Replacement of Soybean Meal with Silkworm Meal In The Diets of White Leghorn Layers and Effects on Performance, Apparent Total Tract Digestibility, Blood Profile and Egg Quality
R Ullah1, S Khan1, NA Khan2, M Mobashar2, A Sultan1, N Ahmad1, J Lohakare3*
1 Department of Poultry Science, The University of Agriculture Peshawar, Peshawar, Pakistan.
2 Department of Animal Nutrition, The University of Agriculture Peshawar, Peshawar, Pakistan.
3 University of Arkansas at Pine Bluff, Pine Bluff, AR, USA.
*Corresponding Author
Jayant Lohakare,
University of Arkansas at Pine Bluff,
Pine Bluff, AR-71601, USA.
Tel: +1-870-575-8540
Fax: +1-870-575-4629
E-mail: lohakarej@uapb.edu
Received: July 27, 2017; Accepted: August 18, 2017; Published: August 21, 2017
Citation: R Ullah, S Khan, NA Khan, M Mobashar, J Lohakare, et al., (2017) Replacement of Soybean Meal with Silkworm Meal In The Diets of White Leghorn Layers and Effects on Performance, Apparent Total Tract Digestibility, Blood Profile and Egg Quality. Int J Vet Health Sci Res. 5(7), 200-207. dx.doi.org/10.19070/2332-2748-1700040
Copyright: J Lohakare© 2017. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
This study elucidated the effects of replacing soybean meal with silkworm meal on production performance, apparent total tract digestibility of nutrients, blood profile, and egg quality traits in white leghorn layers. A total of 150 white leghorn laying birds (52 weeks of age) were randomly divided into fifteen replicate groups (n=10 per replicate), and reared on five experimental diets having three replicates allocated to each treatment group for a period of six weeks. Five iso-nitrogenous and iso-caloric diets (D) were utilized as; 0 (D1), 25 (D2), 50 (D3), 75 (D4) and 100% (D5) as replacement of soybean meal with silkworm meal in commercial layer rations. The weight of the bird, daily feed intake (g/day/bird), hen day production (%), average egg weight (g), and feed conversion ratio did not differ significantly (P>0.05) among the dietary groups. Apparent total tract digestibility of nutrients was not different in the control group compared to treatment groups. Blood profile and egg quality parameters also showed no significant (P>0.05) differences among dietary groups. The egg weight, albumen height, yolk weight and shell thickness were not affected (P>0.05) by dietary treatments. Based on our results, it could be concluded that silkworm meal can be effectively used as an alternative protein sourceto soybean meal without any adverse effects on the layers.
2.Introduction
3.Materials and Methods
3.1 Experimental Birds and Diets
3.2 Performance Parameters
3.3 Egg Quality Analysis
3.4 Nutrients Digestibility
3.5 Blood Profileanalysis
3.6 Statistical Analysis
4.Results
5.Discussion
6.Conclusions
7.Acknowledgments
8.References
Keywords
Soybean Meal; Silkworm Meal; Layer Production; Layer Performance; Nutrient Digestibility; Egg Quality.
Introduction
In the economics of poultry production, feed covers 60-80% of the total production cost [6] and protein cost accounts approximately 15% of feed cost [3, 36] in livestock and poultry farming. Among many alternative protein sources, waste silkworm pupae (SWP) is considered as an important dietary protein source for poultry after proper processing at a reasonable cost. Utilizing SWP as a livestock or poultry feed would partly meet the protein feed deficiency. SWP generates vast resources of nutrients for livestock and poultry. SWP is one of the unconventional good ranked protein (65-75% CP) and lipid [5] feeds which is a waste product of the silk industry and is obtainable four times in a year. Insects can convert waste biomass into high value food and feed resource. A recent study has shown that it is technically feasible to produce insects on a large scale and to use them as an alternative sustainable protein rich ingredient in poultry ration, and the benefit is particularly high, if they are reared on substrates of biowaste and organic side streams [46]. Insects have high nutritive value in proteins as well as in fats, vitamins, and minerals [8].
The growth and development cycle of the silkworm caterpillar starts when it hatches from a tiny black egg laid by the adult moth. After hatching process, caterpillar feeds on mulberry and shear butter leaves constantly and attains full size of 7.5-10cm [8], within 4-6 weeks. When the silkworm enters the pupa phase, it builds a protective cocoon made of raw silk. At the end, the pupa releases an enzyme that creates a hole in the cocoon and the moth emerges. In silk production, the pupae are killed by boiling, drying or soaking in NaOH before they produce the enzyme [13]. The spent pupae are produced in large quantities and are a major by-product of silk-worm production [13]. For 1 kg of raw silk, 8 kg of wet pupae (2 kg of dry pupae) are produced [34]. The silkworm caterpillar meal has high potential as a protein ingredient in poultry ration. The silkworm is the caterpillar of moth butterfly known as Bombyx mori whose cocoon is used to make silk [1]. Conversely, limited work has been done on the use of silkworm meal in feed.
Solomon and Yusuf [39] explored the utilization of silkworm meal as feed ingredient due to its high nutritional value of protein as a substitute to reduce feed cost. It is a waste material of silk industry and contain high quality protein (49.4-60% CP), lipids (14.5-30.3 % crude fat), and amino acid profile is as close to fish meal. The lipid composition of Bombyx mori includes palmitic, oleic, palmitoleic, linoleic, stearic (24.6% of lipid), myristic, linolenic, lauric (14% of lipid) and arachidic acids that are present in a segment of neutral lipids. One third of the total fatty acids constituted of unsaturated fatty acids. Cholesterol are found along with trace amount of campesterol in an oily fraction of sterols. The confirmation on mineral and vitamin content of silkworm pupa are limited [23, 40]. The objective of this study was to investigate the effects of replacing soybean meal with silkworm meal in basal diets on performance, nutrients digestibility, blood profile, and egg quality attributes of laying birds.
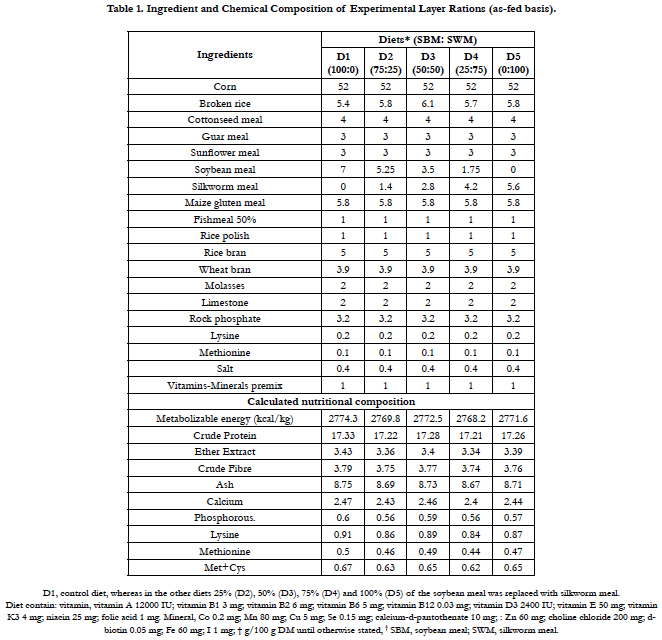
The present study was performed at poultry farm complex, faculty of animal husbandry and veterinary sciences, the University of Agriculture, Peshawar, Pakistan. A total of 150 white leghorn layers (average weight = 1,371 kg) at 52 weeks of age were placed in 15 identical indoor floor pens (150 cm x 120 cm x 90 cm) with 10 birds/pen (saw dust used as bedding material). Five isocaloric and iso-nitrogenous layer rations having D1 (0), D2 (25), D3 (50), D4 (75), D5 (100) percent replacement of soybean meal with silkworm pupae meal were prepared, respectively (Table 1) according to the NRC recommendations [31]. Five treatments were assigned at random to pens, and each treatment had 3 replicate pens. The birds were kept in partial environmentally controlled house with temperature range (23-27°C) and 17 hours lighting. The five rations were formulated according to nutrients requirements of the layers in which soybean meal was step-wise (0, 25, 50, 75, 100%) replaced with silkworm pupae meal in mash form at Khyber poultry feed mill and then pelleted by mini pellet machine in poultry farm complex. Separate feeder and drinker were installed in each pen for provision of water and feed. All the birds were provided feed and water for ad libitum consumption and the birds were housed and handled according to the rules and regulations of ethical committee of the institution. Vaccination to all the birds was carried out according to the locally adopted vaccination schedule. The trial lasted for 42 days.
Amino acid profile of silkworm pupae meal was determined by the method of Cserhati and Forgacs [12]. Briefly, finely ground samples were hydrolyzed by adding 4.83 g barium hydroxide and 5 ml of boiling water to 500 mg of sample. The mixture was evacuated and then heated at 120°C for 8 h. After hydrolysis, the pH was adjusted to three with HCL and diluted to 25 ml with HPLC grade distilled water. One milliliter of sample was vacuum dried using flash evaporator and finally dissolved in citrate buffer (0.1 M; pH 2.2). Acid hydrolysis was carried out with 6N HCL at 110°C to 18-22 h in evacuated and sealed tubes. The hydrolysate was filtered and diluted to 250 ml. One milliliter of the sample was vacuum evaporated at 40°C until dryness was achieved. The content was dissolved in citrate buffer (0.1 M, pH 2.2). 20:1 of this derivatives were injected directly into the HPLC. Detection was accomplished using Shimadzu HPLC detector LC-10A with variable wavelength monitor set at 350-450 nm. Resolution of amino acid derivatives was routinely accomplished using a binary gradient system.
Feed intake, egg weight, hen day production (%) and mortality were noted on daily basis. Feed conversion ratio was calculated as grams of feed intake per dozen of egg produced. All production variables were determined for each replicate of treatment groups.
The egg quality was assessed at 58 weeks of age. A total of thirty eggs (2 eggs per replicate) were collected and weighed for determination of egg quality. Individually, each egg was broken out in plate and kept for 5 minutes. The height of yolk and albumen, the diameter of yolk was recorded by calipers. Yolks were detached from albumen by hand, and were weighed. The egg shell weight, yolk and albumen were divided by the entire egg weight and multiplied by 100 to calculate percentage. Eggshell thickness (without inner and outer shell membranes) was measured at the middle part of egg shell by screw gauge. Haugh units (HU) were calculated from the records of albumen height (H) and egg weight (W) using the following formula:
HU = 100 log 10 (H − 1.7W 0.37 + 7.56), according to Haugh [19].
Nutrients digestibility was determined on day 42 of the experimental trial. For this purpose, 2 birds were chosen randomly from each replicate that represented the penand euthanized with pentobarbitone injection (I/V). Immediately after euthanization each bird was dissected and adigesta sample was collected from the colo-rectal region (just after caeca) to measure the apparent digestibility [42]. For proximate composition, the collected samples were pooled per replicate and freeze-dried, ground with the help of 1-mm mesh screen, and analysedin triplicate. Apparent total tract digestibility was assessed with the help of 0.2% chromic oxide (Cr2O3), as an indigestible marker in the feed. To calculate the nutrients digestibility the following formula was used:
Apparent digestibility = 100 - [100 × (Nutreint (%) in digesta × Cr (%) in diet) / (Nutrient (%) in diet × Cr (%) digesta)]
Where Cr = chromic oxide
At the end of the experiment (42 days of feeding), feed was not withdrawn from the feeder before blood was collected. A total of 30 blood samples (2 birds representative from each replicate, 6 birds per treatment) were collected from the wing vein of the birds using disposable syringe in the tube containing ethylene diamine tetra acetic acid (EDTA) as an anticoagulant. The packed cell volume (PCV), red blood cell (RBC), white blood cell (WBC) and the hemoglobin (Hb) concentrations were measured using the Wintrobes Microhematocrit, improved Neubauer haemocytometer and Cyanomethaemoglobin methods, respectively [10], while mean corpuscular hemoglobin (MCH) levels were calculated according to Bush [7]. Similarly, blood samples collected without anticoagulant were used for the determination of serum biochemical constituents’ viz. albumin, globulin, total protein, glucose, using commercially available analytical kits. Serum was collected in tubes and stored at −20°C until further analysis.
The data generated was statistically analyzed using complete randomized design and general linear model (GLM) [41] using SAS software package. The model comprised of different levels of replacement. Replicate pen was the experimental unit for all analysis. Duncan’s Multiple Range Test [14] was used to compare means. The level of significance was declared at P<0.05.
Results
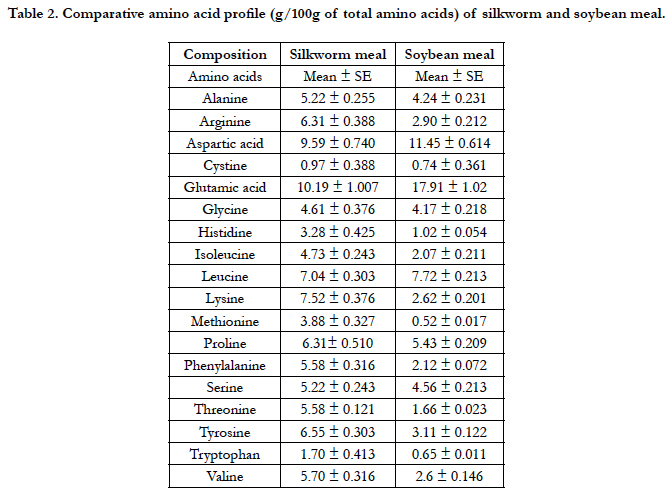
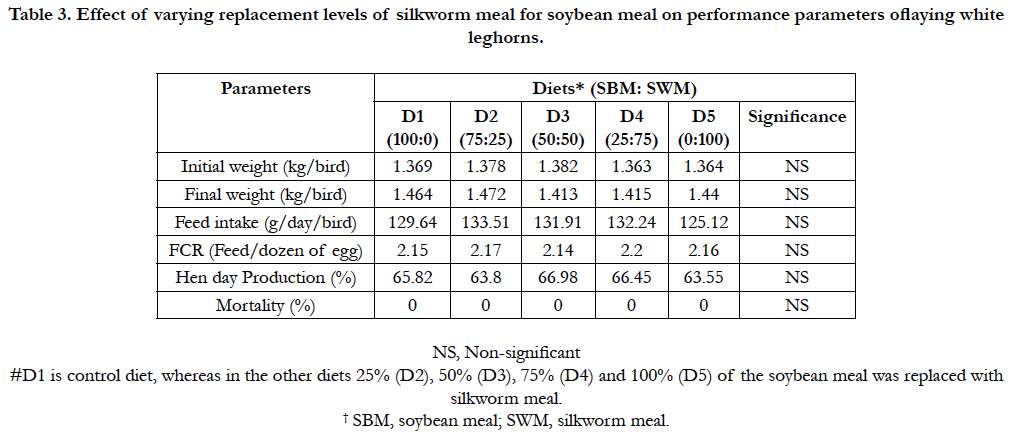
The amino acids profile showed that almost all essential amino acids were observed in silkworm pupae meal used in the present study (Table 2). The body weight, daily feed intake(g/day/ bird), hen day production (%), average egg weight (g) and feed conversion ratio FCR (g feed/dozen egg production) did not differ significantly (P>0.05) among various dietary groups (Table 3). Though there were no significant differences (P>0.05) in the FCR and other performance parameters of the birds across dietary treatments,however, D3 (50%) replacement had a better results in terms of hen-day egg production (66.98%) when compared to other replacement levels and control. Feed conversion expressed as feed required in g/dozen of eggs was also not affected by adding silk worm pupae meal (SWM) in the diet. No mortality was recorded during this feeding trial (Table 3). The replacement of soybean meal with silkworm meal in layer diets did not affect adversely layer performance when compared with control group.
Table 3. Effect of varying replacement levels of silkworm meal for soybean meal on performance parameters oflaying white leghorns.
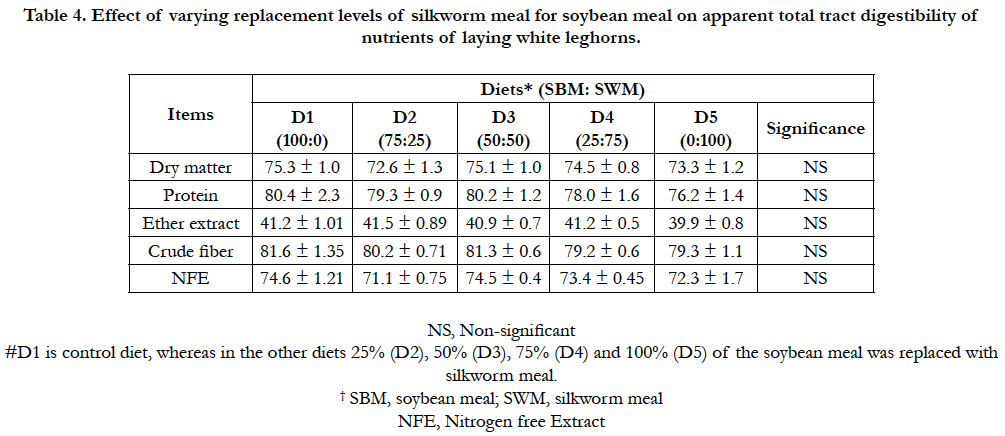
Dry matter digestibility in white leghorn birds was non- significant (P>0.05) in the control group compared to groups D2, D5 and D3 (Table 4). In case of apparent total tract digestibility, no differences in digestibility values were observed among dietary groups for protein, ether extract, crude fiber and nitrogen free extracts.
Silkworm meal replacement in the layer ration had no significant (P>0.05) adverse effect on hematological parameters of laying birds (Table 5). Blood sugar, total protein, albumin, red blood cells, white blood cells, packed cell volume, hemoglobin and mean corpuscular hemoglobin was not different (P>0.05) among various dietary groups (Table 5). The results of egg quality parameters of collected eggs showed no significant differences among dietary groups (Table 6). The egg weight, albumen height, yolk weight and shell thickness were also not affected (P>0.05) with dietary treatments.
Table 4. Effect of varying replacement levels of silkworm meal for soybean meal on apparent total tract digestibility of nutrients of laying white leghorns.
Table 5: Effect of varying replacement levels of silkworm meal for soybean meal on hematological parameters of laying white leghorns.
Table 6. Effect of varying replacement levels of silkworm meal for soybean meal on egg quality parameters of laying white leghorns.
Discussion
Silkworm meal has been used as an alternate substitute for fish meal and soybean meal in the diet as a protein source upto 75% without any adverse effect on growth performance of fish was earlier reported [43]. Limited literature is available on replacement of silkworm in diet of laying hens. However, few studies on fish has been conducted using silkworm meal [30].
Silkworm larvae and pupae were analysed nutritionally and it contained high amounts of protein, (53-54 percent) and very low content of hydrocyanic acid (6.21-50.47 mg/kgDM) [38]. These properties favour to use silkworm as animal feed, aquarium fish food and also as human food. Its high protein content makes it a suitable candidate to be developed as a sustainable high protein food source [37].
The amino acid composition (g/100 g of total amino acids) of the silkworm meal indicates that amino acids lysine (7.52 vs. 6.11) and methionine (3.88 vs. 1.40) were higher in silkworm meal than soybean meal [45]. Solomon and Yusufu [39] and Ji et al., [22] stated that silkworm meal amino acid profile is almost similar but superior to soybean meal for better performance. Moreover, our study showed that silkworm meal had high proportion (51.3%) of essential amino acids (Table 2). Specifically, the silkworm meal contained higher contents (g/100 g of total amino acids) of the essential amino acids, namely, lysine (7.52), methionine + cystine (4.85), arginine (6.31), phenylalanine (5.58) and valine (5.70). However, the ratio of leucine and isoleucine (7.04: 4.73) was lower than that of soybean meal (7.5: 4.6). Leucine and isoleucine ratio is often connected in amino acid antagonism, therefore, it is necessary to include it in the formulation of poultry ration [11]. Moreover, the silkworm meal was limiting in tryptophan. Rao [35] also reported that silkworm meal is deficient in tryptophan and suggested that it would be essential to provide tryptophan along with the feed that comprises silkworm meal. The amino acid contents of the silkworm meal reported in the present study is within the range of literature values [22, 28, 45]. Unlike other animal origin feed ingredients, the fat content of silkworm comprises a proportion of polyunsaturated fatty acids, particularly linolenic acid (18:3n-3), with reported standards ranges from 11 to 45% of the total fatty acids [35, 44]. Venkatesh et al., [47] reported that silkworm containing diet boosts up the growth of cat fish parallel to groundnut cake and meat meal. The silkworm also indicated higher gross energy (4530 ± 81.0 kcal/ kg) than soybean meal (2250 kcal/kg) [31]. The results of present study regarding amino acids profile inspired the replacement of soybean meal with silkworm meal in poultry ration.
Summarising available literature data, Makkar et al., [28] reported that excluding silkworm meal, other insects meal are lacking in lysine and methionine, and addition of silkworm meal in the ration can improve both the performance of the animals and can replace both fish meal and soybean in the diet. The performance of laying hens with the replacement of soybean meal with silkworm meal (up to 50%) could be related to its higher content of essential amino acids, minerals and energy [25]. Likewise, when silkworm and mulberry plant leaves were compared, the nutrient digestibility were better for silkworm meal based diet as compared to mulberry plants leaves [43]. Another study on fish were conducted on substitution of silkworm in the diet beyond 60% resulted in no significant effect on the growth potential.However, Nandeesha et al., [29] found that supplemental silkworm meal up to (30%) had improved the growth potential of common carp. Begun and coworkers [4] suggested that silkworm meal upto 50% in diet have better growth rate, feed conversion ratio and protein efficiency ratio in rohu fish in comparison with fish meal diet. Fagoonee [16] argued that the silkworm meal contain growth prompting factors such as ecdysteroid activity (a hormone involved in protein synthesis and tissue formation), though this has not been confirmed yet. The reasons for comparable results in replacement diets to control diet were the optimal supply of essential amino acid profile in silkworm meal [24]. The reasons for best FCR with the 50% replacement level may be due to a more optimal supply of essential amino acid (predominantly tryptophan), nutrient digestibility and ahigher accumulation rate of protein [24]. Although no direct comparisons could be made due to lack of literature data, some researchers [15, 16, 26] reported that replacing 50% of the fish meal with silkworm meal in broiler ration supported optimum growth performance and improved economic return. Moreover, Ijaiya and Eko [21] reported a higher weight gain with 75% substitute of silkworm on fish meal. However, feed intake and production performance reduced with 100% substitution of silkworm meal on soybean meal [15, 21] which is partly related with the findings of our study. Limited literature is available on the substitution of silkworm meal in the layer diets.
The feed quality affects the blood profile. Hematological studies is an important tool used as a real and sensitive index for monitoring physiological and pathological changes [49]. The assay of blood indices has endorsed to be a valuable approach for analyzing the health status of farmed birds/animals as these indices provide consistent information on metabolic disorders, and stress status in an experimental setting [2]. As it is evident, the hematological parameters remain unchanged; so it indicates that replacement of soybean meal with silkworm pupae meal had no adverse effects on the physiological and performance parameters (Table 5). The diet having various level up to 100% silkworm meal enhanced growth, hematological and physiological performance of cat fish as reported by Olaniyi and Babasanmi [32]. The values noted in treatments groups suggest the nutritional sufficiency of all diets with no malnutrition status [9]. The RBC count showed non-significant differences (P<0.05) among treatments and the values were in normal range as reported by Bhuiyan [6]. These observations found related to health status of the birds and no birds died in our study as well. The values of red blood cells increases in association with high quality protein for disease free animal was reported earlier [18]. The white blood cell count (WBC) were in the normal range of 5 to 13 x 103/mm3 as reported for animals [20].
Limited work has been done on using silkworm pupae meal as a feed ingredient in layer production. The silkworm pupae meal has protein of high nutritional value. Silkworm pupae can serve as a supplemental protein in animal feed. Egg weight was nonsignificantly differed in all the treatment groups that coincide with the findings of Xiaoyun et al., [48]. In the present study the egg quality parameters did not differ significantly (P>0.05). Olteanu et al., [33] reported that the supplemental use of mulberry leaves (feed for silkworm production) up to 6% in layer diets did not disturb adversely layer performance and egg quality. The egg quality parameters such as egg white pH, egg yolk pH and the Haugh unitwas notsignificantly altered in the trial groups related to the control group was also reported by Lokaewmanee et al., [27] similar to our results. There is a dearth of information on the effects of silkworm meal on egg quality parameters, hence, more studies in future are required to study its effect on egg quality and palatability.
Conclusions
Silkworm meal is a potential alternative of soybean meal without adverse effect on layers performance, nutrient digestibility, blood profile and egg quality. Replacement of soybean meal with silkworm meal at 50% replacement in the ration achieved better results in the laying hens. It is suggested to conduct more studies on silkworm meal as feed ingredient.
Acknowledgments
The authors are much thankful to the Pakistan Science Foundation (PSF) for providing funding for this research study under the project PSF/NLSP/KP-AU (293).
References
- Become a member of enchanted learning. Silkworm Moth Butterfly and Moth Printout 2005.
- Bahmani M, KazemiR, Donskaya P (2001) A comparative study of some haematological features in young reared sturgeons (Acipenserpersicus and Husohuso). Fish physiology and Biochemistry, 24: 135-140.
- Banerjee GC (1992) Poultry (Third edition). Oxford and IBH publishing Co. Pvt. Ltd. New Delhi, 168-172.
- Begun NN, Chakarabarthy SC, Zahar M, Abdul MM, Gupta MV (1994) Replacement of fish meal by low cost animal protein as quality fish feed ingredients for the Indian major carp, Labeorohita fingerlings. Journal of food and Agricultural Science, 64: 191-197.
- Bhuiyan AK, Begum, NN, Begum M, Hoq M.E (1989) Survey of potential fish feed ingredients of Bangladesh on the basis of their availability and biochemical composition. Final Report. FRI Research Progress Report 1. Freshwater station, Fri, 70.
- Bhuiyan MZ (1998) Complete replacement of fish meal by full fat soybean and supplementation of lysine and methionine to broilers. M. S. Thesis, Dept. of Poultry Science, Bangladesh Agricultural University, Mymensingh.
- Bush BM (1991) Interpretation of Laboratory Results for Small Animal Clinicians. Blackwell Scientific Publications. London, UK, 32-67.
- Chapman R.F (1998) The insects: structure and function FourthEdition. Cambridge University Press.
- Church JP, Judd JT, Yomg, CW, Kebay TL, Kim WW (1984) Relationship among dietary constituents and specific serum clinical components of subjects eating self-selecting diets. Amer Journal Clinical Nutrition, 40: 1338-1344.
- Coles EH (1986) Veterinary Clinical Pathology. Fourth Edition WB Saunders, Philadelphia, USA.
- Crawshaw R (1994) A Review of nutritional qualities for pigs, poultry and ruminant animals. National Renderers Association, London, UK.1-6.
- Cserhati T, Forgacs E (1999) Chromatography in Food Science and Technology. Technomic Publishing, Lancaster, USA. 158-298.
- Datta SC, Datta R (2007) Intercropping amaranth with mulberry for managing rootknot nematodes and improving sericulture. Sericologia 47: 297- 302.
- Duncan DB (1955) Multiple range and multiple F-test. Biometrics, 11: 1-42.
- Dutta AS, Dutta Kumari S (2012) Growth of poultry chicks fed on formulated feed containing silk worm pupae meal as protein supplement and commercial diet. J. Anim. Feed Res. 3: 303-307.
- Fagoonee (1983) Possible growth factors for chickens in Silkworm pupae meal. British. Poultry Science, 24: 295-300.
- Fudge CS (1999) Laboratory Medicine: Avian and Exotic Pets. WB Saunders, Philadelphia, USA.
- Hackbath HK, BuronSchimansley G (1983) Strain difference in inbred rats: Influence of strain and diet on haematological traits. Lab Anim, 17: 7–12.
- Haugh RR (1937) The Haugh unit for measuring egg quality. U. S. Egg Poult. Mag. 43: 552-555.
- Hillyer EV (1994) Pet Rabbits. The Veterinary Clinics of North America, Small Animal Practice. 24 (1): 25–65.
- Ijaiya AT, Eko EO (2009) Effect of replacing dietary fishmeal with silkworm (Anapheefracta) caterpillar meal on growth digestibility and economics production of starter broiler chicken. Pakistan J. Nut. 8: 845–849.
- Ji H, Jian-Lu Z, Ji-Qin H, Xiao-Fei C, Chao LF (2015) Effect of replacement of dietary fish meal with silkworm pupae meal on growth performance, body composition, intestinal protease activity and health status in juvenile Jian carp (Cyprinuscarpio var. Jian) Aquacul Res. 46: 1209–1221.
- Joachim WH, Pascual FP (2000) Handbook on Ingredients for Aquaculture Feeds, Kluwer Academic Publishers, Dordrecht.
- Khan S, Khan RU, Sultan A, Khan M,HayatSU, Shahid MS (2016) Evaluating the suitability of maggot meal as a partial substitute of soybean on the productive traits, digestibility indices and organoleptic properties of broiler meat. Journal of Animal. Physiology and Animal Nutrition. 100(4): 649-56.
- Khatun R, Howlider MAR, Rahman MM, Hasanuzzaman M (2003) Replacement of fish meal by silkworm pupae in broiler diets. Pakistan Journal of Biological Sciences. 6: 955-958.
- Konwar P, Konwar BK, Ahmed HF, Nath NC, Ghosh MK (2008) Effect of feeding silkworm pupae meal with enzyme supplementation on growth performance of broilers. Indian Veterinary Journal. 85: 47-49.
- Lokaewmanee K, Mompanuon S. Khumpeerawat P, Yamauchi K (2009) Effects of dietary mulberry leaves (Morus alba L.) on egg yolk color. Journal of Poultry Science. 46: 112-115.
- Makkar G. Harinder PS, Tranb B, Valerie Heuze P, Ankers A (2014) Review. State-of-the-art on use of insects as animal feed. Animal Feed Science and Technology. 197: 1–33.
- Nandeesha MC, Gangadhara B, Maniseery JK (1990) Silk worm pupae oil as an additional energy source in the diet of common carp. Cyprinuscarpio. Asian Fisheries Science, 12: 207-215.
- NandeeshaMC, Gangadhara B, Varghese TJ, Keshavanath P (2000) Growth response and flesh quality of common carp, Cyprinuscarpio fed with high levels of non-defatted silkworm pupae. Asian Fish Sci., 13: 235-242.
- NRC (1994) Nutrient requirements of poultry. Ninth Revised Edition, National Academy Press, Washington D.C.
- Olaniyi CO, Babasanmi GO (2013) Performance characteristics of African cat fish (Clarias gariepinus) fed varying inclusion levels of silkworm pupae (Anapheinfracta). Bangladesh. J Anim Sci. 42(1): 76-80.
- Olteanu M, Panaite T, Ciurescu G, Criste RD (2012) Effect of dietary mulberry leaves on performance parameters and nutrient digestibility of laying hens. Indian J Anim Sci. 82(8): 914–917.
- Patil SR, et al., (2013) Utilization of silkworm litter and pupal waste-an ecofriendly approach for mass production of Bacillus thuringiensis. Bio resource Technology. 131: 545-547.
- Rao PU (1994) Chemical composition and nutritional evaluation of spent silk worm pupae. Journal of Agriculture and Food Chemistry. 42: 2201-2203.
- Singh RA (1990) Poultry Production, Third edition. Kalyany publishers, New Delhi, Ludhiana.
- Sirimungkararat S, Atthathom T, Saksirirat W (2002) Development of eri silkworm rearing technique using cassava leaf as food plant and its textile production. Proceedings of the XIXth Congress of the International Sericultural Commission, September Queen Sirikit National Convention Center. Bangkok, Thailand.
- Sirimungkararat S, Wachirapakorn C, Waikakul Y, Wapugpetr B (2004) The preliminary detection for hydrocyanic acid content in eri silkworm, Philosamiaricini B. fed with cassava leaf. Presented at Annual Agricultural Seminar, January Faculty of Agriculture, KhonKaen University, KhonKaen. (Abstract in English.)
- Solomon SG,Yusufu II (2005) The amino acid profile, proximate and mineral composition of silkworm caterpillar (Anapheinfracta) meal as possible alternative to fish meal in the diets of cultural fish species. Department of Fisheries and Aquaculture. University of Agriculture, Makurdi Nigeria, 10-15.
- Sreekantuswamy HS, Siddalingaiah KS (1981) Bombyx mori L.Fette, Seifen, Ansrichimittel. 83(3): 97-99.
- Steel RGD, Torrie JH, Dickey DA (1997) Principles and procedures of statistics; A biometrical approach. 3. Boston: McGraw-Hill.
- Sultan A, Ullah R, Khan S, Khan MT, Khan H, Zaman IU (2014) Nutrient digestibility values and apparent metabolizable energy of corn, wheat and sorghum by pheasants (Phasianuscolchicus). Pak Vet J. 34(4): 479-483.
- Swamy HVV, Devaraj KV (1994) Nutrient utilization by common carp (Cyprinuscarpio Linn) fed protein from leaf meal and silkworm pupae meal based diets. Indian J. Anim. Nutri. 11(2): 67-71.
- Usub T, Lertsatitthanakorn C, Poomsa-ad N, Wiset L, Yang L, Siriamornpun S (2008) Experimental performance of a solar tunnel dryer for drying silkworm pupae. Biosystems engineering. 101: 209-216.
- Valerie H, Tran G, Giger-Reverdin S, Lebas F (2015) Silkworm pupae meal. Feedipedia, a programme by INRA, CIRAD, AFZ and FAO.
- Veldkamp T, van Duinkerken G, van Huis A, Lakemond CMM, Ottevanger E et al (2012) Insects as a sustainable feed ingredient in pig and poultry diets - a feasibility study. Rapport 638-Wageningen Livestock Research.
- Venkatesh B, Mukherji AP, Mukhopadhyay PK, Dehadrai PV (1986) Growth and metabolism of the cat fish Clariasbatrachus (Linn,) fed with different experimental diets. Proc, Indian Academy of Science, 95(4): 457-462.
- Virk RS, Lodhi GN, Ichhponani JS (1980) Nutritive value of untreated, water and acid treated De-oiled silkworm pupae meal for broiler. Indian Journal of Poultry Science. 15: 155-161.
- Xiaoyun Z, Mingyun L, Khalid A, Weinmin W (2009) Comparative of haematology and serum biochemistry of cultured and wild Dojo toachMisgurnusangwillicadatus. Fish physiology and Biochemistry. 35: 435-441.