV Spectrophotometre Method to Determine the Effective Contentof Levo-cetirizine Hydrochloride Tablets. J Huaihua Univ. 2006;25(8): 65-66.
- Wei L, Zhou W, Yu R. Determination of cetirizine in cetirizine HCl syrup by capillary zone electrophoresis. Chin. New Drugs J. 2000;10:694-7.
- Chen Lihui, Yang Ran, Zeng huajin, etc. Determination of Cetirizine Hydrochloride by Capillary Electrophoresis-electrochemiluminescenc. J Zhengzhou Univ (Nat. Sci. Ed.). 2009;41(3):64-67.
- Xu WX, Xie MF. Research on determination of cetirizine hydrochloride. Chin. J Pharm. 2003;34(7):343-4.
- Xie Tianyang, Tuo Honggui. Study on Linear Scanning Polarographic Method of Cetirizine Hydrochloride. Journal of Longyan University. 2006;24(6):58-59.
- Song HJ, Zhang ZJ. Electrochemiluminescent determination of cetirizine hydrochloride based on Ru (bpy)3(2+)immobilized in a nano-titania/nafion membrane. Chin. J Anal Lab. 2007;26(2):1.
- Kedia S, Samson SA, Farmer A, et al. Handheld interface for miniature sensors. In Smart Structures, Devices, and Systems II. International Society for Optics and Photonics. 2005 Feb 28;5649:241-253.
- Rogers AA, Samson S, Kedia S. Far-field evanescent wave propagation using coupled subwavelength gratings for a MEMS sensor. J Opt Soc. 2009 Dec 1;26(12):2526-31.
- Rogers AA, Kedia S, Samson S, Bhansali S. Verification of evanescent coupling from subwavelength grating pairs. J Appl Phys B. 2011 Dec 1;105(4):833-7.
- Kedia S, Wang W. Simulation, design, fabrication, and testing of a MEMS resettable circuit breaker. J Microelectromech Syst. 2015 Feb;24(1):232-40.
- Horning EC. Org. Synth Coll Vol III. NewYork: John Wiley;1955:140.
- XU Xiaoxian, VV Cosfret, Zhang Z. Study on Electrochemicalmpedance Spectroscopy of Drug Sensor. Chemical Sensors. 1991;11(1):45-50.