Hemorrhagic Centrolobar Necrosis and Cytoplasmic Vacuolation of the Hepatocytes in Syzygium Guineense Chronic Treated Mice
Abba S1,2*, Omotoso. O Dare2, Joseph Ibrahim M3, Eyinna O4
1 Department of Anatomy, Institutes of Biomedical Sciences, Mekelle University, Ethiopia.
2 Faculty of Basic Medical Sciences, Department of Anatomy, College of Health Sciences, Kogi State University Anyigba, Nigeria.
3 Faculty of Basic Medical Sciences, Department of Physiology, College of Health Sciences, Kogi State University Anyigba, Nigeria.
4 Faculty of Basic Medical Sciences, Department of pharmacology, College of Health Sciences, Kogi State University Anyigba, Nigeria.
*Corresponding Author
Sunday Abba,
Department of Anatomy, Institutes of Biomedical Sciences,
Mekelle University, Ethiopia
Tel: +2348159344263
E-mail: suneabba007@gmail.com
Received: July 21, 2018; Accepted: September 07, 2018; Published: September 29, 2018
Citation: Abba S, Omotoso. O Dare, Joseph Ibrahim M, Eyinna O. Hemorrhagic Centrolobar Necrosis and Cytoplasmic Vacuolation of the Hepatocytes in Syzygium Guineense Chronic Treated Mice. Int J Anat Appl Physiol. 2018;4(4):99-102. doi: dx.doi.org/10.19070/2572-7451-1800018
Copyright:Abba S©2018. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
A World Health Organization survey indicated that about 70-80% of the world population rely on non-conventional medicine mainly of plant sources in their primary healthcare. In Ethiopia over 85% of the population relies on traditional medicine for the fight against various diseases. Syzygium guineense is one of such medicinal plants in use. It is traditionally used as a remedy for diarrhea, stomach pain and intestinal cramps. The toxic effects of these plants are often not put into considerations when they are being used. In this study we attempted to investigate the toxic effect of Syzygium guineense. On the histopathology of the liver of mice we choose the Liver because it is the major metabolic organ. Chronic administration of the aqueous of the extract of Syzygium guineense was found to be toxic to the hepatocytes as the micrograph showed increase vaculations, and shrinkage of the hepatocytes. There was also a general decrease in body weight and enlarged liver.
2.Abbreviations
3.Introduction
4.Materials and Methods
4.1 Experimental design and Laboratory
4.2 Collection of Plant Materials
4.3 Processing of the Plant Materials
4.4 Experimental Animals
4.5 The LD50 and Tissue Morphology
4.6 Animal Dissection and Tissue Sample Collection
4.7 Tissue Processing
4.8 Light Microscopy
4.9 Statistical Analysis
6.Results
7.Discussion
8.Conclusion
9.References
Keywords
Hepatocyte; Toxic; Syzygium; Guineense; Acqeous.
Abbreviations
EHNRI: Ethiopian Health and Nutrition Research Institute; ANOVA: Analysis of Variance.
Introduction
Plants that are commonly used in traditional medicine are assumed to be safe and their safety is based on their long usage in the treatment of diseases according to knowledge accumulated over centuries. However, recent studies have showed that many plants used as food or in traditional medicine are potentially toxic, mutagenic and carcinogenic [1, 2]. A World Health Organization survey indicated that about 70-80% of the world population rely on non-conventional medicine mainly of plant sources in their primary healthcare [3]. In Ethiopia over 85% of the population relies on traditional medicine for the fight against various diseases [4]. Plant remedies still remain the most important and sometimes the only source of therapeutics for nearly 80% of the population, in Ethiopia and the Sub Saharan Africans [5].
In Ethiopia, there are traditional medicines that have been used by many folk medicine practitioners without taking into consideration their side effects on different parts of the body [6]. Syzygium guineense is one of such medicinal plants in use.
S. guineense grows in most parts of Ethiopia at altitudes of 1200-2500m [7] and is traditionally used as a remedy for diarrhea, stomach pain and intestinal cramps [6].
Both the hydroalcoholic and aqueous extracts of the leaves of S. guineense was found to be effective at the dose of 200mg/ kg in controlling castor oil induced diarrhea in mice by oral administration [8]. Same study also demonstrated that the leaf and stem bark extracts had spasmolytic effect in Guinea pig ileum.
Phytochemical investigation of S. guineense showed the presence of tannins, phytosteriods, flavonoids and saponins in some of the crude extracts and fractionates [8]. Eugenin, an active ingredient found at buds of Syzygium Spp; had antiviral effect against Herpes simplex [6, 9]. According to [10] Syzygium guineense had potent antibacterial effect against diarrhea causing bacteria. Methanolic extract of S. guineense inhibited intrinsic contraction in isolated ileum tissue of rabbit [11]. Eugenia guineense revealed a significant increase in the plasma bilirubin of alkaloidal fraction treated rats, which is suggestive of a possible damage to the liver occasioned by the treatment with the E.guineense stem-bark total alkaloidal fraction [12]. The aim of the present study was to investigate the effect of the extract of S. guineenses on the histopathology of the liver of mice.
It is an experimental case control type of research. The animal laboratory works; histological tissue processing was done in the histology laboratory of anatomy department of Mekelle University Ethiopia. Light microscopy and micrographs were taken in the histology laboratory of Anatomy Department, Kogi State University Anyigba Nigeria.
Fresh leaves of S.guineense were collected from Wolliso area about 116 km south west of Addis Ababa, Ethiopia, during the month of February, 2015. Plant sample was identified and deposited in the National Herbarium of the Department of Biology, AAU with a specimen number of 001, 002, and 003.
Fresh leaves of S.guineense were cleaned with tap water from the extraneous materials, dried at room temperature, and ground to powder in the Phytochemistry Laboratory of the Department of Drug research, Ethiopian Health and Nutrition Research Institute, EHNRI. Extraction of the aqueous extract of the ground fresh leaves was done by maceration of 600 g in 2000 ml of distilled water. The aqueous extract was intermittently agitated for 24 hours by Orbital shaker (DS-500 model 0.75” 1.9cm Cat. No. 444.7018) at room temperature. After 24 hrs, the extract was filtered with gauze and lyophilized to give a powdered material yield of the extract, which was 9.5%. The extract was then kept in desiccators at room temperature till use.
The research was conducted following the approval of the proposal by the Faculty Research and Publication Committee. Male and female animals of Swiss albino mice (25-30 g) were provided from the animal department of EHNRI. The mice were acclimatized for 5 days to the environment of the animal house in the department of drug research, EHNRI. Animals were fed standard commercial diet and water ad libitum. They were maintained at standard conditions of temperature (21 ± 2°C), relative humidity of 65 ± 0.5% and 12 hours light/night cycles till the end of the experiment. The mice were randomly divided into four groups with each group consisting of 10 mice (5 male and 5 female) and separate cages were used for the sexes. Group I served as a control and received the vehicle, i.e., distilled water. Groups II, III and IV were given 200, 400 and 600 mg/kg bw of the extract for each group, respectively, once a day at 24 hour intervals, for six weeks. Administration of the extract was done with intragastric tube, and the volume administered in all cases was 0.5 ml/mouse. The no-effect dose was that inhibited diarrhea in the in vivo study carried out for the effect of crude leaf extract of S. guineense on castor-oil induced diarrhea [8].
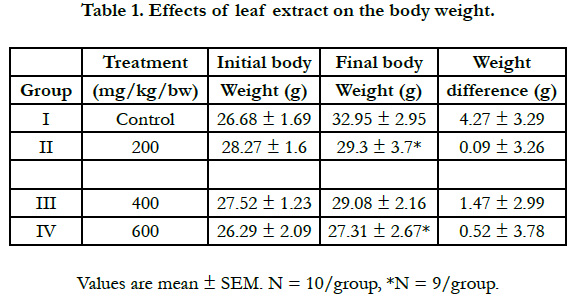
At the beginning and end of six weeks, all the mice were weighed as described by [13]. Body weight taken on the initial date of treatment was considered the initial weight and the weight taken on the last day of administration before anesthesia and dissection; was considered the final weight.
A batch of 28 mice (27-30g) of both sexes were divided randomly into two groups of each containing 14 mice (7male and 7 female), one of which serving as a control. The oral LD50 (median lethal dose) of the aqueous extract was 14.1g/kg bw [15]. The extract was given orally after overnight fasting. The control group was given distilled water orally. Soon, after the death of the animal, dissection was carried out. The tissue samples were collected from the liver and immersed immediately in a fixative (10% buffered neutral formalin solution).
Animals were anesthetize and sacrifice. The whole liver was exposed and carefully removed and blotted on filter paper and weighed quickly on a semi-microbalance sensitive to 0.001mg (S¢IENTECH Boulder Co., USA). Part of the median lobe of the liver were cut in coronal and transverse sections respectively and placed immediately in fixative (10% buffered neutral formalin solution) for tissue processing. Similarly, the liver of mice found dead was also dissected immediately, trimmed and placed using the same methodology.
The tissues were processes following routine tissue processing techniques for hematoxyline and eosine. This was done in the histology laboratory of Anatomy department Mekelle Unversity.
Sections of the liver and kidney were examined under the compound light microscope with different objectives and photomicrographs of selected samples of the liver and kidney tissue were then taken by Fuji iso 200 film using a MC 80 DX Microscopic Camera (Carl Zeiss Jena Gmbh, Germany) and Zeiss Aploscope microscope fitted with a digital camera (Nicon Coolpix 5000). A magnification of ×40 was used to examine changes in the liver tissue. Following the evaluation of control animals, the liver of the remaining groups were evaluated blind to treatment.
All the values in the test are expressed as mean ± SEM. Statistical differences between the means of various groups were evaluated by one-way analysis of variance (ANOVA) using the SPSS version 11.0 programmer followed by student’s t-test. P-values less than 0.05 were considered to be significant.
Results
The results observed from this study are tabulated below and the micrographs are also presented below.
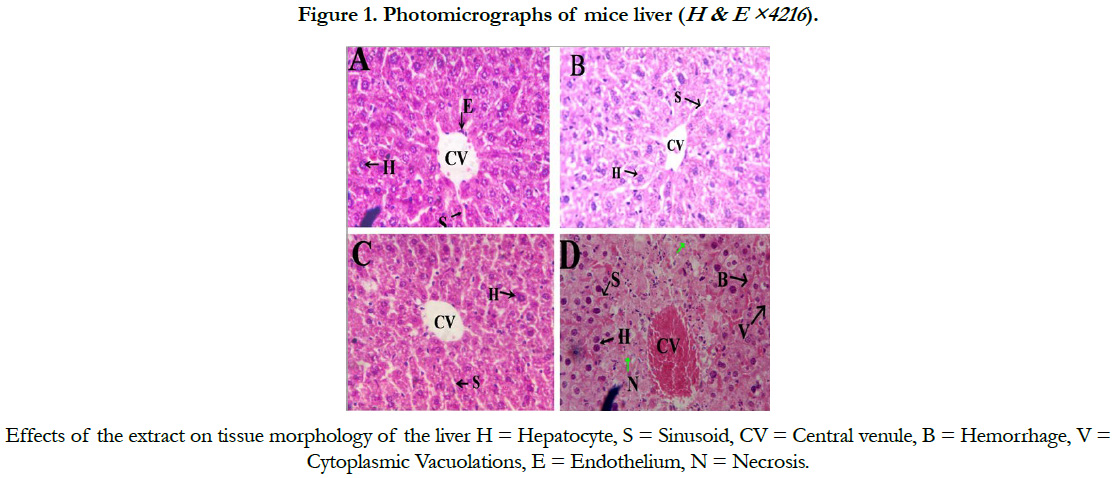
Light microscopic examination of liver tissue of the control mice showed characteristic features showing normal portal triad, interlobular bile duct, radiating hepatic cells and hepatic sinusoids lined by endothelial cells (Figure. 1A).
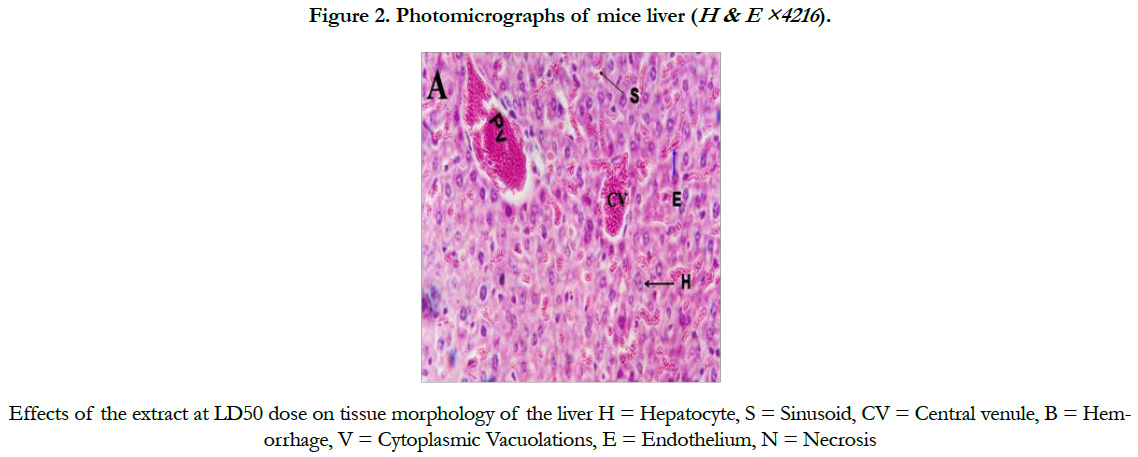
Mice treated at a dose of 200 and 400 mg/kg bw showed no histopathological changes as compared to the control (Figure 1, B and C). However, tissue morphology of mice treated with 600 mg/kg bw of the extract showed hemorrhagic necrosis, and cytoplasmic vacuolations (Figure 1D). Similarly, congestion of liver sinusoids was observed in mice treated with the LD50 of the extract (Figure 2).
Discussion
In this study, significant general body weight gain of mice was observed in mice treated with 200, and 600mg/kg bw as compared to the control group as shown in Table 1. The weight gain difference between the treated and the control groups suggest the presence of toxic constituents in the extract that impaired the growth. This is in agreement with the findings of [14] that rats on 10% Acacia abyssinica diet which showed decrease in body weight gains and development of hepato-nephropathy indicating that the plant contains toxic constituents that impaired growth and reduced their rate of excretion by the injured liver.
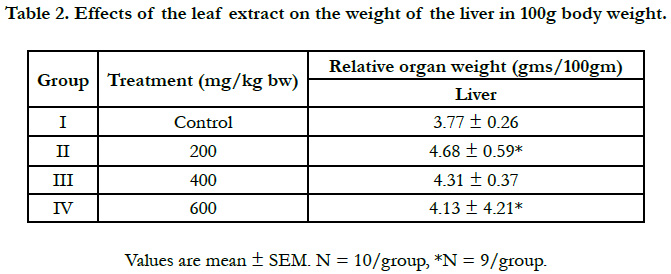
The increase in liver weight with 200 and 400mg/Kg extract is due to the toxic effect of the active component (s) present in the extract leading to abnormal histology of the liver. The increased in liver weight in the present study as shown in Table 2, is consistent with the ones observed in the previous studies done with other plants [15-17].
Chronic administration of 600mg/kg S. guineense aqueous leaf extract in mice revealed centrilobular necrosis of the hepatocytes a of the live, which is in agreement with that of [18], who reported that massive hepatocyte necrosis was observed in the liver of the rats treated with aqueous extract of Securidaca Longepedunculata and described that this abnormal histology of the liver is characterized by focal hepatocellular necrosis similar to the pattern of damage induced by overdose of paracetamol [19].Explained that hepatotoxic drugs and chemicals may act through their metabolites which could typically cause centrilobular necrosis. They demonstrated an autopsy specimen of acetaminophen that disclosed a prominent hemorrhagic necrosis of the centrilobular zones of all liver lobules. A study by [20] reported that mice treated with Senna podocarpus extract revealed congested central veins and sinusoids of the liver.
Conclusion
The aim of this study was to investigate the effect of the extract of Syzygium guineense on the histopathology of the liver of mice. From the result obtained here and in comparison to other studies we conclude that chronic usage of S. guineense is toxic to the hepatocytes, and will result to decrease body weight and enlarged liver.
References
- Kassie F, Parzefall W, Musk S, Johnson I, Lamprecht G, Sontag G, et al. Genotoxic effects of crude juices from Brassica vegetables and juices and extracts from phytopharmaceutical preparations and spices of cruciferous plants origin in bacterial and mammalian cells. Chem Biol Interact. 1996 Sep 27;102(1):1-16. PubMed PMID: 8827059.
- de Sá Ferreira IC, Ferrão Vargas VM. Mutagenicity of medicinal plant extracts in Salmonella/microsome assay. Phytother Res. 1999 Aug;13(5):397- 400. PubMed PMID: 10441779.
- World Health Organization. General guidelines for methodologies on research and evaluation of traditional medicine. Geneva: World Health Organization; 2000.
- Abebe D, Zewdu M, Demissie A. conservation and sustainable use of medicinal plants in Ethiopia Proceeding of the National Workshop on Biodiversity Conservation and Sustainable Use of Medicinal Plants in Ethiopia. Addis Ababa: IBCR; 2002. p. 1-3.
- Abebe D. The role of medicinal plants in healthcare coverage of Ethiopia: The possible benefits of integration. Conservation and sustainable use of medicinal plants in Ethiopia. Institute of biodiversity and research: Addis Ababa, Ethiopia; 2001. p. 6-21.
- Abebe D, Debella A, Urga K. Illustrated checklist: Medicinal plants and other useful plants of Ethiopia. EHNRI, Camerapix Publisher International: Kenya; 2003. p.188-194.
- Edwards S, Tadese M, Hedberg I. Flora of Ethiopia and Eritrea: Addis Ababa, Ethiopia. 1995;2(2):77.
- Nigatu, B. Anti spasmodic, anti diarrheal and LD50 determination of Syzygium guineese in animal model. A thesis submitted to school of graduate studies, AAU; 2004. p. 30-65.
- Takechi M, Tanaka Y. Purification and characterization of antiviral substance from the bud of Syzygium aromatica. Planta Med. 1981 May;42(1):69-74. PubMed PMID: 17401883.
- Tsakala TM, Penge O, John K. Screening of in vitro antibacterial activity from Syzygium Guineense (Willd) hydrosoluble dry extract. Ann Pharm Fr. 1996;54(6):276-9. PubMed PMID: 9008903.
- Malele RS, Moshi MJ, Mwangi JW, Achola KJ, Munenge RW. Pharmacological properties of extracts from the stem bark of Syzygium guineense on the ileum and heart of laboratory rodents. Afr J Health Sci. 1997 Jan-Mar;4(1):43-5. PubMed PMID: 17583982.
- MacGregor FB, Abernethy VE, Dahabra S, Cobden I, Hayes PC. Hepatotoxicity of herbal remedies. BMJ. 1989 Nov 4;299(6708):1156-7. PubMed PMID: 2513032.
- Khleifat K, Shakhanbeh J, Tarawne KA. The chronic effects of Teucrium polium on some blood parameters and histopathology of liver and kidney in the rat. Turkish J Biol. 2002 Apr 2;26(2):65-71.
- Al-Redhaiman KN, Salad MA, Adam SE. Effect of feeding Acacia abyssinica and of its extracts given by different routes on rats. Am J Chin Med. 2003;31(2):259-66. PubMed PMID: 12856864.
- de la Iglesia FA. Chronic toxicity study of the antidiabetic troglitazone in Wistar rats. Toxicol. 1998;42(1):50.
- Herman JR. Subchronic toxicity of the antidiabetic troglitazone in Wistar rats. Toxicol. 1997;36:273.
- Witthawaskul P, Panthong A, Kanjanapothi D, Taesothikul T, Lertprasertsuke N. Acute and subacute toxicities of the saponin mixture isolated from Schefflera leucantha Viguier. J Ethnopharmacol. 2003 Nov;89(1):115-21. PubMed PMID: 14522442.
- Dapar ML, Aguiyi JC, Wannang NN, Gyang SS, Tanko NM. The histopathologic effects of Securidaca longepedunculata on heart, liver, kidney and lungs of rats. Afr J Biotechnol. 2007;6:591-595.
- Rubin E, Farber JL. Pathology: the liver and biliary system. 7th ed. Lippincot Raven Publishers; 1999. p. 756-839.
- Akanmu MA, Iwalewa EO, Elujoba AA, Adelusola KA. Toxicity potentials of Senna podocarpa (Guill. et Perr.) lock pods in rodents. Afr J Tradit Complement Altern Med. 2005;2(3):274-81.