Effect of Enrichment of in vitro Fertilization Medium with Cysteine on Fertilization and Embryo Development in Buffaloes
Abu-El Naga EM1, El-Sheikh Ali H2, Balboula AZ2, Ramdan Badr M3, Abd El-Moneim M2, Said Hussein M4, Abd El-Raouf OH2,5, Zaabel SM2*
1 Faculty of Veterinary Medicine, Theriogenology Department, Aswan University, Egypt.
2 Faculty of Veterinary Medicine, Theriogenology Department, Mansoura University, Egypt.
3 Artificial Insemination and Embryo Transfer Department, Animal Reproduction Research Institute, Al Haram, Giza, Egypt.
4 Faculty of Veterinary Medicine, Department of Theriogenology, Veterinary Teaching Hospital, Mansoura University, Egypt.
5 Faculty of Science, Department of Biology, Taif University, Taif, Saudi Arabia.
*Corresponding Author
Samy Moawad Zaabel,
Faculty of Veterinary Medicine,
Theriogenology Department,
Mansoura University, Egypt.
E-mail: zaabelsamy@gmail.com
Received: June 21, 2017; Accepted: July 12, 2017; Published: July 13, 2017
Citation: Zaabel SM, Abu-El Naga EM, El-Sheikh Ali H, Balboula AZ, Ramdan Badr M, et al., (2017) Effect of Enrichment of in vitro Fertilization Medium with Cysteine on Fertilization and Embryo Development in Buffaloes. Int J Vet Health Sci Res. 5(6), 196-199. dx.doi.org/10.19070/2332-2748-1700039
Copyright: Zaabel SM© 2017. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
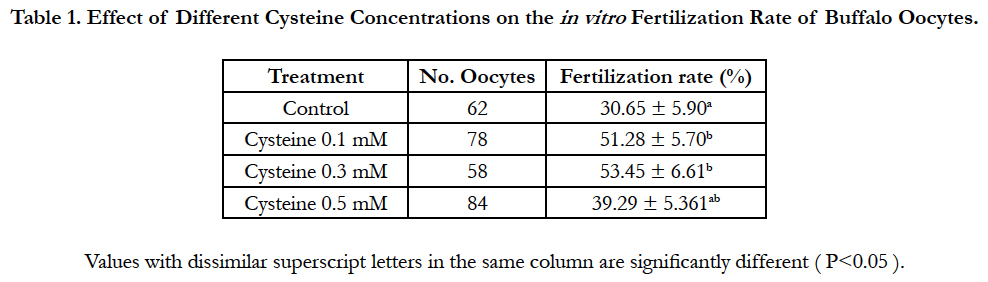
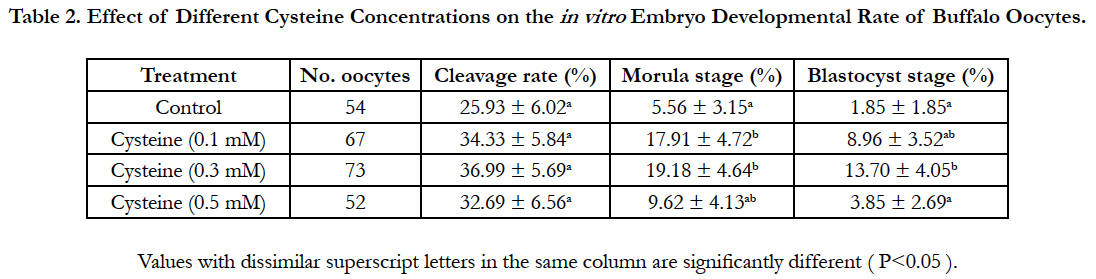
The current study aimed to elucidate the effect of adding various concentrations of cysteine to the in vitro fertilization culture medium on the fertilization rate and following embryonic development of buffalo oocytes. in vitro matured oocytes were fertilized using different concentrations of cysteine (0.5, 1.3 mM) in the presence of 5.0 mM caffeine. After in vitro fertilization, fertilization rate, cleavage rate and developmental rates till the blastocyst stage have been evaluated. The present results revealed that supplementation of the fertilization media with 0.3 Mm cysteine enhanced significantly (P<0.05) the fertilization rate and the development rates till the morula and blastocyst stages (53.45% ± 6.61%, 19.18% ± 4.64% and 13.70% ± 4.05%, respectively) compared to those of the control groups (30.65% ± 5.90%, 5.56% ± 3.15% and 1.85% ± 1.85 %, respectively). The current results demonstrated that addition of 0.3 mM cysteine to a fertilization medium had positively improved in vitro fertilization rates and supported embryonic progress till the blastocyst stage of buffalo oocytes.
2.Materials and Methods
2.1 Cumulus Oocyte Complexes Collection
2.2 in vitro Maturation of Oocytes
2.3 Sperm Preparation and in vitro Fertilization
2.4 in vitro Culture
2.5 Experimental Design
2.6 Statistical Analysis
3.Results
4.Discussion
5.Conclusions
6.References
Introduction
Animal production industry depends on the dissemination of the genetic properties of superior animals. Therefore, in vitro embryo production emerged as a promising strategy to produce embryos with high genetic properties. However, the overall success to improve the in vitro developmental rate of buffalo preimplantation embryos remained very low. Therefore, it is essential to find the suitable approach to improve the developmental competence of bubaline preimplantation embryos.
in vitro embryo development is passively compromised by the oxidative stress induced by suboptimal culture conditions. Oxidative stress perturbs the functional integrity of the cells through the release of reactive oxygen species (ROS) [1]. For example, oxidative stress-induced ROS elevation has the potential to react with the polyunsaturated fatty acids of lipid membranes and induces lipid peroxidation. Moreover, ROS oxidize free amino acid residue side chains resulting in protein aggregation with subsequent cell damage. Furthermore, ROS elevation was identified as one of the main pathways that induce DNA double strand breaks, the leading cause of cancer and apoptosis [2]. However, the cells respond to the oxidative stress by triggering the antioxidant machinery. Accordingly, catalase and superoxide dismutase, as well as the “thiol” components were elevated to act as metabolic lids to neutralize the harmful effect of the ROS [3].
Cysteine is the precursor of intracellular glutathione (GSH) biosynthesis. Therefore, cysteine enrichment has the ability to increase the GSH level, another potent antioxidant enzyme. Previous work revealed that the supplementation of cysteine to the bovine in vitro maturation medium improved the embryonic progress and quality [4] and [5]. However, the effect of cysteine supplementation during in vitro fertilization, the step that is critical for the subsequent embryonic development, has not been studied. This study aimed to elucidate the effect of cysteine supplementation to the in vitro fertilization medium on the fertilization rate and the subsequent developmental competence of buffalo preimplantation embryos.
Ovaries were collected within thirty minutes after slaughter from mature Egyptian buffaloes in a local abattoir and transported within two hours to the laboratory in a sealed flask inclosing sterile PBS (pH 7.35) provided by with 100 IU penicillin G, as well as, 100 μg/mL of streptomycin in 30° [6]. Cumulus oocyte complexes (COCs) have been aspirated from medium size follicles (2-8 mm) using 18-gauge needle attached to a ten mL sterile syringe. Only COCs having multi-layers of cumulus cells, intact zona pellucida and homogeneous cytoplasm have been selected for further processing [7].
The selected COCs have been washed 3 times with Dulbecco’s PBS. Maturation was performed using 10 to 15 COCs in 10×35 mm Petri dishes with 100 μL drops of modified synthetic oviduct fluid (SOF) media comprising Earle's salts (GibcoTM, Ref. 31- 10-035, Invitrogen Corporation, USA) provided with 0.0225 mg/ mL sodium pyruvate, 0.01IU r-hFSH/mL, 0.05mg/mL of sLH (Lutrophin-V, Bioniche Animal Health, Canada) and 10% of fetal calf serum (FCS). The selected COCs have been washed twice in the maturation medium and then incubated in 200 μL drops of SOF covered with sterile mineral oil (Sigma) and incubated in the CO2 incubator in 39° and 5% CO2 in air, and saturated humidity for 24 h. After 24 h, oocytes were examined for cumulus expansion under stereomicroscope. Cumulus cells were then removed mechanically from some COCs using 0.1 % hyaluronidase, and the denuded oocytes have been inspected for the formation of the first polar body extrusion (PBE). Both cumulus expansion and PBE were evaluated to assess the maturation rate [8].
Three straws of frozen buffalo semen have been thawed in a water bath at 38° for 30 seconds. After thawing, swim up technique was used to isolate the most motile spermatozoa have been separated within sperm-TALP medium containing 6 mg/mL BSA, for 30 min [9]. The uppermost layer of the medium in closing the motile spermatozoa was collected. The selected spermatozoa were washed two times by centrifugation (x500g/10 min). The sperm pellet has been reconstituted with the fertilization TALP (F-TALP) medium containing 5 mM caffeine. The prepared sperm was incubated in the CO2 incubator in 38.5°, 5% CO2 for two hours before further use. The matured COCs were washed with F-TALP medium and the prepared sperm was added into the droplets containing matured oocytes to achieve a final concentration of 2×106 sperm cells/mL. Gametes have been coincubated in the fertilization drops under sterile mineral oil for 18 h. At the end of gametes co-incubation, some of inseminated oocytes have been freed of the attached cumulus cells, fixed in acetic acid-ethanol (1:3), stained with 1% aceto-orcein stain and examined under phase-contrast microscope (×400) for assessing the in vitro fertilization rate according to Totey SM, et al., [6].
Putative zygotes were denuded from cumulus cells and the extra spermatozoa by gentle pipetting, and washed three times in modified SOF media. Immediately, 20 to 25 zygotes were randomly distributed in 100 μL drops of modified SOF medium according to [10], with 5% FCS, 20 μL/mL of essential amino acids and 10 μl/mL of non-essential amino acids, under mineral oil in 10×35 mm Petri dishes for 7 days at 38.5° in an atmosphere of 5% CO2 in air with maximum humidity. Developmental competence was assessed by evaluating cleavage, morula and blastocyst rates on days 2, 5 and 7, respectively, according to Totey SM, et al., [6].
Matured COCs were in vitro fertilized in the absence (control) or the presence of different concentrations of cysteine (0.1 mM, 0.3 mM or 0.5 mM) followed by assessing the fertilization rate. The putative zygotes were then in vitro cultured for 7 days. Cleavage, morula and blastocyst rates were evaluated on days 2, 5 and 7, respectively.
Costat Computer Program, Version 3.03 copyright (1986) Cottort Software, and were compared using ANOVA followed by LSD as a post hoc test. Variant were considered significant when P<0.05. Inserting Graphics.
Results
Data regarding the effect of replenishing of the in vitro fertilization medium with different concentrations of cysteine on the fertilization rate was shown in Table 1. Addition of 0.3 mM cysteine to the fertilization medium significantly improved (P<0.05) the fertilization rate (53.45% ± 6.61%) when compared to that of control group (30.65% ± 5.90%). Importantly, although cleavage rate did not vary significantly, supplementation of the fertilization medium with 0.3 mM cysteine increased significantly (P<0.05) morula and blastocyst rates (19.18% ± 4.64% and 13.70% ± 4.05%, respectively) in comparison to those of the control group (5.56% ± 3.15% and 1.85% ± 1.85 %, respectively; Table 2).
Table 1. Effect of Different Cysteine Concentrations on the in vitro Fertilization Rate of Buffalo Oocytes.
Table 2. Effect of Different Cysteine Concentrations on the in vitro Embryo Developmental Rate of Buffalo Oocytes.
Discussion
Elevation of ROS during in vitro fertilization perturbed the fertilization rate and subsequent embryonic development [11]. Oxidative damage to cellular components through the ROS release is one of the main pathways that cause damage to many cellular functions [3]. These observations may explain the results in the present study. The supplementation of different levels of cysteine improved the in vitro fertilization and the development rates of buffalo embryos. These findings were similar to results obtained by previous studies reporting that the addition of cysteine to the in vitro culture medium increased the proportion of fertilized oocytes that developed to morula and blastocyst stages [12-14].
The higher rates of fertilization and embryo progress in 0.3 mM cysteine-treated group may be through increasing GSH level. Previous studies demonstrated that, addition of cysteine during the culture medium stimulated the synthesis of GSH and, accordingly, improved the in vitro embryo production [15]. This improvement may be due to the beneficial antioxidant mechanism of cysteine in neutralizing the reactive oxygen species and their harmful effects. Moreover, It was proved that addition of low molecular weight thiol compounds such cysteine to the culture media or the use of a cysteine-rich medium (TCM 199), promotes the synthesis of GSH, increased its intracellular level and improved embryo development and quality in pig [16] and bovine [17].
There are different mechanisms for controlling cellular ROS levels such as GSH and superoxide dismutase. GSH is a nonprotein sulphydryl compound in cattle cells and considered an important regulator of the ROS. GSH aids as a reservoir for cysteine and plays an essential role in guarding mammalian cells from oxidative stress, and its intracellular synthesis is an important factor in oocyte cytoplasmic maturation [19]. Moreover, GSH has a possible role in sperm nucleus decondensation and regulate spindle microtubule development in the ovum, and protect ova and embryo during in vitro fertilization, thus improving the outcome of pregnancy [20]. Recently, it was shown that GSH has a positive impact on in vitro embryo development [21] and [22].
Conclusions
The current results inferred that cysteine has a positive effect on buffalo oocytes fertilization and subsequent embryo development in a dose dependent trend. Moreover, the addition of 0.3 mM cysteine to the fertilization medium is a promising approach to improve the blastocyst rate in buffaloes.
References
- Halliwel B, Gutteridge JMC (1989) The chemistry of oxygen radicals and other derived species. Free radicals in biology and medicine. Oxford, UK: Oxford University Press. 22-85.
- Halliwel B, Gutteridge JMC, Cross CE (1992) Free radicals, antioxidants, and human disease: where are we now? J Lab Clin Med. 119(6): 598-620.
- Del Corso A, Capiello M, Mura U (1994) Thiol dependent oxidation of enzymes: the last change against oxidative stress. Int J Biochem Cell Biol. 26(6): 745-750.
- De Matos DG, Furnus CC, Moses DF, Baldassarre H (1995) Effect of cysteamine on glutatione level and developmental capacity of bovine oocyte mature in vitro. Mol Reprod Dev. 42(4): 432-436.
- Rahim B, Jalal S, Yosef N (2011) Effect of cysteine supplementation on in vitro maturation of bovine oocyte. Afr J Biotechnol. 10(70): 15830-15833.
- Totey SM, Singh G, Taneja M, Pawshe CH, Talwar GP (1992) in vitro maturation, fertilization and development of follicular oocytes from buffalo (Bubalus bubalis). J Reprod Fertil. 95(2): 597-607.
- Hasler JF, Henderson WB, Hurtgen PJ, Jin ZQ, McCauley AD, et al., (1995) Production, freezing and transfer of bovine IVF embryos and subsequent calving results. Theriogenol. 43(1): 141-152.
- Jainudeen MR, Takahashi Y, Nihayah M, Kanagawa H (1993) in vitro maturation and fertilization of swamp buffalo (Bubalus bubalis) oocytes. Anim Reprod Sci. 31(3-4): 205-212.
- Parrish JJ, Susko-Parrish JL, Leibfried-Rutledge ML, Critser ES, Eyestone WH, et al., (1986) Bovine in vitro fertilization with frozen thawed semen. Theriogenol. 25(4): 591-600.
- Holm P, Booth PJ, Schmidt MH, Greve T, Callesen H (1999) High bovine blastocyst developmental in a static in vitro production system using sofaa medium supplemented with sodium citrate and myo-inositol with or without serum-proteins. Theriogenol. 52(4): 683-700.
- Geshi M, Takenouchi N, Yamauchi N, Nagai T (2000) Effects of sodium pyruvate in nonserum maturation medium on maturation, fertilization, and subsequent development of bovine oocytes with or without cumulus cells. Biol Reprod. 63(6): 1730-1734.
- Caamño JN, Ryoo ZY, Youngs CR (1998) Promotion of development of bovine embryos produced in vitro by addition of cysteine and beta-mercaptoethanol to a chemically defined culture system. J Dairy Sci. 81(2): 369-374.
- Ali AA, Bilodeau JF, Sirard MA (2003) Antioxidant requirements for bovine oocytes varies during in vitro maturation, fertilization and development. Theriogenol. 59(3-4): 939-949.
- Whitaker BD, Casey SJ, Taupier R (2012) The effects of N-acetyl-L-cysteine supplementation on in vitro porcine oocyte maturation and subsequent fertilisation and embryonic development. Reprod Fertil Develop. 24(8): 1048-1054.
- Gasparrini B (2002) in vitro embryo production in buffalo species: state of the art. Theriogenol. 57(1): 237-256.
- Abeydeera LR, Wang WH, Cantley TC, Prather RS, Day BN (1999) Glutathione content and embryo development after in vitro fertilisation of pig oocytes matured in the presence of a thiol compound and various concentrations of cysteine. Zygote. 57(1): 203-210.
- Lott WM, Anchamparuthy VM, McGilliard ML, Mullarky IK, Gwazdauskas FC (2011) Influence of cysteine in conjunction with growth factors on the development of in vitro-produced bovine embryos. Reprod Domest Anim. 46(4): 585-594.
- Sagara J, Miura K, Bannai S (1993) Cystine uptake and glutathione level in fetal brain cells in primary culture and in suspens. J Neurochem. 61(5):1667-1671.
- Luberda Z (2005) The role of glutathione in mammalian gametes. Reprod Biol. 5(1): 5-17.
- Gadea J, Selles E, Marco MA, Coy P, Matas C, et al., (2004) Decrease in glutathione content in boar sperm after cryopreservation; Effect of the addition of reduced glutathione to the freezing and thawing extenders. Theriogenology. 62(3-4): 690-701.
- Badr MR, Abd el Hafez SM, Eman M, Abd el Fatah (2009) Influence of antioxidants on DNA integrity, mitochondrial function and fertilizing potentials of cryopreserved buffalo spermatozoa. Assiut Vet Med J. 55 (120): 296-317.
- Tsuzuki Y, Toyama1 H, Nabenishi1 H, Morita T, Ashizawa K (1998) The effect of various concentrations of taurine during in vitro fertilization on the development of bovine embryos fertilized with spermatozoa from three different bulls. Asian-Aust J Anim Sci 1998; 23: 873-879.