Effect of Cold Pretreatment, Anthers Orientation, Spikelet Position and Donor Tiller on the Callusing Response in Barley Anther In vitro Culture
El Goumi Y1*, Fakiri M1, Benbachir M2, Essayagh S2, Lamsaouri O1
1 Laboratory of Agrifood and Health, Faculty of Sciences and Techniques, Hassan 1st University, Settat, Morocco.
2 Laboratory of Biochemistry and Neuroscience, Faculty of Sciences and Techniques, Hassan 1st University, Settat, Morocco.
*Corresponding Author
Younes El Goumi,
Laboratory of Agrifood and Health, Faculty of Sciences and Techniques,
Hassan 1st University, BP 577, 26000, Settat, Morocco.
E-mail: elgoumiyounes@hotmail.com
Received: September 04, 2017; Accepted: October 05, 2017; Published: October 11, 2017
Citation: El Goumi Y, Fakiri M, Benbachir M, Essayagh S, Lamsaouri O. Effect of Cold Pretreatment, Anthers Orientation, Spikelet Position and Donor Tiller on the Callusing Response in Barley Anther in vitro Culture. Int J Med Biotechnol Genetics. 2017;S2:003:33-38. doi: dx.doi.org/10.19070/2379-1020-SI02003
Copyright: El Goumi Y© 2017. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
The anthers in vitro culture is a useful technique for plant improvement as to create new varieties. However, the success of this technique is limited by several factors. This study was carried out to identify factors as the influence of spikelet position in the spike, position of donor tiller on plate tillering and anther orientation onto the medium as well as cold pretreatment and the genotype that affect the callusing induction in anthers in vitro culture of three cultivars of Moroccan barley (Arig 8, Asni and Tamelalt). Among genotypes, the callusing response was significantly higher in Asni (21.42%). The callusing response was significantly decreased when spikes stemmed from tillers far from the main tiller on the same tillering plate. However, anthers coming from the spikelets of the middle part of the spike showed the highest callusing response (29.85%) compared with the basal (14.83%) and the upper (6.72%) parts. It was also shown that anther cold pretreatment at 4°C for 14 days had a beneficial effect only on the callusing response but it decreased the regeneration of green plants, due to the interaction between cold pretreatment and genotypes to which spring barley is recalcitrant and more predisposed to albinism, especially six-row type (Arig 8). Moreover, anther orientation onto the surface medium had a marked effect on callusing response, anthers placed in upper orientation developed more calli (60.22%) than those in flat position (18.99%). Our findings suggest that the studied factors control the callusing response. Especially, the Anthers orientation that must be taken into account during androgenesis through anthers in vitro culture.
2.Introduction
3.Materials and Methods
3.1 Donor Plant
3.2 Anthers Culture, Induction, and Regeneration
3.3 Statistical Methods
4.Results
4.1 Spikelet Position on Spike
4.2 Tiller Position on the Plate Tillering
4.3 Cold Pretreatment Effect
4.4 Anther Orientation onto Culture Medium
5.Discussion
6.Acknowledgements
7.Conclusion
8.References
Keywords
Albinism; Anther Culture; Barley; Callusing Response; Spikelet.
Introduction
Plant biotechnology offers very valuable tools for crop improvement. Doubled haploid techniques generate homozygous/fixed lines in a shorter time than that necessary with conventional generations of selfing, and allow acceleration of breeding programs. Androgenesis is the most used technique for obtaining doubled haploids (DHs). It has seen vastly applicated especially in cereals such as wheat [1, 2], barley [3, 4], rice [5, 6], triticale [7, 8], oat [9, 10], and maize [11, 12].
Although it is widely used as a method to create DHs, androgenesis yields depend on several factors as the physiological condition of the donor plant, the genotype, the culture medium composition, pretreatments such as stressors and microspore developmental [2, 13, 14]. Androgenesis improvements rely mainly on optimizing the composition of culture media, the duration/ temperature of pretreatments, microspores isolation, hormones, and genotype. However, the androgenic response can also be influenced by factors like the spike position on the tiller [14-16] and the spikelet position on the spike [5, 17, 18]. Furthermore, the yield of androgenesis using in vitro culture of anthers was shown to depend on anther orientation on the culture medium [19-23]. Despite the known influence of these factors, often they are not taken into account during in vitro culture of anthers and their role remains poorly studied.
Hence, the aim of this study was to highlight the influence of spikelet position in the spike, position of donor tiller on plate tillering and anther orientation onto the medium as well as cold pretreatment and the genotype of Moroccan barley on the yield of anthers cultured in vitro. We focused especially on embryos and/or calli induction. We want to highlight the importance of taking these factors into account when improving in vitro anther culture protocols.
Three cultivars of Moroccan spring barley were used namely Arig 8 (six-raw), Asni and Tamelalt (two-raw). Plants were grown in pots in a greenhouse from December to April, without treatment or fertilization, and were watered daily to avoid hydric stress.
Spikes were harvested when microspores were at the mid- to the late-uninucleate stage. The developmental stage was determined by cytological analysis of aceto-carmine stained microspores.
Spikes were subjected to a cold treatment at 4°C for 14 days in the dark. The spikes inside flag leaves were surface sterilized by wetting with 95% ethanol and then removed from the sheath. Anthers from the same spike were excised and transferred onto FHG medium [24] solidified by agarose (8 g/L) for embryos induction, supplemented with 2 mg/L NAA (Naphthaleneacetic acid) and 1 mg/L BAP (6-benzylaminopurine), with adjusted pH (5.6), respecting parts of the spike (base, middle, top). Dishes were sealed with parafilm and incubated in the dark at 25±1°C.
After 3-4 weeks, embryos and/or calli were transferred to regeneration medium contain the same salt elements and vitamins contained in the induction medium, supplemented with 35 g/L sucrose, 0.4 mg/L BAP and solidified by agar (7 g/L); the pH of the medium was adjusted to 5.8. Dishes were kept at 23 ± 2°C under 18 hour light photoperiod of 2000-3500 Lux. After regeneration, shoots were transferred on the same regeneration medium without hormone, containing 20g/L sucrose, and incubated in the same conditions to ensure their growth. Plantlets were afterward planted in small pots with earth then acclimatized.
The calculated parameters in this study were:
Callusing response (I%) = (Number of responding anthers)/ (Number of cultured anthers) × 100
Rebeneration rate (R%) = (Number of regenerated plants)/ (Number of cultured calli) × 100
Albinism rate (A%) = (Number of albinos plants)/(Number of regenerated plants) × 100
Data were analyzed using JMP SAS Pro software (JMP®, Version <12> SAS Institute Inc., 2015). Data normality was verified according to Shapiro-Wilk test and then transformed using Arcsin √x to achieve normality. Data were subjected to analysis of variance (ANOVA) F-test to assess effect sources and interactions. Significance between means was tested by Student’s t-test. For the anthers orientationon medium, statistical analysis was performed using likelihood ratio test [25] as cited by El Goumi et al., [2]. A difference was considered significant at P=0.05.
Results
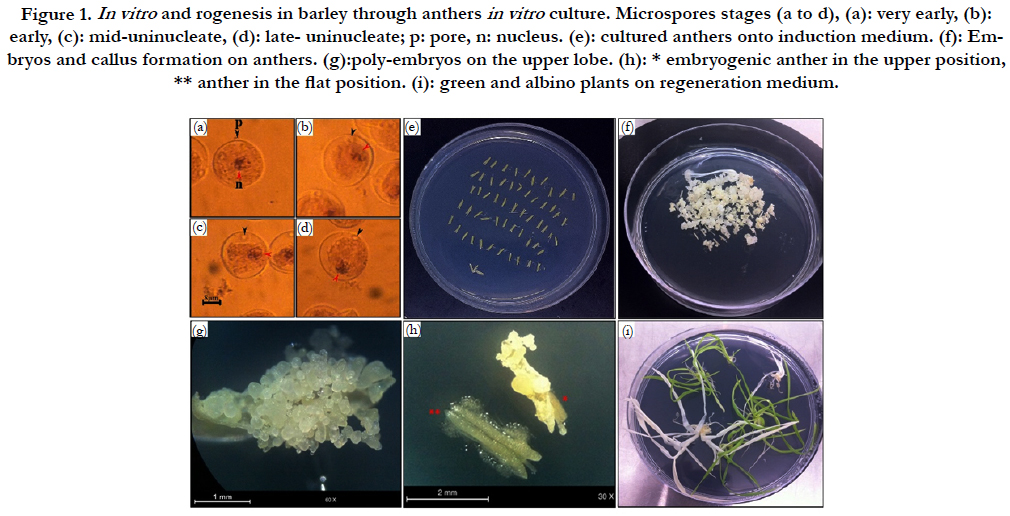
In our study, all genotypes have responded positively to embryos/calli induction (Figure 1, f). 8274 anthers from the various barely genotypes previously cited, were cultured (Figure 1, e). The tests were carried out between March and April in order to obtain the optimal results. For tested cultivars, the majority of microspores were in the appropriate stages (Figure 1, a to d) when the interligule length between flag leaf and penultimate was 2.5 to 5 cm for Asni and Tamelalt, whereas it was 3 to 8 cm for Arig 8.
Figure 1. In vitro and rogenesis in barley through anthers in vitro culture. Microspores stages (a to d), (a): very early, (b): early, (c): mid-uninucleate, (d): late- uninucleate; p: pore, n: nucleus. (e): cultured anthers onto induction medium. (f): Embryos and callus formation on anthers. (g):poly-embryos on the upper lobe. (h): * embryogenic anther in the upper position, ** anther in the flat position. (i): green and albino plants on regeneration medium.
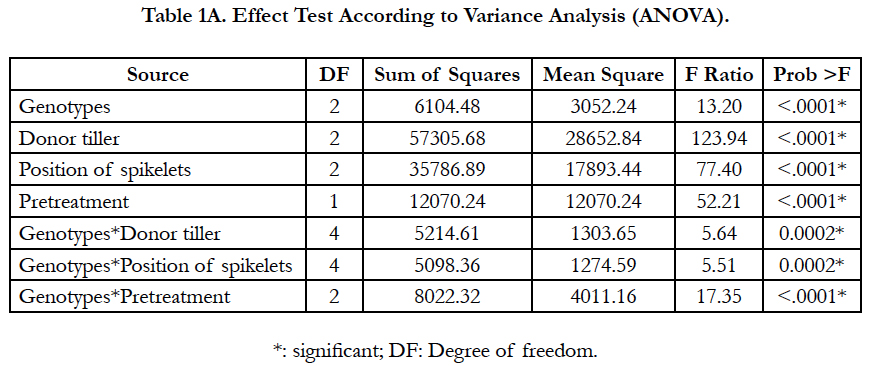
The results revealed that all the studied factors had a significant effect on the callusing response, and the genotype was significantly in interaction with the other factors. This suggested that the responses of the tested genotypes were variable among those factors (Tables 1A-1B).
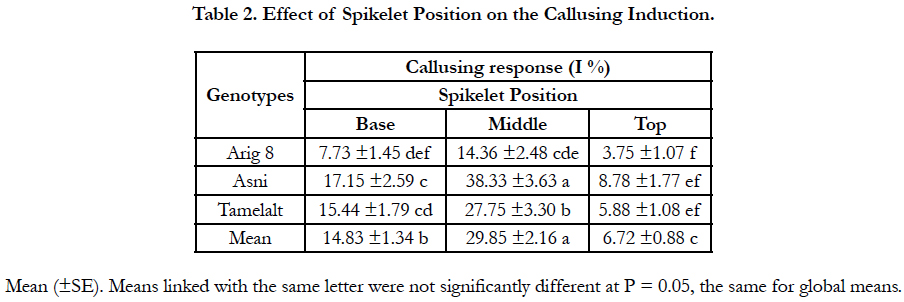
The callusing response from different spike parts is shown in Table 2. The spikelet position had a significant effect on the percentage of responding anthers. Regardless of genotypes, significant differences were observed among spikelet positions (P<0.0001) (Table 1A). The highest callusing response, when averaged over genotypes, was archived by anthers coming from the middle part with a yield of 29.85%. Whereas anthers coming from spikelets of the top and the basal parts yielded the lowest response with 6.72% and 14.83%, respectively. Among genotypes, for all spikelet positions, the highest callusing response was shown in Asni cultivar followed by Tamelalt and Arig 8 cultivars. However, for the top part, the callusing response between genotypes remained with no significant differences.
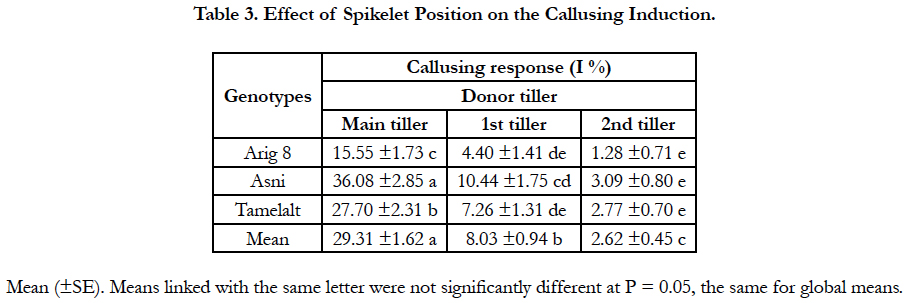
Overall, tiller position on the plate of tillering has an impact on embryos and/or calli induction. Significant differences were found between the tiller positions for callusing anther response (P < 0.001), and among genotypes differences were significant only for the main tiller (Table 1A). The highest callusing anther response (29.31%) was obtained with anthers of spikes coming from main tillers; this response was decreased when the donor spike was far from the main tiller on the tillering plate. Moreover, the cultivar Asni presented the highest average of anthers induction (36.08%), followed by Tamelalt (27.4%) and Arig 8 (15.55%) when spikes coming from the main tiller. The lowest responses were achieved by the second tiller, it was 3.09%, 2.77%, and 1.28% for Asni, Tamelalt and Arig 8, respectively. It is worth noting that the intermediate responses were observed for the first tiller (Table 3).
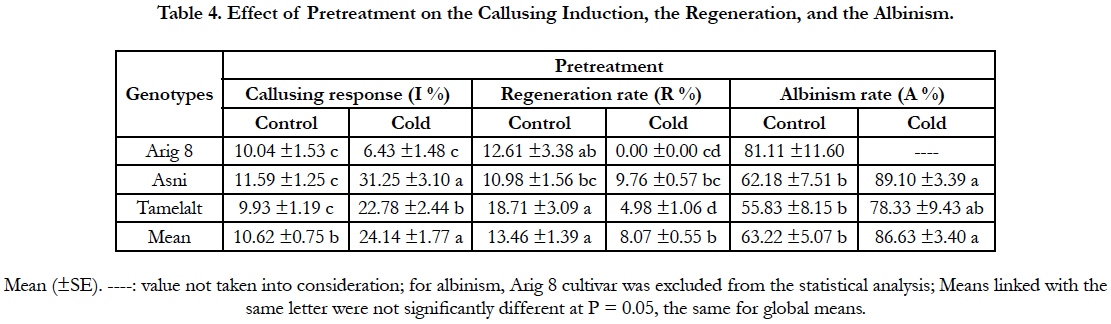
Overall, the cold pretreatment was found to significantly increase the callusing response in comparison with the control (P<0.0001). Anthers coming from cold pretreated spikes displayed the highest callusing induction (24.14%) compared to the control (10.62%). A significant interaction between genotype and pretreatment was found, indicated that the callusing responses of the tested cultivars were variable among pretreatment (Table 1A). The beneficial effect of cold pretreatment was shown in Asni and Tamelalt cultivars by increasing their callusing responses from 11.59% to 31.25% and from 9.93% to 22.78%, respectively. However, this cold pretreatment had a negative effect on Arig 8 cultivar that showed a decrease of its callusing response from 10.04% to 6.43%. The callusing responses of genotypes were significantly different when anthers receiving cold pretreatment, while they were not in the control (Table 4).
Although the beneficial effect of cold pretreatment on the callusing response, our results showed its negative effect on the number of plants regenerated (Figure 1, i). The regeneration rate decreased significantly from the control (13.46%) to the cold pretreatment (2.35%). This decrease was more important in Arig8 and Tamelalt cultivars; it was from 12.61% to 0.00% and from 18.71% to 4.98%, respectively. In addition, the cold pretreatment showed its negative effect on the quality of regenerated plants which albinism rate was increased significantly (P<0.0001) in cold pretreatment (86.63%) compared to the control (63.22%). Arig8 as six-row spring showed the highest albinism rate compared to Asni and Tamelalt, which are two-row spring barley (Table 4).
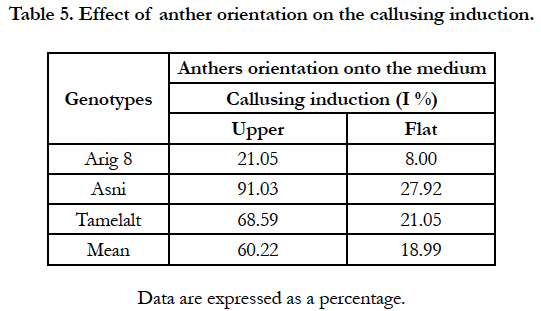
In this experiment, only anthers coming from middle part of the cold pretreated spike of the main tiller were cultivated. Results are shown in Table 5.
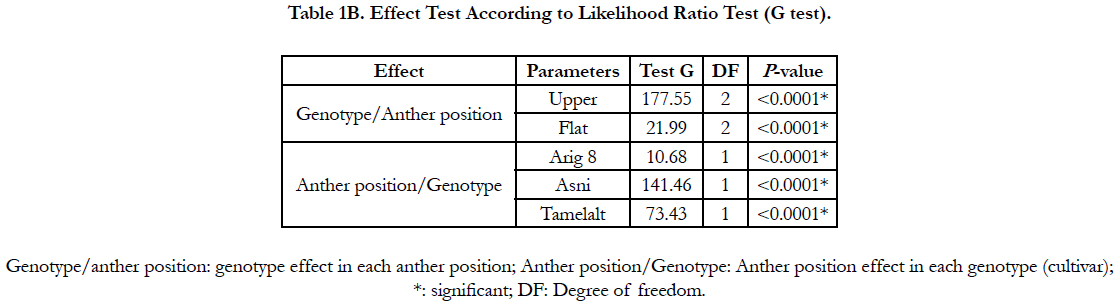
The likelihood ratio test (G test) showed a high significant effect of anthers orientation onto medium and genotype on the percentage of the callusing response (P<0.0001) (Table 1B). When the callusing response was averaged over genotypes, the anthers placed on edge (just one lobe in contact with the medium) expressed the highest callusing response (60.22%) while the anthers lying on two lobes (flat) showed the lowest callusing response (18.99%). In addition, in anthers lying in upper position, only upper lobes showed embryos development (Figure 1, g). Whereas in anthers placed in flat position the microspores mostly were released onto the medium with no development (Figure. 1. h). Among genotypes, the highest callusing response rate was obtained by Asni (59.74%) followed by Tamelalt (44.82%) and Arig 8 (14.53%).
Discussion
In the current work, we have highlighted the effect of a number of factors on the success of anthers in vitro culture in three genetic variants of Moroccan barley. The genotype was found to be a major factor in androgenetic ability; it manifests both in callusing response and in plantlets regenerating. This finding consistent with that described by other authors [18, 26-28]: some genotypes respond negatively to the cold pretreatment, as in Arig 8 in which the induction rate in cold-pretreated spikes was decreased compared to the control. This observation coincides with the results presented by Powell [29] in Igri cultivar. Note that the effect of genotype is noticed since we used spring barleys that are predisposed to albinism. Indeed, Arig 8 is barley variety presenting both spring and six-row type, which explains the high rate of albinism, obtained for this genotype. Similar results have been reported by previous authors describing spring barley recalcitrant genotype, particularly six-row types [4, 26, 30].
Furthermore, the present findings also suggest that spikelet position in the ear had a noticeable effect on the callusing induction. Anthers coming from the middle part responded better than those coming from the lower part and the upper part, which is in agreement with results reported by Fakiri [18] and Afza et al., [5]. These results can be explained by the more advanced stage development of anthers coming from the spikelet of the middle part than that coming from the upper and the lower part of the ear [17]. Furthermore, the determination of the relevant stage of microspore for the in vitro androgenesis generally focuses on spikelets of the central part of the ear.
Regarding the spike position on the donor tiller, the callusing induction response was affected accordingly. A decrease in embryonic induction was unveiled significant between spikes positions when donor tiller was far from the main tiller (main shoot) on the plate of tillering. Our results are consistent with those described for barley by Jacquard et al., [14] and for wheat by Brazauskas [16] and Yldrm et al., [15]. This difference is due to a competitiveness of tillers for nutrients in a negative gradient of assimilates from the main shoot to the nth tiller [31]. This competitiveness increased the number of sterile florets when tillers were far from the main shoot [32], which explains the callusing induction decrease for spikes of the 1st and 2nd tillers.
For the orientation of the anther on the surface of the solid medium, the results revealed a large fluctuation around 41% of the callusing induction rate between the two studied positions. Consistent with findings by Hunter [20], Shannon et al., [21] and Perera et al., [23], we found that anthers cultured in the upper position (on one lobe) were more likely to callusing induction, confirming the influence of this factor on the success of androgenesis. Anther in flat position absorbed liquid held around it and released microspores on the surface of the medium [21]. Moreover, the isolated microspores, which were located in this liquid, accumulated starch and die [33]. In anthers cultured in flat position the development of embryonic formations when existent was slow, whence the low rate of callusing induction [23].
As to the cold pretreatment at 4°C, our finding is in agreement with Fakiri [18], Lazaridou et al., [28] and El Goumi et al., [2]; these findings showed the beneficial effect on the callusing induction There are several possible explanations for these beneficial effects. Firstly, the effect of cold pretreatment causes degradation of the tapetum before the spores begin to divide. This disrupts the normal developmental stages of microspore and induces the release of microspores in the anther's locule with more nutritional fluid [34]. Secondly, cell death of tapetum cells emits signals that can lead to sensitive microspores entering the sporophytic development pathway [35]. Thirdly, the cold pretreatment causes the decomposition of starch in the microspore, which is a determining factor of recalcitrance in androgenesis [36]. Finally, in addition to its advantage in stopping the fragmentation of DNA in anther tissue cells, DNA restoration is performed at the end of the pretreatment, as well as the increased viability of the microspore. This viability is provided by an increase in ABA levels that protects microspores from dying by apoptosis [37]. The observations of the current study do not support that cold pretreatment is beneficial for all barley genotypes, corroborating similar observations by Powell [29] for Igri cultivar.
Across all genotypes, the negative effect of cold pretreatment was apparent in the regeneration phase. Compared to control, a decrease in the regeneration rate was recorded. This may be due to the duration of the pretreatment (14 days) in combination with genotype; Lazaridou et al., [28] listed the same remarks. The negative effect of this cold pretreatment was clearly represented in the quality of regenerating, there were more albinos plantlets regenerated in the cold pre-treated series. This is probably due to alterations that can reach the plastid DNA as the deletion or amendment of certain sequences [36, 38], but also the prevention of differentiation of plastids chloroplasts under the conditions of culture [26].
Acknowledgements
We thank Dr. H. Ouabbou responsible of the gene bank (National Institute for Agricultural Research of Morocco, Settat Centre.) for the delivery of the grains used in this study. We thank Dr. P. Fourgeois director of the biotechnology laboratory at Institut de Genech (France) who gave me the opportunity for an apprenticeship in his laboratory.
Conclusion
The present study was designed to identify several factors that affect the callusing induction in anthers in vitro culture for barley. In addition to cold pretreatment, it appears to be more important to concentrate on the main tiller, the middle part of the spike and the anther position onto the surface medium for the best callusing induction. As for regeneration, spring barley genotypes in particular six-row types are more predisposed to albinism, which should be avoided to increase the number of green regenerated plantlets or seek for alternatives to overcome this hurdle such as the culture of isolated microspores, gynogenesis or interspecific or intergeneric crosses.
References
- Cherkaoui S, Lamsaouri O, Saidi N, Chlyah B, Chlyah H. Regeneration of haploid green plants through anther culture in durum wheat (Triticum turgidum ssp. durum) using liquid media. Actes Inst Agron Vet. 1997;17(4): 201-208.
- El Goumi Y, Fakiri M, Lamsaouri O, Benchekroun M, Hassani MF. Analy sis of the androgenetic capacity of three durum wheat cultivars (Triticum durum) and three cultivars of common wheat (Triticum aestivum). Leban Sci J. 2014;15(1): 85-98.
- Sibi M, Fakiri M. Androgenesis and gynogenesis, source of vitrovariation and salinity tolerance in barley Hordeum vulgare? Secheresse. 2000 Jun;11(2):125-132.
- Esteves P, Belzile F. Improving the efficiency of isolated microspore culture in six-row spring barley: I-optimization of key physical factors. Plant Cell Rep. 2014 Jun;33(6): 993-1001. PubMed PMID:24563120. doi:10.1007/s00299-014-1583-x
- Afza R, Shen M, Zapata-Arias FJ, Xie J, Fundi HK, Lee K, et al. Effect of spikelet position on rice anther culture efficiency. Plant Sci. 2000 Apr;153(2):155-159. PubMed PMID: 10717321. doi:10.1016/S0168-9452(99)00266-6
- Talebi R, Rahemi MR, Arefi H, Nourozi M, Bagheri N. in vitro plant regeneration through anther culture of some Iranian local rice (Oryza sativa L.) cultivars. Pak J Biol Sci. 2007 Jun;10(12): 2056-2060. PubMed PMID: 19093446.
- Immonen S, Robinson J. Stress treatments and ficoll for improving green plant regeneration in triticale anther culture. Plant Sci. 2000 Jan;150(1): 77-84. doi:10.1016/S0168-9452(99)00169-7.
- Asif M, Eudes F, Randhawa H, Amundsen E, Spaner D. Induction medium osmolality improves microspore embryogenesis in wheat and triticale. in vitro Cell Dev Biol Plant. 2013 Aug 13;(50): 121-126. doi:10.1007/s11627-013-9545-5.
- Slusarkiewicz-Jarzina A, Ponitka A. The effect of physical medium state on anther culture response in polish cultivated oat (Avena sativa L.). Acta Biologica Cracoviensia Series Botanica. 2007;49(2): 27-31.
- Ferrie AMR, Irmen KI, Beattie AD, Rossnagel BG. Isolated microspore culture of oat (Avena sativa L.) for the production of doubled haploids: effect of pre-culture and post-culture conditions. Plant Cell, Tissue and Organ Culture (PCTOC). 2014 Jan;116(1): 89-96. doi:10.1007/s11240-013-0385-0
- Ambrus H, Darko É, Szabo L, Bakos F, Király Z, et al. in vitro microspore selection in maize anther culture with oxidative-stress stimulators. Protoplasma. 2006 Aug;228(1-3): 87-94. doi:10.1007/s00709-006-0159-1. PubMed PMID: 16937059.
- Ismaili A, Mohammadi PP. Effect of genotype, induction medium, carbohydrate source, and polyethylene glycol on embryogenesis in maize (Zea mays L.) anther culture. Acta Physiologiae Plantarum. 2016 Mar;38: 74. doi:10.1007/s11738-016-2085-y.
- Bouharmont J. Cytology of Microspores and Calli After Anther Culture in Hordeum vulgare. Caryologia. 1977;30(3): 351-360. doi:10.1080/0008711 4.1977.10796708.
- Jacquard C, Asakaviciute R, Hamalian AM, Sangwan RS, Devaux P, et al. Barley anther culture: effects of annual cycle and spike position on microspore embryogenesis and albinism. Plant Cell Rep. 2006 May;25(5):375-381. doi:10.1007/s00299-005-0070-9
- Yldrm M, Bahar B, Genc I, Hatipoglu R, Atis I. Relations among Anther Culture Ability and Some Field Based Characters in Wheat. American-Eurasian J Agric Environ Sci. 2008;4(1):30-33.
- Brazauskas G. The effect of tiller numbers in wheat haploid production. Agronomijas vēstis (Latvian j agronomy). Jelgava: Latvia University of Agriculture. 2011;11:39-42.
- Foroughi-Wehr B, Wenzel G. Andro- and parthenogenesis. In: Hayward MD, Bosemark NO, Romagosa I, Cerezo M, editiors. Plant Breeding: Principles and prospects. Dordrecht: Springer Netherlands;1993. 261-277.doi:10.1007/978-94-011-1524-7_18
- Fakiri M. Obtained in barley (Hordeum vulgare) regeneration by androgenesis and in vitro gynogenesis under salt stress conditions. Application to three Moroccan genotypes. 1995;169.
- Misoo S, Yokota F, Matsubayashi M. Effect of incubation ways of anthers on the pollen mitosis and plantlet formation in tobacco anther culture. Rep Soc Crop Sci Breed Kinki. 1981;26: 44-48
- Hunter CP. The effect of anther orientation on the production of microspore- derived embryoids and plants of Hordeum vulgare cv. Sabarlis. Plant Cell Rep. 1985 Oct;4(5): 267-268. PubMed PMID: 24253985.doi:10.1007/bf00269374
- Shannon PRM, Nicholson A, Dunwell J, Davies DR. Effect of anther orientation on microspore-callus production in barley (Hordeum vulgare L.). Plant Cell Tiss Org Cult. 1985 Sep;4(3):271-280. doi:10.1007/bf00040201
- Mercy ST, Zapata FJ. Position of anthers at plating and its influence on anther callusing in rice. Plant Cell Rep. (6): 318-319. PubMed PMID: 24248469. doi:10.1007/bf00272008
- Perera PIP, Yakandawala DMD, Hocher V, Vendeil JL, Weerakoon LK. Variations among anthers and their orientation during in vitro culture for inducing androgenesis in coconut. In: Nainanayake NP, Everard JM, editors. Export competitiveness through quality improvement, 2nd Symposium on Plantation Crop Research. Colombo, Sri Lanka; 2008. 104-110.
- Hunter CP. Plant regeneration from microspores of barley (Hordeum vulgare L.). [Ph.D. thesis]: Wye College, University of London; 1988.
- Sokal RR, Rohlf FJ. Biometry: the principles and practice of statistics in biological research. San Francisco. WH Freeman Publ, San Fransico; 1969. 776.
- Caredda S, Doncoeur C, Devaux P, Sangwan RS, Clément C. Plastid differentiation during androgenesis in albino and non-albino producing cultivars of barley (Hordeum vulgare L.). Sex Plant Reprod. 2000 Sep;13(2):95-104. doi:10.1007/s004970000043.
- Kasha KJ, Simion E, Oro R, Yao QA, Hu TC, et al. An improved in vitro technique for isolated microspore culture of barley. Euphytica. 2001 Aug;120(3):379-385. doi:10.1023/a:1017564100823.
- Lazaridou TB, Lithourgidis AS, Kotzamanidis ST, Roupakias DG. Anther Culture Response of Barley Genotypes to Cold Pretreatments and Culture Media. Russ J Plant Physiol. 2005 Sep;52(5):696-699. doi:10.1007/s11183-005-0104-8.
- Powell W. The influence of genotype and temperature pre-treatment on anther culture response in barley (Hordeum vulgare L.). Plant Cell Tiss Org Cult. 1988 Jan;12(3):291-297. doi:10.1007/bf00034371.
- Castillo AM, Vallés MP, Cistué L. Comparison of anther and isolated microspore cultures in barley. Effects of culture density and regeneration medium. Euphytica. 2000 May; 113(1):1-8. doi:10.1023/a:1003937530907.
- Gu J, Marshall C. The effect of tiller removal and tiller defoliation on competition between the main shoot and tillers of spring barley. Ann Appl Biol. 1988 Jun; 112(3):597-608. doi:10.1111/j.1744-7348.1988.tb02096.x
- Guo Z, Schnurbusch T. Variation of floret fertility in hexaploid wheat revealed by tiller removal. J Exp Bot. 2015 Sep;66(19):5945-5958. PubMed PMID: 26157170.doi:10.1093/jxb/erv303
- Peng M, Wolyn DJ. Improved callus formation and plant regeneration for shed microspore culture in asparagus (Asparagus officinalis L.). Plant Cell Rep. 1999 Aug;18(11): 954-958. doi:10.1007/s002990050690
- Sunderland N, Huang B, Hills GJ. Disposition of Pollen in situ and its Relevance to Anther/Pollen Culture. J Exp Bot. 1984 Apr 1;35(4):521-530. doi:10.1093/jxb/35.4.521
- Wang M, Van Bergen S, Van Duijn B. Insights into a Key Developmental Switch and Its Importance for Efficient Plant Breeding. Plant Physiol. 2000 Oct;124(2):523-530. PubMed PMID:11027703.doi:10.1104/pp.124.2.523
- Caredda S, Devaux P, Sangwan R, Proult I, Clément C. Plastid ultrastructure and DNA related to albinism in androgenetic embryos of various barley (Hordeum vulgare L.) cultivars. Plant Cell Tiss Org Cult. 2004 Jan;76(1): 35-43.doi:10.1023/a:1025812621775
- Wang M, Hoekstra S, Van Bergen S, Lamers GE, Oppedijk BJ, et al. Apoptosis in developing anthers and the role of ABA in this process during androgenesis in Hordeum vulgare L. Plant Mol Biol. 1999 Feb;39(39): 489-501.
- Dunford R, Walden R. Plastid genome structure and plastid-related transcript levels in albino barley plants derived from anther culture. Curr Genet. 1991 Sep;20(4):339-347. doi:10.1007/bf00318524