Development and Characterization of Mucoadhesive Film of Nitrendipine for Buccal Administration
Nitish Chauhan1*, Ashu Mittal1, U.K.Bajaj2
1 Department Of Pharmaceutics,K.I.E.T. School Of Pharmacy, Murad Nagar, Ghaziabad, Uttar Pradesh, India.
2 Department Of Pharmacology, K.I.E.T. School Of Pharmacy, Murad Nagar, Ghaziabad, Uttar Pradesh, India.
*Corresponding Author
Nitish Chauhan
Department Of Pharmaceutics,
K.I.E.T. School Of Pharmacy, Murad Nagar,
Ghaziabad, Uttar Pradesh,
India
E-mail: nschauhan84@gmail.com
Article Type: Review Article
Received: June 27,2013; Accepted: July 26, 2013; Published: July 29, 2013, 2013
Citation: Chauhan N, Mittal A, U.K.Bajaj (2013) Development and Characterization of Mucoadhesive Film of Nitrendipine for Buccal Administration, Int J Nano Stud Technol, 02(03), 23-28. doi: dx.doi.org/10.19070/2167-8685-130005
Copyright: Nitish Chauhan© 2013. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Mucoadhesive buccal patches containing nitrendipine were prepared using the solvent casting method. Chitosan was used as bio-adhesive polymer and different ratios of chitosan to PVP K-30 were used. The pre-formulation study using DSC revealed the compatibility of drug and polymer. Patches were evaluated for their physical characteristics like weight variation, drug content uniformity, folding endurance, surface pH, in vitro drug release, and in vitro buccal permeation study. Patches exhibited controlled release for a period of 8 h. The mechanism of drug release was found to be non-Fickian diffusion and followed the first-order kinetics. Incorporation of PVP K-30 generally enhanced the release rate. Swelling index was proportional to the concentration of PVP K-30. Optimized patches (F6) were characterized by moderate swelling; a convenient residence times as well as adequate drug release (93.4%). The surface pH of all patches was between 5.7 and 6.3 and hence patches should not cause irritation in the buccal cavity. Good correlation was observed between the in vitro drug release and in vitro drug permeation with a correlation coefficient of 0.99.
2.Introduction
3.Materials And Methods
4. Preparation of Mucoadhesive Buccal Patches
5. Evaluation of Buccal Films of Nitrendipine
5.1 Physical appearance
5.2 Surface texture
5.3 Weight variation
5.4 Thickness and size
5.5 Folding Endurance
5.6 Surface pH
5.7 Drug content
5.8 Swelling studies
5.9 In-vitro drug release
5.10 In vitro buccal permeation study
6.Result and Discussion
7.Conclusion
8.References
Key Words
Nitrendipine, Chitosan, In-Vitro Drug Release, Buccal Patch, Swelling Index.
Introduction
"Mucoadhesive drug delivery systems may be defined as drug delivery system which utilizes property of bioadhesion of certain water soluble polymers which becomes adhesive upon hydration and hence can be used for targeting a drug particular region of the body for extended time of period". The oral transmucosal drug delivery by passes liver and avoids pre-systemic elimination in the GI tract and liver especially peptides and proteins. Buccal delivery involves the administration of the desired drug through the buccal mucosal membrane lining of the oral cavity. Unlike oral drug delivery, mucoadhesive buccal delivery system presents a hostile environment for drugs, especially proteins and polypeptides, due to acid hydrolysis and the hepatic “firstpass” effect, the mucosal lining of buccal tissues provides a much milder environment for drug absorption. Chitosan has been used in a wide variety of biomedical applications like sustained release of drugs. In general the permeability of the oral mucosa decreases in order sublingual>buccal>palatal. The distinct problems that is present in the sublingual route like the drug dissolving in the saliva and unpleasant taste and odor felt by the patient are absent in the mucoadhesive route. (Madgulkar et al. 2009).
During the last decade, bioadhesive polymers received considerable attention as platformsfor buccal controlled delivery due to their ability to localize the dosage form in specificregions to enhance drug bioavailability [2]. In the present study, the natural bioadhesivepolymer chitosan was selected [3] for the development of controlled release buccalmucoadhesive devices. Chitosan is the N-deacetylated product of the polysaccharide chitin [4]. Chitosan is gaining increasing importance in the pharmaceutical field due to its goodbiocompatibility, after both intravenous and oral administration, and its non-toxicity andbiodegradable [5]. From the technological point of view, chitosan has also been demonstratedto be a promising matrix carrier for sustained drug release [6] and it possesses excellentfilm-forming properties (Gavin et at.2009). In spite of this, only a few studies have so far been performedon the usefulness of chitosan films as drug delivery systems (Gibaldi et al 1985).
Nitrendipine is a calcium channel blocker used in the treatment of mild to moderate hypertension, chronic stable angina pectoris and Prinz metal’s variant angina. It undergoes extensive hepatic first pass metabolism and its oral bioavailability is 11±5%. Its duration of action ranges from 4-48 h. There is a need for an alternative route of administration. It is a potent molecule (20 mg once daily) with low molecular weight and lipid solubility. These drug characteristics favor its absorption via buccal route. Buccal delivery of nitrendipine may circumvent hepatic first pass metabolism to improve its bioavailability. Hence in this work fabrication of mucoadhesive buccal films of nitrendipine using bioadhesive polymers was executed.
The main objective of the present study was to establish and evaluate matrix type transdermal therapeutic systems containing new polymeric Combinations (Chitosan/PVP K 30) as polymers and nitrendipine as a model drug which can release the drug into the body via mucus for longer duration of action (8 hrs),improved bioavailability, better compliance and better management of hypertension on long term basis.Several advantages of muccoadhesive systems wereanticipated e.g. increased patient compliance, better therapeutic efficacy, reduced side effects and a reduced total dose.
Materials and Methods
Nitrendipine (NTD) was obtained as a gift sample from Concept Pharmaceuticals, India. Chitosan were purchased from Hi-media. Polyvinylpyrrolidone K-30 (PVP K-30) was purchased from SD Fine chemicals Ltd, India. All additives were used as such. Gift sample of nitrendipine was subjected to tests specified in monograph of British Pharmacopoeia 2004.
Preparation of Mucoadhesive Buccal Patches
Patches containing different drug and chitosan proportions were prepared by the solvent casting method. 200mg of chitosan was dissolved in 100 mL 1.5% (V/V) acetic acid under occasional stirring for 48 h. The resulting viscous chitosan solution was filtered through nylon gauze to remove debris and suspended particles. To improve patch performance and release characteristics, a water-soluble hydrophilic additive, PVP K-30, was added in different concentrations. The drug and PVP K-30 were added into the chitosan solution under constant stirring. Propylene glycol (5%,V/V) was added into the solution as plasticizer under constant stirring. This viscous solution was left overnight at room temperature to ensure a clear, bubblefree solution. The solution was poured into a glass Petri dish (16 cm diameter) and allowed to dry at ambient temperature till a flexible film was formed. Dried films were carefully removed, checked for any imperfections or air bubbles and cut into patches of 16 mm in diameter, containing 10 of drug per patch. The patches were packed in aluminum foil and stored in an airtight glass container to maintain the integrity and elasticity of the patches. Table 4 shows the composition of different buccal patches.
Evaluation of Buccal Films of Nitrendipine
The films were observed visually for their physical appearance such as color and transparency.
The surface texture of the films was evaluated by pressing the film with finger.
Four films of each formulation were taken weighed by using single pan balance and average weight films were calculated and standard deviations were computed.
Four Films of each formulation were taken and the thickness of the film was measured using screw gauge at different places. The average film thickness and standard deviation were computed.
The folding endurance was measured manually. A small strip of film 2 cm2 of each formulation was taken and folded at the same place till it breaks. The number of times a film could be folded at the same place gave the value of folding endurance. Average of three determination were calculated and standard deviation were computed. (Sharma et al 2012)
The surface pH of the film was determined by allowing the film to swell by keeping them in contact with 0.5 ml of distilled water (pH 6.5±0.05) for 1 hour in 50 ml glass beaker. The surface pH was noted by bringing a combined glass electrode near the surface of the film for 1 min using pH meter. The pH was recorded and average of three determination and standard deviation was computed.
Drug content uniformity was determined by tacking film area of 1.5 cm2 from each formulation and it was placed in 50 ml of volumetric flask contained 50 ml of phosphate buffer of pH 6.4. It was kept aside for 6 hours and volume was made up to 100 ml with the buffer of pH 6.4. The content of (drug) nitrendipine was calculated using standard graph. Averages of three determinations were calculated.
Buccal films of 2 cm2 area from each formulation were taken accurately weighed by using single pan balance (W1 gms) and it was placed in a petridish contained 50 ml distilled water. After different time intervals film was removed and blotted with filter paper and weighed again. The weight of the film was noted (W2). The swelling index was calculated by the formula (Panchal et al. 2012)
Swelling index = (W2-W1) / W2 × 100
Where W2 = Wet weight of the film
W1 = Dry weight of the film
The USP 23 (24) rotating paddle method was used to study the drug release from buccal patches. The dissolution medium consisted of 200 mL of isotonic phosphate buffer pH 6.4. The release was performed at 37 ± 0.5 °C, at a rotation speed of 50 rpm. One side of the buccal patch was attached to a glass disk with instant adhesive (cyanoacrylate). The disk was put in the bottom of the dissolution vessel so that the patch remained on the upper side of the disk. Samples (5 mL) were withdrawn at pre-determined time intervals and replaced with fresh medium. The samples were filtered through 0.45 mm Whatman filter paper with appropriate dilutions with phosphate buffer pH 6.4 and were assayed spectrophotometrically at 355 nm.( Margret et al 2009).
The in vitro study of Nitrendipine permeation through the sheep buccal mucosa was performed using a Keshary- Chien type glass diffusion cell at 37 ± 0.2 °C. Sheep buccal mucosa was obtained from a local slaughterhouse (used within 2 h of slaughter). Freshly obtained sheep buccal mucosa was mounted between the donor and receptor compartments so that the smooth surface of the mucosa faced the donor compartment.
The patch was placed on the mucosa and the compartments clamped together. The donor compartment was filled with 1 mL of isotonic phosphate buffer pH 6.4. The receptor compartment (15 mL capacity) was filled with isotonic phosphate buffer pH 6.4 and the hydrodynamics in the receptor compartment was maintained by stirring with a magnetic bead at 100 rpm. One mL sample was withdrawn at predetermined time intervals and analyzed for drug content at 355 nm.
Results and Discussion
Buccal delivery of drug provides an attractive alternative to the oral route of drug administration, particularly in overcoming deficiencies associated with the latter mode of dosing. A combined polymers were used (Chitosan combination with PVP K-30) for the preparation of buccal films contained Nitrendipine as a model drug.
The drug was subjected to various pre-formulation studies which include solubility, melting point, and partition coefficient. The melting point was found to be 158oC. The partition coefficient was found to be Log P (n-butanol/water), 2.88.In the next phase the DSC thermograms for physical mixture (drug + Chitosan+ PVP K-30) was taken to understand the drug polymer interaction.
The DSC thermogram of physical mixture showed peak at 158.64oC which is very close to melting point of pure nitrendipine, since the peak of drug was found to be intact in the mixture, it is concluded that that there was no interaction between drug and the excipients.
Prepared mucoadhesive buccal films were evaluated for their various physical parameters such as appearance, surface texture, and weight variation, thickness, folding endurance, surface pH and drug content. The results are depicted in Table:1.
Appropriate swelling behavior of a buccal adhesive system is the essential property for uniform and prolonged release of the drug and effective mucoadhesion. The swelling study indicated that the swelling index was higher in patches containing a higher amount of PVP K-30. Examination of the patches during the dissolution studies also revealed that the patches showed considerable swelling, especially at higher concentrations of PVP K-30. The weak aqueous solubility of the cationic polymer (chitosan) limited the swelling of the patches, which was observed in placebo patches. Addition of the hydrophilic polymer PVP K-30 increased the surface wettability and consequently water penetration within the matrix. Patches did not show any appreciable changes in their shape and form during the 3 h that they were kept on a 2% agar gel plate. The optimized patch (F6) showed a 22.9 ± 1.2% swelling index due to water absorption within 3 h. swelling behavior of patches as a function of time is shown in Table 2. It was observed that medicated patches had a higher swelling index compared to plain patches.
In-vitro release studies were carried out using Dissolution apparatus (USP 23 rotating paddle method) in phosphate buffer of pH 6.4.
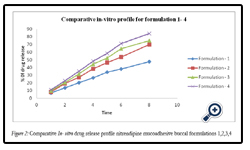
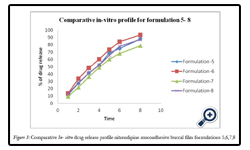
The percentage release of drug from all formulation F1 to F8 is as follows: 47.65, 69.13, 74.93, 84.19, 88.27, 93.4, 78.64, and 87.65 at the end of 8hrs respectively. Among various formulations F6 has released maximum amount of drug within 6hrs. The order of retardation time for different films was as follows F1 > F2 > F3 > F7 > F4 > F5 > F8> F6.The reason might be due to water permeability characteristics of the film i.e., the lower the water permeability co-efficient the greater the extended drug release characteristics observed. The drug released increased linearly with the increasing concentration of PVP K- 30 from batches F1 to F6. The maximum in vitro release was found to be 93.4 ± 1.3% over a period of 8 h in batch F6, containing the highest amount of PVP K–30, which could be attributed to its high rate and extent of swelling.
This finding was also supported by the results of swelling studies where the highest swelling index was also exhibited by batch F6, indicating that the increase in water-soluble polymer PVP-K30 content results in faster swelling and release from patches.
Figure 2: Comparative In- vitro drug release profile nitrendipine mucoadhesive buccal formulations 1,2,3,4.
Figure 3: Comparative In-vitro drug release profile nitrendipine mucoadhesive buccal film formulations 5,6,7,8.
Table 4: R2 values of drug release kinetic models for nitrendipine mucoadhesive buccal film formulations.
The kinetic values obtained from different plots are listed in Table No 4. The data was subjected to first order kinetics by plotting Cumulative % drug retained vs time in min. Fairly linear plots were obtained for all formulation with regression value between (0.9784 to 0.9967) indicated that the rate of drug release was followed first order kinetics.
Further the data was fitted with Higuchi equation and showed linear plots with their high regression co-efficient value between (0.9907 to 0.9919) indicated that the mechanism was diffusion controlled.
To know whether the fickian and non-fickian diffusion, the data was further subjected to Korsmeyer’s equation. Fairly linear plots were obtained with the regression co-efficient values 0.9916 to 0.9996 and The value of release exponent “n” calculated as a slope defines the release mechanism. The value of “n” obtained for all the formulations was > 0.5 - > 0.89 suggested that the drug release followed non-fickian anomalous diffusion.
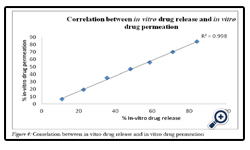
F6 patches were characterized by moderate swelling as well as adequate drug release. These patches were subject to investigation of in vitro drug release. They showed 93.95% drug permeation in 8 h through sheep buccal mucosa. Good correlation was observed between in vitro drug release and in vitro drug permeation with a correlation coefficient of 0.99 (Fig. 4.)
Conclusion
From the present investigation, one can conclude that the optimized buccoadhesive patch (F6) of Nitrendipine with the combination of chitosan and PVP K-30 can meet the ideal requirements for buccal devices, which can be a good way to bypass the extensive hepatic first pass metabolism.
References
- Madgulkar A, Kadam S , and Pokharkar V. Development of buccal adhesive tablet with prolonged antifungal activity: optimization and ex vivo deposition studies. Indian J Pharm Sci, 2009; .71 (3) : 290–294.
- Amir HS. Buccal mucosa as a route for systemic drug delivery: A review. J Pharm Pharmceut Sci,1998; 1 (1):15-30.
- Bhanja S B, Ellaiah P, Martha SK, Kar RK and Panigrahi BB . Buccoadhesive Drug Delivery System of Captopril. Formulation and In Vitro Evaluation. Journal of Pharmacy Research. 2010; 3,( 2) : 335-340.
- Consuelo ID, Falson F, Guy RH and Jacques Y, . Ex Vivo Evaluation of Bioadhesive Films for Buccal Delivery of Fentanyl. Journal of Controlled Release. 2007; 122 :135–140.
- Feng SS,. Nanoparticles of biodegradable polymers for newconcept chemotherapy. Expert Review of Medical Devices. 2004; (1):. 115-125.
- Sharma Gaurav Kumar, Sharma Pramod Kumar, Bansal Mayank . A Review on Mucoadhesive Buccal Patch as A Novel Drug Delivery System. An International Journal of Pharmaceutical Sciences. 2012; 3 (2): 0976-7908
- Gavin P. Andrews, Thomas P. Laverty, david S. jones . Mucoadhesive polymericplatforms for controlled drug delivery. European Journal of Pharmaceutics and Biopharmaceutics. 2009; (71): 505-518
- Gibaldi M, Harris D and Robinson JR . The number of drugs administered buccally is increasing. Clinical Pharmacology. 1985; (3): 49-56.
- Gupta SK S.K.Gupta, Singhvi I.J, Shirsat M, Karwani G, Agarwal & Agarwal Aditi., A Buccal adhesive drug delivery system: A review. Asian J Biochem Pharm Res. 2010; 1(2): 105-14.
- Harshad GP, Janak JJ, Tarun KP,Vishnu MP . Buccal patch: A technical note. Int J PhamSci Rev Res. 2010; .4, (3): 178-82.
- Hassan N, Ali M, Ali J. Development and evaluation of novel buccoadhesive wafers of nimodipine for treatment of hypertension. Drug Delivery. 2010; 17 (2): 59-67.
- J Thimmasetty, 2008. Design And In Vivo Evaluation of Carvedilol Buccal Mucoadhesive Patches. Pak. J. Pharm. Sci.2008 ;2: 21-26.
- Kashappa Goud H. Desai and T.M. Pramod Kumar. Preparation and Evaluation of a Novel Buccal Adhesive System. AAPS PharmSciTech. 2004;5, (3):1-9.
- Kawarkhe Shilpa and Poddar S.S, Design of mucoadhesive vaginal metronidazole films. Acta Pharmaceutica Sciencia. 2010; 52: 181-189.
- Kolli CS, Gannu R, Yamsani VV, Kishan V and Yamsani MR . Development of Mucoadhesive Film for Buccal Administration of Prochlorperazine: Evaluation of In Vitro Release and Mechanical Properties. International Journal of Pharmaceutical Sciences and Nanotechnology 2008; 1 (1): 64-70.
- Margret Chandira ,Mehul, Debjit, Chiranjib,Kumudhavalli, .Jayakar B., Formulation design and development of buccoadheshive tablets of verapamil hydrochloride. Int J PharmTech Res. 2009; 1(4): 1663-1677
- Panchal Amish V, Mehta Markand, Shah Viral H, Upadhyay Umesh. Formulation and in-vitro evaluation of mucoadhesive bilayered buccal tablets of Rosuvastatin calcium. International Journal of Pharmaceutical Science and Research. 2012; 3(8): 2733-2740.