Acceptability and Detection of High-Risk Human Papilloma Virus using Self-Collected Sampling for Cervical Cancer Screening among HIV-Positive and HIV-Negative Women in Tanzania
Kandali Samwel1*, Julius Mwaiselage1, Redempta Mbatia2, Madalyn Nones3, Pendo Bukori2, Francis Nyakubene2, Benedicta Masanja2, Richard F. MacLehose3, Keith J. Horvath4, Shalini Kulasingam3
1 Ocean Road Cancer Institute, Dar es Salaam, Tanzania (ORCI).
2 Tanzania Health Promotion Support, Dar es Salaam, Tanzania (THPS).
3 Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, Twin Cities, Minneapolis, MN, USA.
4 Department of Psychology, San Diego State University, San Diego, CA, USA.
*Corresponding Author
Kandali Samwel (MD, MSc),
Ocean Road Cancer Institute, Box 3592, Chimara/Obama Street, Dar es Salaam, Tanzania.
Tel: 255 762 600987
Fax: 255-22-2118704
E-mail: kandalisamwel@gmail.com
Received: November 16, 2024; Accepted: December 04, 2024 Published: December 12, 2024
Citation: Kandali Samwel, Julius Mwaiselage, Redempta Mbatia, Madalyn Nones, Pendo Bukori, et al., Acceptability and Detection of High-Risk Human Papilloma Virus using Self-
Collected Sampling for Cervical Cancer Screening among HIV-Positive and HIV-Negative Women in Tanzania. Int J Chronic Dis Ther. 2024;9(2):148-153.
Copyright: Kandali Samwel�2024. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Objective: Cervical cancer is responsible for the greatest number of cancer-related cases and deaths among Tanzanian women.
Although a national cancer screening program using visual inspection with acetic acid (VIA) was established in Tanzania
in 2010, participation remains low. Self-sampled human papillomavirus (HPV) tests have recently been recommended as a
method to increase screening participation. We evaluated the acceptability and detection of high-risk HPV (Hr-HPV) using
self-collected sampling for cervical cancer screening among Tanzanian HIV-negative and HIV-positive women.
Methods: A cross-sectional study design was used to recruit women from five clinics offering cervical cancer screening in
Dar es Salaam and the surrounding Pwani Region. Eighteen women (9 HIV+ and 9 HIV-) were recruited from each clinic
using convenience quota sampling. A trained nurse instructed women who provided informed consent on the self-collection
of vaginal samples. A survey to assess self-sampling acceptability was administered after self-collection was completed. All
women were then screened with VIA. The self-collected samples were genotyped for Hr-HPV using the AmpFire� Multiplex
High Risk HPV Assay.
Results: Among 90 women who participated in the study, the median age was 34 years (IQR: 28 - 37). Thirty-four percent of
women indicated they preferred self-collection, 30% indicated that either method was fine and 36% indicated that they would
prefer clinician- collected sampling. A high proportion of all women (>90%)found self-sampling acceptablebased on six different
indices including convenience and ease. Thirty-eight (42.2%) women were Hr-HPV positive. Hr-HPV prevalence was
28.9% (13 women) for HIV-negative women and 55.6% (25 women) in HIV-positive women.
Conclusion: Self-collected samples were well accepted in this sample of HIV-positive and negative women. Although selfcollection
may address the low cervical cancer screening participation in Tanzania, if confirmed in larger studies, cost-effective
strategies to triage the potentially high proportion of HPV positive women are needed.
2.Introduction
7.Conclusion
5.References
Keywords
Human Papilloma Virus; Cervical Cancer; Self-Collected Test; Human Immunodeficiency Virus; Screening.
Introduction
Cervical cancer is the leading cause of cancer-related morbidity
and mortality among Tanzanian womenwith approximately
10,241 diagnosed with and 6,525 dying from cervical cancer in
the last year.[1] Current estimates indicate an age-standardized
incidence rate of 62.5 cases per 100,000 women and an agestandardized
mortality rate of 42.7deaths per 100,000 women.[2]
In comparison, Europe has an age-standardized cervical cancer
incidence of 10.7 per 100,000 women with a mortality rate of
3.76 per 100,000 women and globally the incidence rate is approximately
13.3 cases per 100,000 women and the mortality rate
is 7.25 deaths per 100,000 women.[2] Additionally, approximately
5% of Tanzania�s adult population lives with HIV,[3] which is an
independent risk factor for cervical cancer.[4-6]
Cervical cancer screening with visual inspection with acetic acid
(VIA) has been available as part of a national program since
2010.[7] In 2021, approximately 1.5 million women were screened
for cervical cancer by VIA, which is about 40% of the eligible
population for screening in Tanzania.[8] A number of factors are
associated with low uptake of cervical cancer screening among
women in Tanzania, including a low clinician-to-patient ratio, a
lack of knowledge about screening, as well as fear about screening
procedures and results. [9-11] There are also identifiable subgroups
in Tanzania, such as women with lower education levels
or high parity as well as those who reside in rural areas, for whom
screening rates are lower than for women as a whole.10Given
these concerns and the relatively high prevalence of HIV, increasing
screening uptake is an important public health priority.[12]
In 2021, theWorld Health Organization recommended on the
use of human papillomavirus (HPV) testing for cervical cancer
screening in low and middle-income countries, including Tanzania.[
13] Reasons for this include the fact that the test can be
conducted from a self-collected sample or a clinician-collected
sample and importantly has a high sensitivity for the detection
of high-grade dysplasia and cancer compared to VIA. In a study
conducted in Tanzania, HPV testing was shown to be significantly
more sensitive than VIA using detection of HSIL on cytology as
the outcome.[4] Studies conducted inTanzania have shown that
self-collection is acceptable.[14, 15] In a study by Katanga et al.
most women (79.8%) preferred self-collection to clinician-collected
samples (16.5%).[14] Previous studies, including a systematic
review of HPV self-collection studies conducted in Africa,
have also demonstrated moderate to strong agreement between
clinician-collected samples and self-collected samples, suggesting
the feasibility of self-administered tests to adequately detect HPV.
[14, 16] Based in part, on these findings and the WHO recommendation,
the Tanzanian Ministry of Health recently developed
a plan to integrate HPV-based testing into the national cervical
cancer screening program.[17] The success of this plan will depend,
however, on understanding how best to integrate HPV selfcollection
based testing with a screening program that�s currently
structured based on VIA and also how HPV self-collection may
perform in a mixed population of HIV-positive and HIV negative
women.
To address this, we conducted a pilot study to assess self-sampling
acceptability and compare HPV test results to VIA test results
stratified by HIV status in 90 women attending healthcare facilities
across Tanzania.
Patients and Methods
Sample Selection
IRB approval for this study was provided by the Tanzanian National
Institute for Medical Research and the University of Minnesota
Institutional Review Board. This cross-sectional study consisted
of women selected from five sites: the Ocean Road Cancer
Institute (ORCI) in Dar es Salaam, Bagamoyo District Hospital
(located in a rural coastal areanorth of Dar es Salaam), Mkuranga
District Hospital (located in a rural area south of Dar es Salaam),
Chalinze District Hospital (in a rural region west of Dar es Salaam)
and Kisarawe District Hospital(located in a semi-urban area
west of Dar es Salaam). The ORCI is a designated cancer hospital
while the remaining study sites are all district-level hospitals. The
5 study sites were selected based on their geographic range to ensure
inclusion of women from a variety of urban and rural areas
from Dar es Salaam and the surrounding Pwani region. All sites
are government funded hospitals that provide regular cervical
cancer screening services and HIV care. For this pilot study, eighteen
women (nine HIV-positive women and nine HIV-negative
women) were selected from each study site. Convenience quota
sampling was used for study recruitment to ensure equal numbers
of HIV-positive and HIV-negative women. Enrollment took
place over nine months from February 2019 to October 2019. To
participate in the study, women had to be 25 years of age or older,
provide informed consent, not have a current diagnosis of cervical
cancer, provide a medical history to confirm HIV status, and
have the ability to self-collect a sample for HPV testing.
Following an informed consent process, each participant was administered
a demographic and health-related questionnaire. Detailed
instructions were then provided by a trained study nurse for
HPV self-collection; an instructional poster was placed in the selfcollection
room for additional guidance. Women were provided
with all equipment necessary to obtain the specimen, including
a collection brush and a private setting for sample collection.
After self-collection, participants underwent VIA performed by
a trained nurse; women who were VIA-positive were treated by
cryotherapy or LEEP based on their VIA results.[13]
The self-collected samples were stored and shipped at room
temperature to the Ocean Road Cancer Institute laboratory, in
Dar-es-salaam, Tanzania for processing. This site has trained molecular
technicians who perform HPV testing for research studies
conducted in Tanzania.[14] Samples were extracted with the Qiagen
DNeasy Blood and tissue kit (Qiagen Inc, Valencia, California
USA; cat number 69506) according to the manufacturer's instructions.
The concentrations of the extracted DNA were determined
using a nanodrop spectrophotometer. DNA samples were then
stored at -20�C until further analysis. Genotyping was done using
the Ampifire� Hr- HPV assay BIO-RAD Real-time PCR according
to the manufacturer�s instructions.[18, 19] Only samples that
tested positive for Hr-HPV DNA were considered HPV-positive
for this study. The assay identifies 15 high-risk Hr-HPV genotypes
(16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, and
68) as has been previously described.[18, 19] Results for Hr-HPV
were shared with the study nurse who then contacted each participant.
HPV positive women were asked to follow up with their
clinic for a VIA screening.
Post sampling survey
Each participant completed a survey immediately following selfcollection.
The survey was adapted from a survey administered to
women in Malaysia.[20] For our study, the survey was translated
into Swahili, focused on self-collection only, and administered
after collection was completed (post-collection); a copy is available
from the authors. Participants were asked if they preferred
self-collection of HPV samples, clinician-collected sampling, or
if either was fine. Acceptability of self-collection was measured
according to six separate indices:experience with self-collection,
ease of collection, convenience, embarrassment associated with
self-collection, discomfort associated with the collection procedure,
and confidence in the ability to correctly collect a sample.
These indices were measured on a five-point Likert scale with �1� indicating the lowest level of satisfaction and �5� indicating the
highest level of satisfaction. For all Likert scales, a score >4 was
considered a �high rating/positive score� and a score <4 was considered
a �low rating/negative score.�
Statistical Analysis
All survey data and test results were entered in R (version 4.2.1
for Windows) for descriptive analysis and hypothesis testing. Participant
age was categorized as <30 or > 30 years; the number of
sexual partners in the last 5 years was categorized as <2 or >2
partners; age at first intercourse was categorized as <18 or >18
years. Cut-points were based on a previous study evaluating HPV
prevalence by HIV status as well as the median value for variables
such as age at first intercourse and past sexual partner number.
[21] Sampling preference was categorized into three levels: preference
for self-collection, preference for clinician-collection, or
either.
The proportion of women who were positive based on their selfcollected
HPV tests was compared to the proportion positive by
VIA, since the latter is currently recommended for screening in
Tanzania. Concordance between a positive result for any HPV
type from self-collected specimens and VIA results was evaluated
using kappa statistics. Data on demographic and sexual history
were used to identify potential characteristics associated with
an HPV-positive test result and/or an abnormal VIA test result.
Crude prevalence ratios and 95% confidence intervals were used
to estimate the association between participant characteristics and
preferred sampling method (self vs clinician) as well as the association
between HIV positivity and HPV infection. Acceptability
of HPV self-collection was also assessed, stratified by HIV status.
Results
Demographics
The final sample consisted of 90 women (half (n=45) of whom
were HIV-positive) with a median age of 34 years (IQR: 28 - 37).
No difference was observed in participant age distribution across
the study sites. When considering marital status, 51 women (56.7%)
were married, 24 women (26.7%) were widowed, divorced or separated,
and 15 women (16.7%) were single. Seventy-two women
(80%) reported a formal education (primary school, secondary
school, college, or university) while 20% of women reported no
formal education. The median age of first sexual intercourse was
18 years (IQR: 17 - 18.6). Seven (10.8%) of the 65 women who
responded to the question regarding contraceptive use indicated
the recent use of condoms. All HIV-infected women were using
ART and self-reported adherence to their current regimen. The
median duration of usage was 36 months (IQR: 12 - 84).
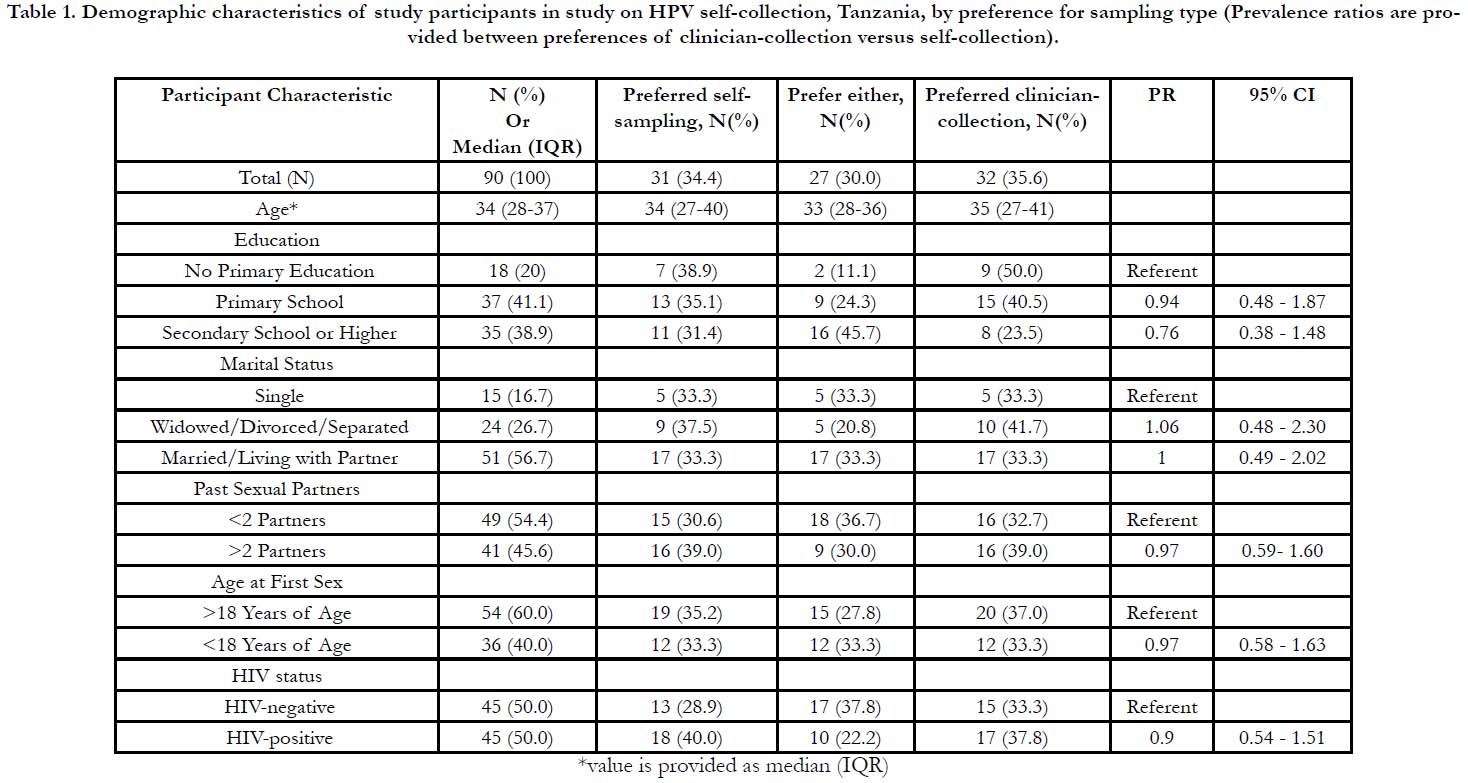
Acceptability and Preference of HPV Sampling
In terms of preferences for HPV self-collection sampling, 31
women (34.4%) indicated that they would prefer self-collection
while 32 women (35.6%) indicated that they would prefer clinician-
collected sampling and 27 women (30%) indicated that
either method was fine. Of the women who preferred clinician
collection, 26(81.3%) indicated that they felt more confident with
clinician-collection. There were no significant differences between
preferences for self-collected versus clinician-collected samples
for any of the assessed demographic variables, including HIVstatus
(Table 1). The demographic distribution for women who
preferred self-collection was similar to that of the entire sample.
Of those that preferred self-collection, 18 women (58.1%) were
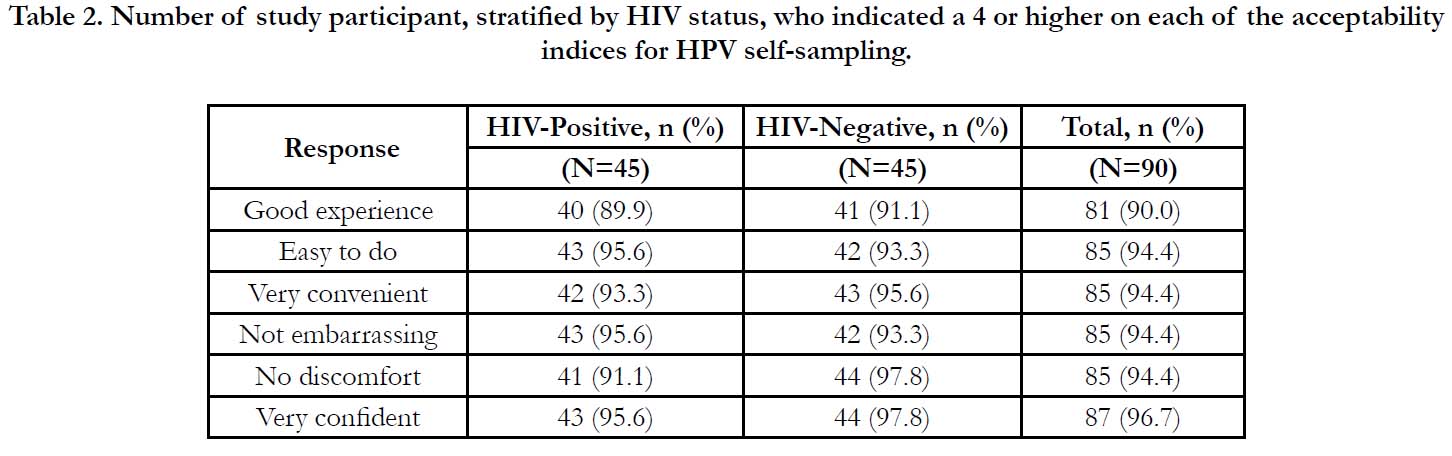
HIV-positive (Table 1). Each of the acceptability indices had a
high level of positive scores: ninety percent of women scored the
experience highly. Eight-five women (94.4%) scored the indices
of easy-to-do, convenient, not embarrassing, and no discomfort
or pain as high. Eighty-seven women (96.7%) scored a four or
above for confidence in self-sampling ability (Table 2).
Table 1. Demographic characteristics of study participants in study on HPV self-collection, Tanzania, by preference for sampling type (Prevalence ratios are provided between preferences of clinician-collection versus self-collection).
Table 2. Number of study participant, stratified by HIV status, who indicated a 4 or higher on each of the acceptability indices for HPV self-sampling.
HPV Prevalence in HIV-positive and HIV-negative Women
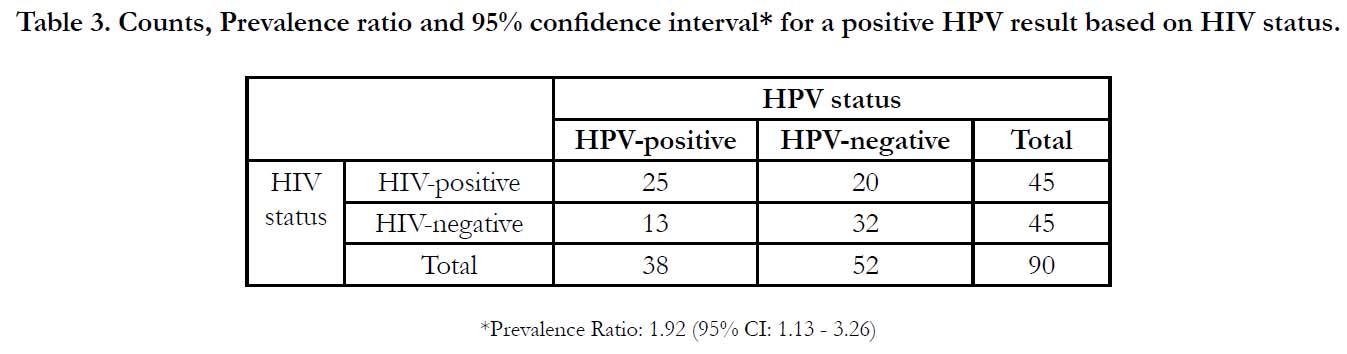
All 90 samples were suitable for testing for the presence of HrHPV. Overall, thirty-eight (42.2%) women were Hr-HPV positive (Table 3). Hr-HPV prevalence was 28.9% for HIV-negative women and 55.6% in HIV-positive women. Fifteen (39.5%) of the 38 Hr-HPV positive samples were positive for multiple HPV types. The prevalence of being HPV-positive for HIV-positive women was 1.92 times (95% CI 1.13 - 3.26) that of HIV-negative women (Table 3). Among HPV-positive women, the prevalence of testing positive for multiple Hr-HPV types in HIV-positive women was 1.74 times (95% CI 0.36 � 9.90) that of HIV-negative women. The most commonly detected Hr-HPV types were HPV 16 (18.4%), HPV 39 (26.3%), HPV 56 (13.2%), HPV 59 (15.8%) and HPV 68 (15.8%). Among women co-infected with HIV and HPV, HPV 39 was the most commonly detected Hr-HPV type (32.0%). Among HIV-negative women with HPV, HPV 16 (15.0%) and HPV 68 (15.0%) were the most commonly detected Hr-HPV types.
Table 3. Counts, Prevalence ratio and 95% confidence interval* for a positive HPV result based on HIV status.
Comparison of HPV testing and VIA
Two women were VIA-positive. Of these, one was HPV-positive and HIV-negative while the other woman was HPV-negative and HIV-positive. Cohen�s ? was 0.008 (95% CI -0.06�0.08) for the concordance between HPV testing and the VIA results indicating very poor agreement.
Discussion
In this study of self-collected HPV tests, a slightly lower proportion
of participants preferred self-collection compared to
clinician-collected sampling. However, the post-collection survey
on acceptability showed a high proportion of both HIV-positive
and HIV-negative women found self-collectionacceptable based
on different indices including experience with self-collection as
well as ease and convenience. Previous studies have assessed
women�s acceptability of self-collected HPV samples in similar
low-resource settings, including Tanzania and have found a high
acceptance of HPV self-sampling.[14,16] However, in contrast
to our study, some of these studies also demonstrated an overall
preference for self-collection over clinician collection. The main
reason provided was greater confidence in a clinician-collected
sample, which is consistent with the preference for clinician collection
provided in other studies.[14,16] Other reasons for the
slight difference in preference noted may be due to our small
sample size, differences in the number of choices provided for
the response for this survey compared to others and/or clarity of
the question since the Swahili version of the survey was not validated
or evaluated in terms of reliability.[22] These results suggest
the need for education and ways to reassure women about HPV
self-collection.
This study found that both HIV-positive and HIV-negative women
were infected with high-risk HPV types. The prevalence of
Hr-HPV in HIV-negative (28.9%) and positive (55.6%) women
in our study is similar (38.1% and 50.9%, respectively) to another
study that included women from Tanzania but used clinician-collected
HPV tests.[21] However, in contrast, our estimates of Hr-
HPV are higher than those from a large study also conducted in
Tanzania, which reported 17.2% and 46.7% for HIV-negative and
HIV positive women, respectively.[23] Differences may be due to
our smaller sample size and the fact our sample was younger and
more women were from rural areas, both of which are associated
with a higher prevalence of HPV. Other reasons could be the
assay used, which can affect estimates of prevalence, as well as
mode of collection (clinician versus self-collection).[23, 24]
Although no statistically significant differences were found when
we compared sampling preference by HIV status, HIV-positive
women had a significantly higher prevalence of positive Hr-HPV
results and were more likely to positive for multiple HPV types,
which is consistent with findings from other studies. [23, 25] Taken
together, these results suggest that self-collected samples can
be successfully used for HPV testing to determine the presence
of Hr-HPV types for both HIV positive and negative women.
However, a limitation of our study is that we did not collect biopsies
on women. Thus, while the higher prevalence of HPV in our sample suggests the ability of HPV testing to better detect
high-grade disease and cancer, we were not able to determine test
accuracy in relationship to biopsy-confirmed CIN 3 or cancer.
This is an important consideration, especially for HIV positive
women, given the higher prevalence of hr-HPV and potential
for overtreatment. Other limitations, as noted previously, are our
relatively small sample size which limits our ability to detect significant
differences based on demographic characteristics of our
sample. In addition, our sample was recruited using convenience
sampling, and included relatively young women from Dar es Salaam
and hospitals in the surrounding Pawani regionwhich limits
comparability of our estimates of HPV prevalence to those expected
from older populations and/or sampled from other regions
across Tanzania.
Although self-collection was well accepted by both HIV positive
and negative women and provided samples that were adequate
for HPV testing, it is important to consider the effectiveness of
self-collection as a cervical cancer screening tool in comparison
to VIA, which has, until recently been recommended and used
for screening in Tanzania. In our study, one participant was discordant
with VIA-positive and HPV-negative results. This could
be due to a false positive VIA test result, as found in Katanga
et al. (2019) or the presence of non-HPV lesions.24The other
participant who was VIA-positive also tested positive for Hr-
HPV. Of note, this study foundthe majority of those who were
HPV-positive were VIA-negative. This notable difference in test
performance may be due to our small sample size, so should
be interpreted with caution. However, in a large study of 3,767
women in Tanzaniabv Dartell et al. (2014), 4.5% of women were
VIA positive compared to 20.1% who were Hr-HPV positive.[4]
These results suggest that follow-up from a positive HPV result
will not only add additional costs to the healthcare system but may
also overburden clinics that have until now, provided VIA-based
screening to a limited number of women. Use of VIA, which is
widely available in Tanzania, as well as genotyping may help identify
women who need immediate treatment and those who can
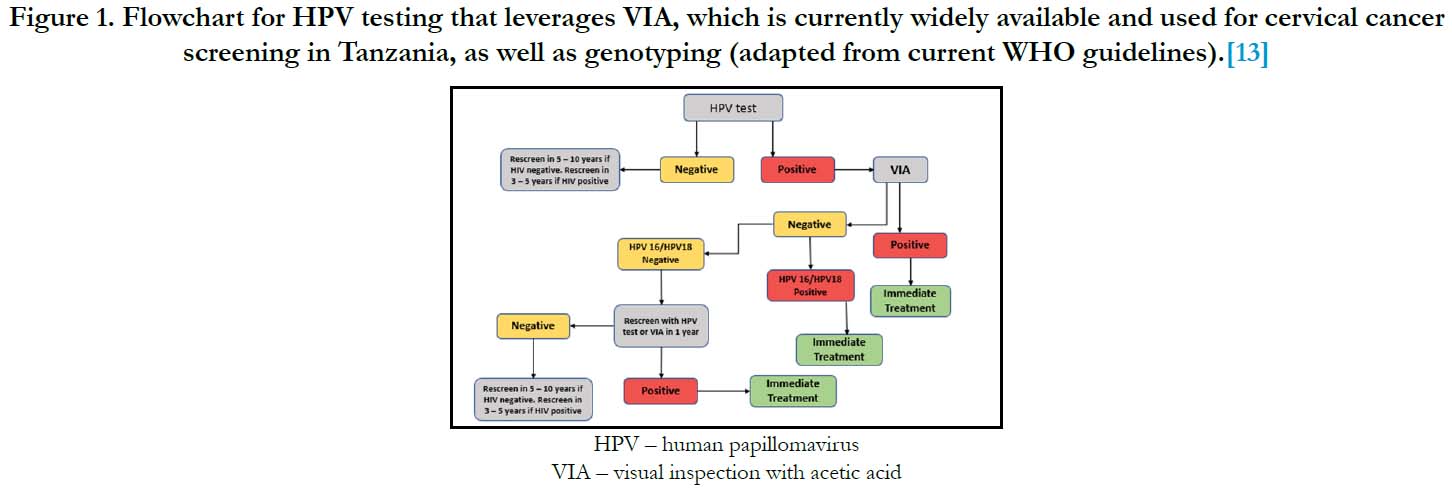
undergo rescreening at a later date. An example algorithm, which
is a slight variation on that proposed by the WHO is presented in
Figure 1.13 The effectiveness and cost-effectiveness of different
algorithms that combine these options,especially for HIV-positive
women and that can be implemented across Tanzania remains to
be determined.
Figure 1. Flowchart for HPV testing that leverages VIA, which is currently widely available and used for cervical cancer screening in Tanzania, as well as genotyping (adapted from current WHO guidelines).[13]
In conclusion this pilot study highlights the potential for HPV self-collection as a cervical cancer screening option in Tanzaniathat could increase screening participation.In this study, all the self-collected samples were adequate for testing, and we were able to detect Hr-HPV types in both HIV-positive and HIV-negative women. Additionally, self-collection was well-accepted in women irrespective of HIV status. However, given the high Hr-HPV positivity compared to VIA positivity in this study, especially among HIV positive women,if confirmed in larger studies, countries such as Tanzania will need to consider how best to incorporate this approach to screening into their existing infrastructure to avoid over burdening healthcare facilities and providers.
Acknowledgment
We thank the administration and nursing staff at Ocean Road
Cancer Institute, Bagamoyo District Hospital, Chalinze District
Hospital, Mkuranga District Hospital and Kisaware District Hospital
cervical cancer screening clinics for their assistance with recruitment
and support of the study.
Funding: This study was supported by pilot grants from the University
of Minnesota, Twin Cities Division of Epidemiology and
Community Health and the Center for Global Health and Social
Responsibility.
References
-
[1]. ICO/IARC Information Centre on HPV and Cancer. Tanzania. Human
Papillomavirus and Related-Cancers. Fact Sheet. 2023.
[2]. Bruni L, Albero G, Serrano B, Mena M, Collado JJ, G�mez D, et al. ICO/ IARC Information centre on HPV and cancer. Human Papillomavirus and Related Diseases in the World. Summary Report. 2023 Mar.
[3]. Country progress report - United Republic of Tanzania. 2023 Oct 19. Available from:https://www.unaids.org/sites/default/files/country/documents/ TZA_2020_countryreport.pdf
[4]. Dartell MA, Rasch V, Iftner T, Kahesa C, Mwaiselage JD, Junge J, et al. Performance of visual inspection with acetic acid and human papillomavirus testing for detection of high-grade cervical lesions in HIV positive and HIV negative Tanzanian women. Int J Cancer. 2014 Aug 15;135(4):896-904. Pubmed PMID: 24391021.
[5]. Tugizov SM, Herrera R, Chin-Hong P, Veluppillai P, Greenspan D, Michael Berry J, et al. HIV-associated disruption of mucosal epithelium facilitates paracellular penetration by human papillomavirus. Virology. 2013 Nov;446(1-2):378-88.Pubmed PMID: 24074602.
[6]. Stelzle D, Tanaka LF, Lee KK, Khalil AI, Baussano I, Shah AS, et al. Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob Health. 2021 Feb 1;9(2):e161-9.
[7]. The United Republic of Tanzania Ministry of Health and Social Welfare. NCCS. 2013.
[8]. Ministry of Health Tanzania. District Health Information System 2 (MOHDHIS- 2) Report. 2018.
[9]. Runge AS, Bernstein ME, Lucas AN, Tewari KS. Cervical cancer in Tanzania: A systematic review of current challenges in six domains. Gynecol Oncol Rep. 2019 May 21;29:40-47.Pubmed PMID: 31309135.
[10]. Kahesa C, Kjaer S, Mwaiselage J, Ngoma T, Tersbol B, Dartell M, et al. Determinants of acceptance of cervical cancer screening in Dar es Salaam, Tanzania. BMC Public Health. 2012 Dec 19;12:1093.Pubmed PMID: 23253445.
[11]. Bateman LB, Blakemore S, Koneru A, Mtesigwa T, McCree R, Lisovicz NF, et al. Barriers and Facilitators to Cervical Cancer Screening, Diagnosis, Follow-Up Care and Treatment: Perspectives of Human Immunodeficiency Virus-Positive Women and Health Care Practitioners in Tanzania. Oncologist. 2019 Jan;24(1):69-75.Pubmed PMID: 29934410.
[12]. Louie KS, De Sanjose S, Mayaud P. Epidemiology and prevention of human papillomavirus and cervical cancer in sub-Saharan Africa: a comprehensive review. TM & IH. 2009 Oct;14(10):1287-302.
[13]. WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention [Internet]. 2nd ed. Geneva: World Health Organization; 2021.Pubmed PMID: 34314129.
[14]. Katanga JJ, Rasch V, Manongi R, Pembe AB, Mwaiselage JD, Kjaer SK. Concordance in HPV Detection Between Self-Collected and Health Provider- Collected Cervicovaginal Samples Using careHPV in Tanzanian Women. JCO Glob Oncol. 2021 Jun;7:985-991.Pubmed PMID: 34181439.
[15]. Bakiewicz A, Rasch V, Mwaiselage J, Linde DS. "The best thing is that you are doing it for yourself" - perspectives on acceptability and feasibility of HPV self-sampling among cervical cancer screening clients in Tanzania: a qualitative pilot study. BMC Womens Health. 2020 Mar 31;20(1):65.Pubmed PMID: 32234028.
[16]. Nodjikouambaye ZA, Adawaye C, Mboumba Bouassa RS, Sadjoli D, B�lec L. A systematic review of self-sampling for HPV testing in Africa. Int J Gynaecol Obstet. 2020 May;149(2):123-129.Pubmed PMID: 32037532.
[17]. Tanzania Ministry of Health. Integrating HPV DNA Testing in Cervical Cancer Screening and Treatment Services, Operational Manual. 2023.
[18]. Zhang W, Du H, Huang X, Wang C, Duan X, Liu Y, et al. Evaluation of an isothermal amplification HPV detection assay for primary cervical cancer screening. Infectious Agents and Cancer. 2020 Dec;15:1-6.
[19]. Tang YW, Lozano L, Chen X, Querec TD, Katabi N, Moreno-Doc�n A, et al. An Isothermal, Multiplex Amplification Assay for Detection and Genotyping of Human Papillomaviruses in Formalin-Fixed, Paraffin-Embedded Tissues. J Mol Diagn. 2020 Mar;22(3):419-428.Pubmed PMID: 31978559; PMCID: PMC7081142.
[20]. Ma'som M, Bhoo-Pathy N, Nasir NH, Bellinson J, Subramaniam S, Ma Y, et al. Attitudes and factors affecting acceptability of self-administered cervicovaginal sampling for human papillomavirus (HPV) genotyping as an alternative to Pap testing among multiethnic Malaysian women. BMJ Open. 2016 Aug 4;6(8):e011022.Pubmed PMID: 27491667.
[21]. Chachage M, Parikh AP, Mahenge A, Bahemana E, Mnkai J, Mbuya W, et al. High-risk human papillomavirus genotype distribution among women living with and at risk for HIV in Africa. AIDS. 2023 Mar 15;37(4):625- 635.Pubmed PMID: 36398743.
[22]. Nelson EJ, Maynard BR, Loux T, Fatla J, Gordon R, Arnold LD. The acceptability of self-sampled screening for HPV DNA: a systematic review and meta-analysis. Sex Transm Infect. 2017 Feb;93(1):56-61.Pubmed PMID: 28100761.
[23]. Dartell M, Rasch V, Kahesa C, Mwaiselage J, Ngoma T, Junge J, et al. Human papillomavirus prevalence and type distribution in 3603 HIV-positive and HIV-negative women in the general population of Tanzania: the PROTECT study. Sex Transm Dis. 2012 Mar;39(3):201-8.Pubmed PMID: 22337107.
[24]. Katanga J, Kjaer SK, Manongi R, Wu CS, Iftner T, Waldstrom M, et al. Performance of care HPV, hybrid capture 2 and visual inspection with acetic acid for detection of high-grade cervical lesion in Tanzania: A cross-sectional study. PLoS One. 2019 Jun 19;14(6):e0218559.
[25]. Okoye JO, Ofodile CA, Adeleke OK, Obioma O. Prevalence of high-risk HPV genotypes in sub-Saharan Africa according to HIV status: a 20-year systematic review. Epidemiol Health. 2021;43:e2021039.Pubmed PMID: 34044477.