Intestinal IL-17 Expression in Canine Inflammatory Bowel Disease
Kinjo T1, Azuma YT2*, Nishiyama K2, Fujimoto Y2, Miki M2, Kuramoto N1
1 Laboratory of Molecular Pharmacology, Setsunan University Faculty of Pharmaceutical Sciences, Hirakata, Osaka, Japan.
2 Laboratory of Veterinary Pharmacology, Division of Veterinary Science, Osaka Prefecture University Graduate School of Life and Environmental Science, Izumisano, Osaka, Japan.
*Corresponding Author
Yasu-Taka Azuma Ph.C., Ph.D.,
Laboratory of Veterinary Pharmacology, Division of Veterinary Science,
Osaka Prefecture University Graduate School of Life and Environmental Science,
Izumisano, Osaka 598-8531, Japan.
Tel: +81 (0)72-463-5264
E-mail: azuma@vet.osakafu-u.ac.jp
Received: February 23, 2017; Accepted: March 27, 2017; Published: April 04, 2017
Citation: Kinjo T, Azuma YT, Nishiyama K, Fujimoto Y, Miki M, et al., (2017) Intestinal IL-17 Expression in Canine Inflammatory Bowel Disease. Int J Vet Health Sci Res. 5(3), 171-175. DOI : dx.doi.org/10.19070/2332-2748-1700035
Copyright: Azuma YT© 2017. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
IL-17 plays important roles in human inflammatory bowel disease (IBD). However, little is known about the possible relation of IL-17 in canine IBD. For comparative medicine and canine disease, it is useful to demonstrate the role of IL-17 in canine IBD. In this study, 7 cases of the dog with IBD typed of lymphocytic-plasmacytic enteritis without Intestinal lymphoma were examined immunohistochemically for possible relation with IL-17. Here, we found that IL-17 is expressed in intestinal mucosa of the dog with IBD. Among 7 cases, there are strong expressions of IL-17 in cases 2, moderate expression of it in cases 2, weak expression of it in cases 2, and very low expression of it in case 1. The score of IL-17 expression is not correlated with sex, age, and the breed. Using biopsy specimen, our results revealed the immunopathological relevance of IL-17 in the dog with IBD.
2.Introduction
3.Materials and Methods
3.1. The Case Animals
3.2. Immunohistochemistry (IHC)
3.3. Histology
3.4. Statistical Analysis
4.Results
5.Discussion
6.References
Abbreviations
CD: Crohn’s Disease; IBD: Inflammatory Bower Disease; IHC: Immunohistochemistry; UC: Ulcerative Colitis.
Introduction
The gastrointestinal tract is crucial for the immunological system in host defense in addition to food intake, food digestion, and nutrient absorption within humans and animals. Epithelium barrier in the intestine plays to block pathogens from the lumen. Inflammatory bowel diseases (IBDs) in dogs, as well as humans, are characterized by dysregulated intestinal inflammation and mucosal tissue damage in parts of the gastrointestinal tract [9, 11-13, 15, 18, 24, 28]. Regarding of histologic criteria, ulcerative colitis (UC) and Crohn’s disease (CD) are the two types of human IBD. Despite having a common basis in over responsiveness to mucosal antigens, UC and CD have considerably different pathophysiologies. In contrast, IBD in dogs divided into the several types by histological criteria of inflammatory infiltrate such as neutrophilic, eosinophilic, lymphocytic, plasmacytic and granulomatous. However, little is known whether these types reveal the common basis or different basis. Some reports show the pathology is different between humans and dogs, whereas other reports show common mucosal immune system between human and dogs [1,6, 8, 19].
The maintenance of intestinal homeostasis and the development of IBD involve interactions among the intestinal microflora, epithelium, and host mucosal immune system [25, 28]. Especially, the host mucosal immune system was controlled by the balance among inflammatory mediators, anti-inflammatory mediators and regulatory cytokines then contributed to the intestinal homeostasis. Cytokines have important roles in the regulation of the mucosal immune system of dogs as well as humans. In human, CD is associated with a Th1 response mainly driven by IL-12 and IFNγ [21], while UC is driven by Th2 cytokines, such as IL-4, IL-5, and IL-13 [27]. In contrast, dogs with IBD express with a mixture of both Th1 and Th2 cytokines, including IL2, IL4, IL5, IL10, IL12, IFNγ, TNFα, and TGFβ, in intestinal mucosa [17]. However, little is known about the expression of IL-17 in the dog with IBD. Data implicating mucosal IL-17 in the pathogenesis of dog with IBD are limited. Th17 is responsible for the IL-17 production, a key inflammatory cytokine and increased in human IBD [29]. The aim of the present study was to report the expression and localization of IL-17 in 7 cases of dogs with IBD typed of lymphocytic-plasmacytic gastroenteritis.
The details of 7 cases of dogs with IBD typed of lymphocyticplasmacytic gastroenteritis are given in Table 1. In all cases, a biopsy was submitted for microscopical evaluation. A sample of biopsy was fixed in 10% neutral buffered formalin, processed routinely and embedded in paraffin.
Table 1. The Prevalence of Physical and Clinical Observed Abnormalities from Adami Tulu and Adama feedlots.
IHC of 3-μm-thick paraffin-embedded sections was performed as previously described [20], with minor modifications. We used primary antibodies against IL-17 (ab79056; Abcam, Cambridge, MA, USA). To absorption the antibody, we used IL17 peptide (ab190773; Abcam). Immunoreactivity was detected using peroxidase-conjugated anti-rabbit polymers (Histofine, Nichirei, Tokyo, Japan) and a DAB substrate kit (Vector Laboratories Inc., Burlingame, CA, USA).
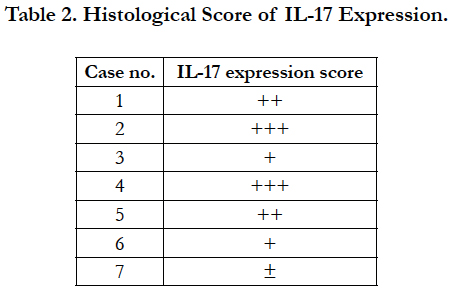
The histological score of IL-17 expression was graded by ranging from 0 to 3 as follows: 0, no expression; 1, low expression; 2, moderate expression; 3, high expression. Scores were obtained by a pathologist.
Histological score was statistically analyzed using the Mann-Whitney U test. Differences with P values of less than 0.05 were considered significant.
Results
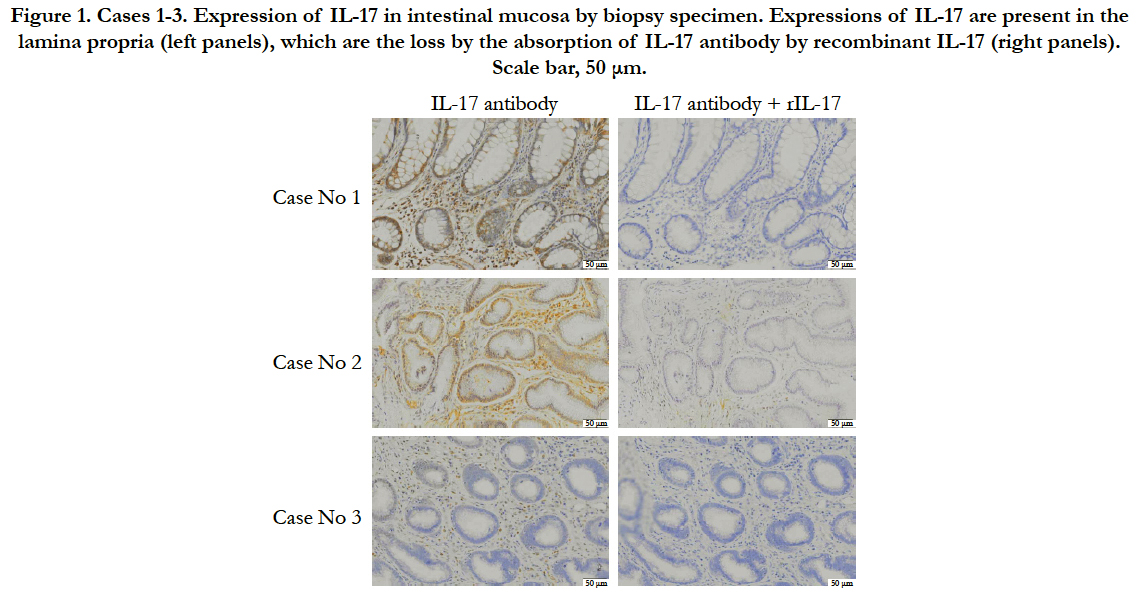
All of 7 cases investigated in this study (Table 1) were diagnosed IBD typed of lymphocytic- plasmacytic enteritis by histological criteria of inflammatory infiltrate. In addition, these 7 cases did not include intestinal lymphoma. IHC was performed to investigate the expression and localization of IL-17-positive cells in the intestinal mucosa of dogs with IBD. The treatment of recombinant IL-17 in addition to IL-17 antibody indicated as negative controls (each right panel). In Figure 1, the breed was all Dachshund. In case 1, the IL-17 was expressed in lamina propria (Figure 1). The expression of IL-17 in case 2 is stronger than it in case 1 (Figure 1). In contrast, the IL-17 was weakly expressed in lamina propria of case 3 (Figure 1). These results indicate that IL-17-positive cells are present in the lamina propria, and the expression level of IL-17 varies by case.
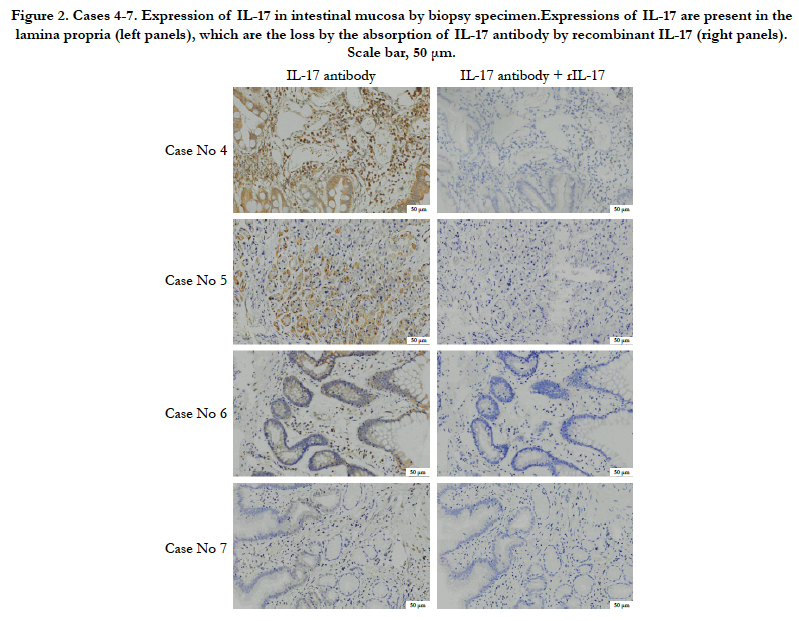
In case 4, there are dense IL-17-positive cells in lamina propria (Figure 2). In case 5, the IL-17 was expressed in lamina propria (Figure 2). In case 6, there is a little expression of IL-17 in lamina propria (Figure 2). In case 7, there is no marked expression of IL-17 in lamina propria (Figure 2). These results also indicate that the expression level of IL-17 varies by case.
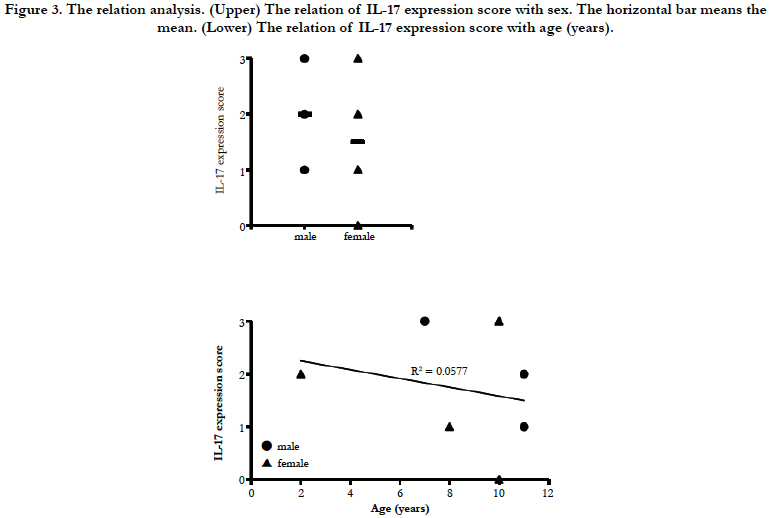
A histological score of the IL-17 expression in all 7 cases is shown in Table 2. Regarding of the score of IL-17 expression in lamina propria, there are no significant differences in sex of dogs with IBD (Figure 3 upper). Furthermore, no significant differences in age were observed in the score of IL-17 expression in lamina propria (Figure 3 lower). These results suggest that the expression level of IL-17 may vary by the case rather than the breed.
Figure 1. Cases 1-3. Expression of IL-17 in intestinal mucosa by biopsy specimen. Expressions of IL-17 are present in the lamina propria (left panels), which are the loss by the absorption of IL-17 antibody by recombinant IL-17 (right panels). Scale bar, 50 μm.
Figure 2. Cases 4-7. Expression of IL-17 in intestinal mucosa by biopsy specimen.Expressions of IL-17 are present in the lamina propria (left panels), which are the loss by the absorption of IL-17 antibody by recombinant IL-17 (right panels). Scale bar, 50 μm.
Figure 3. The relation analysis. (Upper) The relation of IL-17 expression score with sex. The horizontal bar means the mean. (Lower) The relation of IL-17 expression score with age (years).
Discussion
In this study, we defined the mucosal IL-17 expression in intestinal biopsies from dogs with IBD. Namely, we observed marked expression of IL-17 and the different pattern of localization of IL-17 even the expressed in the intestinal mucosa in dogs with IBD. We press that the expression pattern of intestinal mucosa IL-17 did not depend on the breed type of dogs with IBD. These indicate that IL17-mediated immune responses are related to the development of dogs with IBD, similar to human UC, at large. The classified etiology of dogs with IBD is not well understood although dogs with IBD are compared with human IBD such as UC and CD [5, 15]. Similar to human IBD, histologic features of dogs with IBD show infiltration of mononuclear cells into the lamina propria, goblet cell depletion, crypt hyperplasia and epithelial erosion [15]. T-cells also are related to onset and development of dogs with IBD, because CD3+, CD4+, and CD8+ T cells are elevated in the mucosa [10, 14, 16, 26].
Our strength of this study is that dog with IBD expressed IL-17 in duodenal mucosa. Up to the present, little has been reported on possible relation of IL-17 in canine diseases including IBD. These previous reports and our result show that IL-17 plays important role in canine as well as human. Our other strength is that a well-normalized and validated methodology of IHC is useful to evaluate the expression of interleukins in endoscopic biopsy [8, 23].
Cases 1, 2, 4 and 5 show high expression of IL-17, indicating increased infiltration of Th17 into the intestinal mucosa. In contrast, cases 3, 6 and 7 indicate low expression of IL-17, suggesting two possibilities. The first possibility is that Th17 population is low in spite of increased infiltration of the T-cells. The second possibility is that infiltration of the T-cells is not increased into the mucosa.
Unbalance among inflammatory mediators, anti-inflammatory mediators and regulatory cytokines result in IBD. Dysregulated balance between Th1/Th17-mediated inflammatory cytokines and Th2/Treg-mediated immunoregulatory cytokines results in patients with IBD and mice models with colonic inflammation like human IBD [2-4, 7, 10, 20, 22, 23].
A limitation of our results is that dogs with IBD in each case were not almost same by age (years). Aging dogs make a spontaneous progress of terrible and prolonged enterocolitis with diarrhea and body weight loss. Further studies will be needed to assess the relation of IL-17 expression level and age of dogs. Like a human with IBD, genetic factors may contribute to the pathogenesis of dogs with IBD, because an enhanced risk for the development of IBD is related to especial breeds including Basenjis, French bulldogs, German shepherd dogs and soft-coated Wheaton terriers [10, 15]. Further studies are also needed for the expression levels of IL-17 in these breed types.
Our results indicate the increased expression of IL-17 as an inflammatory cytokine in dogs with IBD, and constitute one of the several potential immune mechanisms that can contribute to canine IBD pathology. In conclusion, our results provide the strong insight into the important roles of IL-17 in dogs with IBD. Our results are also able to contribute the relationship among IL-17 expression, canine IBD pathology, drug therapeutics, and clinical outcomes, due to the reduced number of cases. IL-17 and its receptor will be an important consideration in the design of therapeutic agents in dogs with IBD.
References
- Allenspach K, Bergman PJ, Sauter S, Gröne A, Doherr MG, et al., (2006) P-glycoprotein expression in lamina propria lymphocytes of duodenal biopsy samples in dogs with chronic idiopathic enteropathies. J Comp Pathol. 134(1): 1-7.
- Azuma YT, Hagi K, Shintani N, Kuwamura M, Nakajima H, et al., (2008) PACAP provides colonic protection against dextran sodium sulfate induced colitis. J Cell Physiol. 216(1): 111-119.
- Azuma YT, Matsuo Y, Kuwamura M, Yancopoulos GD, Valenzuela DM, et al., (2010) Interleukin-19 protects mice from innate-mediated colonic inflammation. Inflamm Bowel Dis. 16(6): 1017-1028.
- Azuma YT, Nishiyama K, Matsuo Y, Kuwamura M, Morioka A, et al., (2010) PPARα contributes to colonic protection in mice with DSS-induced colitis. Int Immunopharmacol. 10(10): 1261-1267.
- Baumgart M, Dogan B, Rishniw M, Weitzman G, Bosworth B, et al., (2007) Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn's disease involving the ileum. ISME J. 1(5): 403-418.
- Cerquetella M, Spaterna A, Laus F, Tesei B, Rossi G, et al., (2010) Inflammatory bowel disease in the dog: differences and similarities with humans. World J Gastroenterol. 16(9): 1050-1056.
- Fujimoto Y, Azuma YT, Matsuo Y, Kuwamura M, Kuramoto N, et al., (2017) Interleukin-19 contributes as a protective factor in experimental Th2-mediated colitis. Naunyn Schmiedebergs Arch Pharmacolin press.
- German AJ, Hall EJ, Day MJ (2001) Immune cell populations within the duodenal mucosa of dogs with enteropathies. J Vet Intern Med. 15(1): 14- 25.
- German AJ, Hall EJ, Day MJ (2003) Chronic intestinal inflammation and intestinal disease in dogs. J Vet Intern Med. 17(1): 8-20.
- German AJ, Helps CR, Hall EJ, Day MJ (2000) Cytokine mRNA expression in mucosal biopsies from German shepherd dogs with small intestinal enteropathies. Dig Dis Sci. 45(1): 7-17.
- Grimble RF (2003) Inflammatory response in the elderly. Curr Opin Clin Nutr Metab Care. 6(1): 21-29.
- Jacobs G, Collins-Kelly L, Lappin M, Tyler D (1990) Lymphocytic-plasmacytic enteritis in 24 dogs. J Vet Intern Med. 4(2): 45-53.
- Jergens AE (1999) Inflammatory bowel disease. Current perspectives. Vet Clin North Am Small Anim Pract. 29(2): 501-521.
- Jergens AE, Gamet Y, Moore FM, Niyo Y, Tsao C, et al., (1999) Colonic lymphocyte and plasma cell populations in dogs with lymphocytic-plasmacytic colitis. Am J Vet Res. 60(4): 515-520.
- Jergens AE, Moore FM, Haynes JS, Miles KG (1992) Idiopathic inflammatory bowel disease in dogs and cats: 84 cases (1987-1990). J Am Vet Med Assoc. 201(10): 1603-1608.
- Jergens AE, Moore FM, Kaiser MS, Haynes JS, Kinyon JM (1996) Morphometric evaluation of immunoglobulin A-containing and immunoglobulin G-containing cells and T cells in duodenal mucosa from healthy dogs and from dogs with inflammatory bowel disease or nonspecific gastroenteritis. Am J Vet Res. 57(5): 697-704.
- Jergens AE, Sonea IM, O'Connor AM, Kauffman LK, Grozdanic SD, et al., (2009) Intestinal cytokine mRNA expression in canine inflammatory bowel disease: a meta-analysis with critical appraisal. Comp Med. 59(2): 153-162.
- Kleinschmidt S, Meneses F, Nolte I, Hewicker-Trautwein M (2007) Characterization of mast cell numbers and subtypes in biopsies from the gastrointestinal tract of dogs with lymphocytic-plasmacytic or eosinophilic gastroenterocolitis. Vet Immunol Immunopathol. 120(3-4): 80-92.
- Locher C, Tipold A, Welle M, Busato A, Zurbriggen A, et al., (2001) Quantitative assessment of mast cells and expression of IgE protein and mRNA for IgE and interleukin 4 in the gastrointestinal tract of healthy dogs and dogs with inflammatory bowel disease. Am J Vet Res. 62(2): 211-216.
- Matsuo Y, Azuma YT, Kuwamura M, Kuramoto N, Nishiyama K, et al., (2015) Interleukin 19 reduces inflammation in chemically induced experimental colitis. Int Immunopharmacol. 29(2): 468-475.
- Monteleone G, Monteleone I, Fina D, Vavassori P, Del Vecchio Blanco G, et al., (2005) Interleukin-21 enhances T-helper cell type I signaling and interferon-gamma production in Crohn's disease. Gastroenterol. 128(3): 687-694.
- Niessner M, Volk BA (1995) Altered Th1/Th2 cytokine profiles in the intestinal mucosa of patients with inflammatory bowel disease as assessed by quantitative reversed transcribed polymerase chain reaction (RT-PCR). Clin Exp Immunol. 101(3): 428-435.
- Ridyard AE, Nuttall TJ, Else RW, Simpson JW, Miller HR (2002) Evaluation of Th1, Th2 and immunosuppressive cytokine mRNA expression within the colonic mucosa of dogs with idiopathic lymphocytic-plasmacytic colitis. Vet Immunol Immunopathol. 86(3-4): 205-214.
- Roth L, Walton AM, Leib MS, Burrows CF (1990) A grading system for lymphocytic plasmacytic colitis in dogs. J Vet Diagn Invest. 2(4): 257-262.
- Sartor RB (2006) Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 3(7): 390-407.
- Stonehewer J, Simpson JW, Else RW, Macintyre N (1998) Evaluation of B and T lymphocytes and plasma cells in colonic mucosa from healthy dogs and from dogs with inflammatory bowel disease. Res Vet Sci. 65(1): 59-63.
- Tanaka J, Saga K, Kido M, Nishiura H, Akamatsu T, et al., (2010) Proinflammatory Th2 cytokines induce production of thymic stromal lymphopoietin in human colonic epithelial cells. Dig Dis Sci. 55(7): 1896-1904.
- Xavier RJ, Podolsky DK (2007) Unravelling the pathogenesis of inflammatory bowel disease. Nature. 448(7152): 427-434.
- Zhu J, Wang Y, Yang F, Sang L, Zhai J, et al., (2015) IL-33 alleviates DSSinduced chronic colitis in C57BL/6 mice colon lamina propria by suppressing Th17 cell response as well as Th1 cell response. Int Immunopharmacol. 29(2): 846-853.