Development and Evaluation of an Aqueous Polymeric Dispersion of Eudragit L 100-55 Using Emulsification Technology
Shruti Singh1, Ashu Mittal1, Neha Gupta1, Nitesh Chauhan1, Sanjar Alam1*
1 Department of Pharmaceutics, KIET School of Pharmacy, Ghaziabad, U.P, India.
*Corresponding Author
Sanjar Alam
Department of Pharmaceutics,
KIET School of Pharmacy,
Ghaziabad, U.P, India.
Tel: +91- 9891674226
E-mail: sanjaralam10@gmail.com
Article Type: Research Article
Received: November 07,2013; Accepted: November 21, 2013; Published: November 22, 2013
Citation: Alam S, et al. (2013). Development and Evaluation of an Aqueous Polymeric Dispersion of Eudragit L 100-55 Using Emulsification Technology, Int J Nano Stud Technol, 02(05), 33-39. doi: dx.doi.org/10.19070/2167-8685-130007
Copyright: Alam S© 2013.This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Aqueous polymer dispersions (APD) are preferred on environmental and safety grounds. APD offer several advantages over polymers dissolved in organic solvents including lower spraying viscosities, higher solids loading, higher spray rates, no solvent environmental, toxicity, or flammability issues, and reduced energy requirements. The purpose of this work is to prepare and characterize an aqueous-based pseudolatex dispersion of Eudragit L100-55 using emulsification technology with superior stability. The prepared APD is evaluated on the basis of organoleptic, physical, chemical and in-vitro drug release performance. APD film is prepared by casting method and evaluated. Coating is done over Diclofenac sodium tablet and In vitro drug release profile of APD coated tablet and organic coated tablet is studied and shows better release in case of APD as compared to market formulation and organic coating.
2.Introduction
2.1 Types of aqueous polymeric dispersion
2.2 Different techniques used in APD
3.Materials and Methods
3.1 Materials
3.2 Development of an APD
3.3 Pseudolatex (APD) characterization
3.4 Evaluation of APD film
4. Result and Discussion
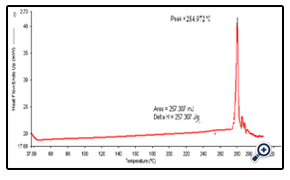
4.1 DSC of drug
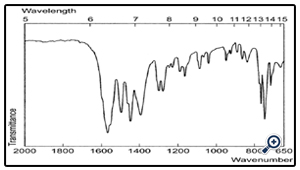
4.2 FTIR study
4.3 HPLC method for diclofenac sodium
4.4 APD Development
4.5 Characterization of APD
4.6 Evaluation of film
4.7 Drug release
4.8 Drug release kinetics
4.9 Stability studies
5.Conclusion
6.Acknowledgement
7.References
Key Words
Aqueous Polymeric Dispersion (APD); Emulsification Solvent Evaporation; Latex; Pseudolatex; TEM; DSC.
Introduction
Coating with water insoluble polymers is an important technique for targeted or controlled release formulations and the taste-masking of drugs, during which the aqueous polymer dispersions are widely used as an alternative to an organic polymer coating system [1] .Water soluble polymers are always being in good demand because of their certain advantages over organic solvents with respect to ecological, toxicological and manufacturing safety concern, high cost of organic solvents, solvent toxicity [2].
Aqueous polymeric dispersions, latexes, or pseudo latexes are all colloidal systems in which high molecular weight polymers are homogeneously dispersed in submicron sizes with the aid of surfactant(s) and other stabilizing agents. aqueous dispersion containing hydrophobic material that are useful as reduced volatile content may be used as a coating , resin, or an additive [3].
Aqueous polymeric dispersions that are widely used by the pharmaceutical industry are manufactured using different processes and different starting materials. They are classified into:
Latexes are aqueous polymeric dispersions produced by emulsion polymerization. The homogeneous and heterogeneous nucleation models have been proposed to describe such processes. As a direct outcome of the manufacturing process, latex may contain not only polymers, but also surfactants, as well as traces of initiators and monomers that must be eliminated as completely as possible from the lattice used in pharmaceutical applications [4,19,20].
Pseudo latexes are prepared by dispersion of the bulk polymer in an aqueous medium. The pseudolatex dispersion, a coating formulation often includes plasticizers to enhance the flexibility of the film and facilitate polymer sphere coalescence, antiadherents to prevent substrate agglomeration during both the coating process and storage, surfactants to promote spreading of the atomized droplets own the substrate surface, and pigments. The addition of other excipients can significantly impact the physical stability of the dispersion, drug release, and film quality [5,19,20].
An APD can be prepared in two different forms:
a. Ready to use: milky white liquid
b. Freeze dried /spray dried: powdered
Water insoluble polymers can be converted into aqueous dispersions by emulsion polymerization, emulsion solvent evaporation, phase inversion, or solvent change method.
1. Emulsification polymerization
»» Direct Emulsification/ emulsion solvent evaporation
»» Self Emulsification
»» Phase Inversion Emulsification or Solvent change method
2. Nanotechnology
3. pH modification
4. Phase separation/ coacervation
Eudragit L100-55 was received as a gift sample from Evonik industries, Mumbai. Diclofenac sodium was purchased from Omega pharmaceuticals, India and Poloxamer 407 & Sodium lauryl sulphate (SLS) were supplied by Central Drug House (P) Ltd., Mumbai. All other chemicals were of either reagent or analytical grade as received.
Several formulations were developed using the emulsification methodology. Solvents for the organic phase was acetone, SLS was taken as surfactant and poloxamer 407 as stabilizer [10,15,-18].
The Eudragit L100-55 dispersion was prepared by emulsion solvent evaporation method. Accurately weighed Eudragit L100-55 was dissolved in acetone, and the resulting solution was dispersed in 50 ml of aqueous phase having surfactant and stabilizer, at room temperature using a Mechanical stirrer (Hicon Instrument Manufacture Co. Ltd. India). The type of the emulsion was determined by diluting the emulsions with either water or acetone. Then the emulsion was subjected to rota evaporator (Perfit, India).To optimize the formulation various batches were made which is given in Table 2.
»» Organoleptic characterization: On the basis of color, odour and appearance APD was characterized [11].
»» Viscosity: Viscosity was measured with a Brookfield DV III Ultra programmable rheometer at 20°C, 30 rpm [11,12,13].
»» Differential scanning Calorimeter (DSC): DSC study is done for the authentication of the drug [14].
»» Particle size distribution: It is measured by zeta sizer instrument Malvern Instrument outsourced from Jamia Hamdard [14].
»» Scanning electron microscopy: Scanning electron microscopy has been used to determine the surface morphology and texture. SEM studies were carried out by using Zeiss scanning microscope [16,17].
»» Transmission electron microscopy: Transmission electron microscopy has been used to determine the surface morphology and texture [18,19].
»» Zeta Potential: Zeta potential is measured by zeta potential analyzer outsourced from Jamia Hamdard [14,20]
»» Solid content (wt%): The solid content of the dispersion was measured as follows: an accurately weighed amount of the dispersion (about 1 g) was spread on a disc and dried for at least 3 h at 110°C to constant weight (according to Ph. Eur.2.2.32d). The solid content (wt%) was the ratio of the dried residue weight to the original weight of the dispersion [21,22].
% Solid Content =((Weight of dish+residue) -Weight of dish) / ((Weight of sample) X100)
The dissolution test for APD coated tablets was performed according to USP (USP 2007) adopting method B in pH 1.2 and pH 6.8 buffers. Drug release was measured in a USP dissolution bath using apparatus II at 50 rpm. In the first stage ( pH 1.2); the tablets were putted in 900 ml of 0.1 N hydrochloric acid in a USP dissolution bath equilibrated to a temperature of 37±0.5°C. The paddle stirring rate was set at 50 rpm. Six tablets were introduced into the apparatus and the apparatus was run for 2 h. After the operation outlined above, an aliquot of the fluid was drawn, and the second stage (pH 6.8) was commenced. This last consisted of a phosphate buffer of pH 6.8 prepared by mixing 0.1 M hydrochloric acid with 0.20 M tribasic sodium phosphate (3:1). The apparatus was operated for a further 45 minutes. At the end of the time period, an aliquot of the fluid was drawn. Samples were assayed by UV method, at 273.4 nm wave length [23,24].
The following characteristics were determined for a dispersion obtained with a good yield, i.e. which formula could be used for an industrial scale-up. The pH was determined by using a pH meter.
For the development of APD film 10% concentration of TEC was optimized as plasticizer. APD film was evaluated on the basis of physical parameters e.g., weight, thickness, folding endurance, percentage moisture uptake and percentage moisture content.
The films were observed visually for their completeness, uniformity, surface texture and flexibility.
The prepared films were marked, then weighed using shimadzu (AU x 220) digital balance individually and kept in a desiccators containing activated silica at room temperature for 24 h. The films were weighed again and again individually until it showed a constant weight. The percentage moisture content was calculated as a difference between initial and final weight with respect to final weight
A weighed film kept in desiccators at normal room temperature for 24 hours was taken out and exposed to 84% relative humidity (saturated solution of potassium chloride) in desiccators until a constant weight for the film was obtained. The percentage of moisture uptake was calculated as the difference between final and initial weight with respect to initial weight (Arora et al., 2002).
The thickness of the film was measured at three different points using a micrometer screw gauge and average thickness recorded.
Folding endurance was determined by repeatedly folding the film at the same place until it broke. The number of times the film could be folded at the same place without breaking was the folding endurance value.
DSC study shows the melting point of the dug at 284.9ºC which is corroborated with the literature value.
The FT-IR of the drug is shown in fig 2 whereas the APD coating is shown in fig 3. which shows that the drug is coated with APD.
a. Equipment: Shimadzu HPLC with an attached UV detector.
b. Column: C18 reversed- phase column (25 cm×4.6 mm, particle size 5μm)
c. Mobile phase: Acetonitrile: water (60:40)
d. Flow rate: 1 ml/min.
e. Detection: UV, 273.4 nm
f. Injection: 20 μl
The calibration curve of Diclofenac was prepared by HPLC method for the determination of drug content and in vitro analysis. The HPLC chromatogram of drug is shown in fig.4
During preliminary studies, several surfactants were studied as stabilizers for the preparation of the pseudolatexes. Using cetyl alcohol as the stabilizer, the produced pseudolatexes were converted into gel-like substances within one hour. A stable dispersion was obtained with poloxamer 407 (Pluronic F 127) given in table 3.
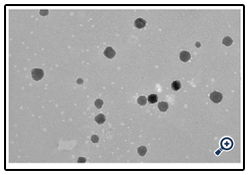
TEM image shows the spherecity of the APD developed and the smoothness of the surface texture as shown in fig 5.
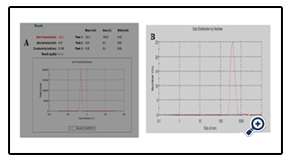
The stability of the dispersions was found to be dependent on the amount of poloxamer 407 (wt% of the polymer). The particle size decreased significantly on increasing the amount of Poloxamer 407, while no significant changes were observed above 6%. Increasing the amount of poloxamer 407 produced little change in the zeta potential. The final optimized dispersion (solid content percentage about 15.9%) was obtained with an average particle size of 611 nm given in fig 6 (A), and a pH of 3.50. A zeta potential of -30.3 mV was measured given in fig 6(B).
The physical evaluation of the developed APD such as thickness, moisture content, moisture uptake as well as folding endurance is given in table 4.
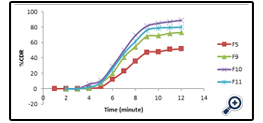
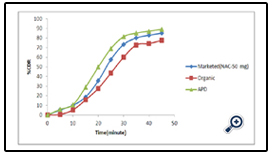
The drug release profile of the different APD is given in fig 7 whereas comparative release profile of the optimized APD with respect to the marketed as well as organic based polymeric dispersion is given in fig 8.
Model fitting of drug release profile is shown in table 5. The high R2 value is near to unity and is maximum that is 0.958 in case of first order for optimized APD. So its follows the first order kinetics. The slope(n) in case of korsmeyer peppas model was found to be more than 0.5 which further suggest non- fickian diffusion method
The optimal formulation was evaluated for stability studies by storing at 40 degree Celsius at 75% RH & tested for 3 months. The APD was analyzed for physical parameters given in table 6. The dispersion was stable and no sedimentation occurred after 3 month storage
Conclusion
APD of Eudragit L100-55 was prepared by the emulsification technology (emulsion solvent evaporation method) using Poloxamer 407 as the stabilizer and SLS as a surfactant. A stable dispersion was obtained through the optimization of surfactant and stabilizer. The optimized concentration of stabilizer and surfactant was 6% w/w of polymer and 2% w/v, respectively for Eudragit L-100-55 dispersion. Different concentration of APD was prepared and evaluated.
On the basis of Organoleptic properties, chemical characterization and in vitro drug release performance formulation F10 (10% APD) was found to be better than the other formulations. Organoleptic and chemical characterization included appearance, odour, pH, viscosity, particle size-shape, zeta potential and % solid content. Appearance of 10% APD was milky white and pH was 3.5. Viscosity was 8.0 mPa.s measured by Brookfield rheometer using 3no.spindle, 30 rpm. Particle surface morphology was done by SEM analysis. Particle size was measured by zeta sizer and was found to be 611 nm. Zeta potential of the optimized APD was -30.3mV.The present dispersion exhibited superior stability with regard to temperature and pH changes. The evaluation parameters of the free film were well controlled by the plasticizer concentration.
Formulation of uncoated tablet of Diclofenac sodium was done by wet granulation method. Enteric coating was successfully done using 10 % aqueous dispersion of Eudragit L 100-55. In vitro drug release of 10% APD was 88.95%. In vitro drug release of APD coated tablet was higher than organic coated tablet. FTIR and UV studies did not show any evidence of interaction between the drug and the polymers. The preparation of this aqueous dispersion is easy and requires short time which decreases the cost of the coating process. The results of the present study suggest that the aqueous- based dispersion of acrylic polymers can be used in a wider variety of conditions for coating.
Acknowledgement
Authors are thankful to the principal KIET school of Pharmacy Ghaziabad for providing the best research facilities available for conducting the research work. Authors are also thankful for the support provided by Advanced Instrumentation Research Facility (AIRF), Jawaharlal Nehru University and Nano Medicine lab, Faculty of Pharmacy, Jamia Hamdard, New Delhi.
References
- Xinke Caoa, C, Qizhen Gaob, Ping Gaoa, Pingtian Dinga, , Zibin Gaoa, Xiyang Suna. Preparation And Characterization Of A Novel Aqueous Dispersion Of Eudragit E For Coating Dispersions Of Eudragit E For Coating, Asian Journal Of Pharmaceutical Sciences.2007;2: 29-37
- Pawar Avinash S., Bageshwar Deepak V., Khanvilkar Vineeta V., Kadam Vilasrao J. Advances In Pharmaceutical Coatings, International Journal Of Chemtech Research. 2010;2: 733- 737
- Jones David.Pharmaceutical Application Of Polymers For Drug Delivery.2004;15:3-5
- Raizada Ankita, Bandari Anil, Kumar Brijesh. Polymers In Drug Delivery: A Review, International Journal Of Pharma. Research And Development.2002;2:9-20
- Ronald Obei.Aqueous Dispersion Utilizing Corboxyalkyl Cellulose Esters And Water Reducible Polymers,United State Patent
- Mehta Naveen, Gupta Mahesh Kumar, Jain Anurekha. Aqueous Polymeric Dispersion(APD) a Third Generation (3G) Pharmaceutical Coating System,Inventi Journal.2011:103-106
- Ms Mittal N Zala .Techniques For Powder Particle Coating: A Review ,International Journal Of Pharma World Research, 2011;2:1-5
- Yang Zhenzhong, Xu Yuanze, Zhao Delu. Preparation Of Water Based Dispersions Of Epoxy Resin By Phase Inversion Emulsification Technique Study On Rheological Behavior Of Phase Inversion.1998
- Vanderhoff J.W. Inversion Emulsion Polymerization of Acrylamide: Polymerization Kinetics and Process Development, Journal Of Dispersion Science And Technology. 1984;5:323-363
- Hua Zou, Shishan Wu, and Jian Shen. Polymer/Silica Nanocomposites: Preparation, Characterization, Properties, and Applications, Chem. Rev. 2008;108: 3893–3957
- Valter Castelvetro, Cinzia De Vita, Nanostructured Hybrid Materials From Aqueous Polymer Dispersions. Advances in Colloid And Interface Science. 2004 :167–185
- Percy M. J. et.al. , Synthesis of Vinyl Polymer−Silica Colloidal Nanocomposites Via Aqueous Dispersion Polymerization. 2003;19:, 2072–2079
- Allémann E. Preparation of Aqueous polymeric Nanodispersions By A Reversible Salting-Out Process: Influence Of Process Parameters On Particle Size, International Journal Of Pharmaceutics. 1992;87: 247–253
- Hideki Murakami, Masao Kobayashi, Hirofumi Takeuchi, Yoshiaki Kawashima. Preparation Of Poly(Dl-Lactide-Co-Glycolide) Nanoparticles By Modified Spontaneous Emulsification Solvent Diffusion Method,International Journal Of Pharmaceutics .1999;187: 143–152
- James W Macginity, Linda A. Felton,Aqueous Polymeric Coatings For Pharmaceutical Dosage Forms,Third Edition. 176:1-47
- Takashi Takayanagis, Masaaki Yamabe, Progress of fluoropolymers on coating applications Development of mineral spirit soluble polymer and aqueous dispersion Progress in Organic Coatings .2000;40:185–190
- Bodmeier R., Paeratakul, O.,Dry and wet strength of polymeric films prepared from an aqueous colloidal polymer dispersion, Eudragit RS30D. Int J Pharm.1993; 96: 129-138.
- Brown GL., Formation of films from polymer dispersions, J Polym Sci.1966; 22(102): 423–434.
- Chevalier Y et al., Film formation with latex particles. Colloid Polym Sci. 1992; 270 (8): 806–821.
- Dillon RE, Matheson LA, Bradford EB.,Sintering of synthetic latex particles. J Colloid .Sci.1951; 6(2):108–117.
- F. T. Hesselink, A. Vriz., On the theory of the stabilization of dispersion by adsorbed macromolecules II. Interaction between two paticles. J. Phys. Chem. 1971; 75: 2094-2103.
- H. P. Nilesh, C. P. Stuart., Tensile properties of free film cast from aqueous ethylcellulose dispersions. Pharm. Res.1993; 10: 810-815.
- Hahn K, Ley G, Oberthür R.., on particle coalescence in latex films (II). J Colloid Polym Sci .1988; 266(7): 631–639.
- Valter Castelvetro,Cinzia De Vita.,Nanostructured Hybrid Materials From Aqueous Polymer Dispersions. Advances In Colloid And Interface Science.2004; 108(109)167–185.