Effects of Condensed Tannin-Rich Pine Bark Diet on Experimentally Infected With Haemonchus Contortus in Meat Goats
B. R. Min1*, E. A. Wilson1, S. Solaiman1, J. Miller2
1 Department of Agricultural and Environmental Sciences, Tuskegee University, Tuskegee, USA.
2 Department of Animal and Meat Sciences, Louisiana State University, Baton Rouge, LA, USA.
*Corresponding Author
B. R. Min,
Department of Agricultural and Environmental Sciences,
Tuskegee University,
Tuskegee, AL 36088, USA.
Tel: +1 334 727 8321
Fax: +1 334 727 8552
E-mail: minb@mytu.tuskegee.edu
Article Type: Review Article
Received: February 13, 2015; Accepted: March 27, 2015; Published: March 30, 2015
Citation: B. R. Min, E. A. Wilson, S. Solaiman, J. Miller (2015) Effects of Condensed Tannin-Rich Pine Bark Diet on Experimentally Infected with Haemonchus Contortus in Meat Goats. Int J Vet Health Sci Res. 3(3), 49-57. doi: dx.doi.org/10.19070/2332-2748-1500013
Copyright: B. R. Min© 2015. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
This study evaluated the effects of dietary supplementation of a novel condensed tannin-rich pine bark (PB) mixture diet on an in vitro gas production, in vivo animal performance, internal parasites, carcass production, and plasma metabolites in meat goats. Fifteen Kiko-cross meat goats (Capra hircus; body weight (BW) = 28.0 ± 1.0 kg) were randomly assigned to two experimental diets (control vs. PB supplementation): 1) control diet (70% grain mixture and 30% wheat straw (WS) and 2) 70% grain mixture with 30% PB. Animals were dewormed on day 10 and animals were artificially inoculated (day 20) with 5,000 infective stage (L3) Haemonchus contortus larvae. Feed intake and performance were monitored for 87 days. Blood samples were taken twice, once at the beginning and once at the end of the experiment. Fecal egg (FEC) was determined approximately every 2 week. At the end of the experimental period, goats were slaughtered, and abomasum parasite load was determined. Abomasal worms were identified and counted. All analyses were conducted using a GLM or MIXED procedure of SAS, but in vitro gas analysis was conducted using non-linear procedure of SAS. Overall, there were no differences (P >0.10) in dry matter intake, body weight, and carcass traits between diets. H.contortus adult worm numbers were lower (P <0.05) for goats that were fed PB diet than for control. On day 84, control goats on control WS diet had greater (P = 0.01) FEC than PB diet group. It was concluded that feeding ground PB as a feed ingredient has the potential to decrease internal parasite infection without detrimental effects.
2.Introduction
2.1 Implications
3.Materials and Methods
3.1 Experimental Procedures
3.2 In vitro rumen fermentation
3.3 Performance Measurement
3.4 Feed sample collection and analysis
3.5 Blood Sample and Analysis
3.6 Harvest Measures
3.7 Fecal Collection and Sampling
3.8 Parasite Identifications
4.Statistical Analysis
5.Results
5.1 Diet Composition
5.2 In vitro rumen digestibility and gas production
5.3 Intake and growth performance
5.4 Carcass characteristics
5.5 Blood parameters and metabolites
5.6 Fecal egg counts
5.7 Worm burdens
6.Discussion
6.1 In vitro rumen fermentation
6.2 In vitro rumen digestibility and gas production
6.3 Feed intake and growth performance
6.4 Carcass traits
6.5 Hemogram and serum chemistry
6.6 Fecal egg count and worm burdens
7.Conclusion
8.Acknowledgement
9.References
Keywords
Condensed tannins; Animal performance; Parasites; Pine bark.
Introduction
Infestation with gastro-intestinal nematodes (GIN) in small ruminants can cause economic losses and endanger animal welfare. Although sheep and goats get numerous types of parasites, Haemonchus contortus is the most clinically significant nematode parasite and most important with respect to dewormer resistance. Unfortunately, the long term, heavy use of dewormers has leaded to parasite resistance to these products. That means that on some farms, there are no dewormers left that will effectively eliminate parasites. We have developed alternative natural dewormer that can control internal parasites infestation in goats. A noble approach of such practical strategy will help to make recommendation to improve the management of animal health and well being, especially organic livestock farmers.
Gastrointestinal (GI) parasites (especially Haemonchus contortus) present the greatest danger to the goat and sheep industry. Infected animals have lower growth rates, reduced reproductive performance, and have higher rates of illness and death. In the past, sheep and goat producers relied heavily on anti-parasite drugs. Unfortunately, GI parasites have become increasingly resistant to many of the anthelmintics. Alternative methods of GI parasite control for animals raised primarily on forages are vital for the sustainability and profitability of sheep and goat farms in the southeastern U.S. Consequently, alternative, sustainable, and affordable methods of parasitic control are required that are practical and realistic for introduction into farm production systems. Research has shown that legumes such as sericea lespedeza (Lespedeza cuneata) contain condensed tannin (CT) have anti-parasitic properties. The anti-parasite properties of CT have been demonstrated to reduce GI parasitic infection in goats at Oklahoma [1] and with hay-feeding trials with goats at Georgia [2] and sheep at Louisiana, U.S.A [3]. Recently, researchers in Tuskegee University [4] found potential benefits of pine bark (PB) supplementation on anti-parasitic effects and improved feed efficiency. Pine bark is one of the abundant forest by-products in the southern U.S. and contains 11–13% condensed tannins (CT) on a dry matter basis [4].
However, relationships among CT levels in PB, experimentally infected worm burden, feed intake, animal performance, blood metabolites, and carcass characteristics have not been explored in meat goats. The aim of this study was to assess possible anthelmintic effects of PB powder supplementation against H. contortus in infected goats and the associated consequences on the animal production and health.
Materials and Methods
This experiment was conducted at Tuskegee University’s Small Ruminant Research and Education Unit over an 87-day period (16 weeks). This experiment was piloted in compliance with Tuskegee University Institutional Animal Care and Use Committee regulations.
Fifteen male 9 month old Kiko-cross meat goats (Capra hircus; BW = 28.0 ± 1.0 kg) were randomly assigned to two experimental diets (control vs. PB supplementation): 1) 0% pine bark (PB) and 30% wheat straw (WS) and 2) 30% ground PB and 0% WS (Table 1). The remainder of each diet was a mix of 85% grain and 15% bermuda grass hay. Experimental goats for this experiment were strategically dewormed on day 0 with 5 mL/100 lbsBW of Cydectin® (Moxidectin, Fort Dodge Animal Health, Fort Dodge, IA; 5mg/mL) followed by 3 mL/100 lbs BW of Valbazin (500 mL, Pfizer Animal Health) under supervision of a veterinarian to reduce or eliminate gastrointestinal parasites. Animals was inoculated on day 20 with 5000 infective stage (L3) H.contortus dominant larvae. The goats were individually housed in 1.1m x 1.2 m pens with plastic-coated expanded mesh floors to allow simple passage of feces and urine to minimize parasite re-infestation. Feed intake and performance were monitored for 87 day. Blood samples were taken twice, once at the beginning and once at the end of the experiment. Fecal egg count (FEC) was determined approximately every 2 weeks. At the end of the experimental period, goats were slaughtered, and GI parasite load was determined. Abomasal worms were identified and counted.
The ingredients composition of the concentrate mix and bermuda grass hay offered to goats is summarized in Table 1. The fresh PB was donated by a wood processing company (West Fraser Timber Co. Ltd, Opelika, AL) and air-dried before processing. Freshly dried PB (11.0 % condensed tannins (CT)) was ground (Hammer Mill Model 1250; Lorenz MFG Co., Benson, MN) to approximately 3 mm particle size before mixing with remaining ingredients of the diets. The WS (0.03% CT) was also ground (3 mm) and incorporated in the grain mix portion of the diet. The final concentration of CT in 30% PB diet was 3.2 % DM. Mixtures containing ground PB and WS were commercially prepared at the local feed mill (Eclectic Feed Mill, Eclectic, AL). Experimental diets met all animals’ requirements for growth and gain according to NRC (2007). The mixed rations (% DM) in control (WS) and pine bark (PB) contained rations consisted of ground PB (0, 30), ground wheat straw (30, 0), corn (20, 20), soy bean meal (19,21), soy hulls (4, 4), alfalfa meal (5, 3), molasses (6, 6), vitamins and mineral mix (0.5, 0.5), salt (0.5, 0.5), ammonium chloride (0.5, 0.5), and chopped (1-2 cm) bermuda grass hay (15, 15) on an asfed basis, respectively.
Additional vitamins and trace mineral salt blocks were provided to secure growing goats’ micronutrients requirements. Animals were fed once a day at 8:00 am and had free access to water and trace mineral salt block. Grain mixes and hay were offered separately, and refusals were recorded daily for 87 days. Amounts of feed offered were adjusted every 3 to 4 days to maintain the preferred daily refusal rate of 5 to 10%.
Rumen contents were collected 2 h post diet supplementation using a stomach tube. Rumen fluid was assayed using the method described by Min et al. [5]. Samples were obtained (about 100 mL) from 4 animals using a stomach tube and transported to the laboratory within 30 min of collection. The rumen fluid was immediately placed on 300 ml thermal bottle wormer that was capped and immediately returned to the Lab for determination of rumen gas production. Rumen gas production was determined by combining, in 18 x 150 mm crimp top tubes, 5 ml mixed rumen fluid with 5 mL anaerobic artificial saliva [6] containing 0, 5, 10 mg/mL of ground PB (to pass 2 mm sieves). These tubes were capped, attached with 60 mL syringe, and incubated at 39°C under H2:CO2 (50:50 mix) atmosphere. In vitro gas production was measured as plunger displacement (mL) at 0, 1, 2, 3, 4, 5, 6, 8 and 12 h incubation periods [6]. In vitro incubation was under taken in duplicate. Total in vitro gas produced was corrected to blank incubations (i.e. no ruminal fluid). All gases were collected from the in vitro rumen incubation for total gas and methane gas production analyses [7].
Feed intake was calculated as difference between feed offered and refused. Feed intake and refusal was treated as daily measures for 87 days for growth performance and gain efficiency determination. Average daily gain (ADG) was calculated by difference in initial and final BW divided by 87 days of growth performance. Gain-to-feed ratio was calculated as ADG (g/d) divided by total dry matter intake (DMI; g/d). The animals used in this study were cared for according to the Live Animal Use in Research Guidelines of the Institutional Animal Care and Use Committee of the Tuskegee University, Tuskegee, AL.
Diet samples were collected every 2-week. Composite samples for grain mixes and ingredient samples for bermuda grass hay, PB, and WS (n = 3) were dried for 48 h at 55°C in a convection oven (model 420, NAPCO, Pittsburgh, PA). Samples were ground to pass a 1-mm screen (Wiley Mill, Arthur Thomas Co., Philadelphia, PA). Ground composite samples were analyzed for DM, lignin, ether extract, and minerals according to the methods described by AOAC [8]. Crude protein was determined using a Kjeldahl-N method [8] by multiplying N by 6.25. Dietary neutral detergent fiber (NDF) and acid detergent fiber (ADF) were sequentially determined using an ANKOM200 Fiber Analyzer (ANKOM Technology, Macedon, NY) according to the methodology supplied by the company, which is based on the methods described by Van Soest et al. [9]. Total extractable CT concentration in experimental diets was determined using a butanol-HCl colorimetric procedure [10].
Blood samples were taken before (day 0) and after inoculated (day 60). Blood samples were collected using 20 gauge 1 inch needles via jugular vein, in EDTA (3 mL) and non-EDTA (10 mL) containing vacutainer tubes (BD, Franklin Lakes, NJ) and blood samples were placed on ice immediately following collection and relocated to Tuskegee University Clinical Pathology Laboratory for analysis. Samples were analyzed for complete blood counts using CELL-DYN 3700 Model System (Abott Diagnostic Division, Chicago, IL), packed cell volume (PCV), white blood cells (WBC) differential count, red blood cell count (RBC), hematocrit (HCT), hemoglobin (Hbg), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), red cell distribution width (RDW), platelet count, and mean platelet volume (MPV) and blood serum metabolites [11] were analyzed at the Tuskegee University Diagnostic Laboratory, immediately after sampling. Total white blood cell numbers were determined by the method of Natt and Herrick [12]. Lymphocyte population was evaluated from stained blood smears with brilliant cresyl blue [13].
Final body weight (BW) was obtained after 87 days and animals were transported to Auburn University Lambert-Powell Meats Lab for harvesting according to the USDA approved guidelines [14]. All of the internal organs with the exception of the abomasum and the kidneys were disposed of after slaughter. The Postmortem necropsy examination and dissecting the kidney and liver were conducted according to the method of Nietfeld [15].
Hot carcass weight (HCC) was determined on the day of slaughter, and carcasses were chilled at 4°C for 24 h. Then, cold carcass weight (CCW) and carcass shrink weight were measured. Carcasses were ribbed between the 12th and 13th rib for further evaluation. Fat depth over the midpoint of longissimus muscle (LM) at the 12 th rib, body wall fat (BWF) measured at lower point of the12th rib, kidney-pelvic fat (KPF), dressing percentage (DP; HCW/live wt.), and LM area, were determined by certified USDA grader 24 h postmortem.
Fecal samples were taken every two weeks using fecal bags for collection. Goats for this experiment were strategically dewormed on day 10 as mentioned above to reduce or eliminate gastrointestinal parasites; however, most resistant worms and coccidian survived under controlled environment. Fecal samples for fecal egg counts (FEC) were obtained on d0 (in quarantine d0-21), 10, 24, 40, 49, 56, 72, and 84. Fecal egg counts were determined by a modification of the McMaster technique [16].
After harvest, the rumen of each animal was obtained where the abomasum was sectioned off and secured individually into an air locked zip locked bag and labeled. The abomasum was frozen and placed into two Styrofoam containers to be kept frozen and shipped to the School of Veterinary Medicine on the campus of Louisiana State University (LSU) for parasite count and identification. Abomasum was thawed and washed by opening the entire length and emptying the contents into a 20 L bucket, and then adjust by tap water up to 10 L (final volume). Collected digesta was washed 3-4 times with 10 L of fresh water each time, and mixed well; two 100 ml (1%) samples were taken from each bucket and mixed with 100 ml of 10% (v/v) formalin solution, then examined under a stereoscope at 30X magnification.
Individual parasite larvae or adults examine were morphologically confirmed according to the methods of Merck Veterinary Manual [17] and Zajac [18]. In the process of parasite identification, a100 mL sample was taken from the 10 L size of plastic bottle and was placed into a 1 L beaker. The sample was then washed using small amounts of water through a 200 mesh sieve. Remain samples were placed into the 1 L beaker and water was added until the original volume (100mL) was reached. A drop of small amounts of the sample were poured into a Petri dish (with grids) and decolorized with hypochlorite solution. Using a dissecting microscope, the contents were slowly scanned to search for adult and larval worms. Using a tuberculin syringe, worms were transferred from the solution to the slide with a drop of lactophenol. The worms were counted, sexed, and identified to determine worm burden and worm percent distribution within each section of the abomasum for each animal. Once the worms on the slides have been counted the differentiated (%) for a given section of GI tract in a given animal is calculated. For calculating total worm count, a dilution factor of 100 (10 L water in washtub, with 1 L sub-sample, and 100 mL sample that was analyzed) was applied to sample worm count.
Statistical Analysis
The study was conducted to two comparable treatments (control vs. PB supplementation) as the main effect using a completely randomized design. The two factors were dietary amendments (PB powder or wheat straw supplementations). Goats were assigned randomly to one of the two treatment combinations. All analyses were conducted using SAS (SAS Inst., Inc., Cary, NC). For characteristics measured only once on the experimental unit (goat), the GLM procedure was used. For characteristics measured more than one time on the experimental unit, the MIXED procedure using the repeated option was used [19]. To evaluate treatment differences in fecal egg counts and worm counts, the GLIMMIX procedure was used with a Poisson error distribution, the log link function and Satterthwaite’s approximation of the denominator degrees of freedom [19]. The level of significance was P<0.05.
An in vitro gas production rate was measured repeatedly and calculated using the exponential equation of Φrskov and McDonald [20]: Y = a + b(1-e-ct).
Where Y was defined as gas production in time t; a, b, and c being constants of the exponential equation where a = the gas production at time 0, b = the proportion of gas production during time (t), and c = the rate of gas production of the ‘b’ fraction. The constants b and c for each treatment were calculated with the method described by Min et al. [6] using the Non-Linear Regression (NLIN) procedure from SAS Institute (SAS Inst. Inc., Cary,NC). Cumulative in vitro gas production in each time point was analyzed using the MIXED procedure of SAS Institute. The Ftest- protected least squares means procedure of SAS was used to separate treatment means.
Results
Chemical composition of the experimental diets including PB and WS are presented in Table 1. The grain mixes were isonitrogenous. Total CT concentration in PB and WS on a DM basis was 11.0% and 0.03%, respectively. However, total CT concentration in these diets (0% PB and 30% PB) was 0.19 and 3.2% CT DM, respectively. According to Table 1, ADF, NDF, and lignin content were higher for PB than for control diet. Total digestible nutrient (TDN) was higher for WS than for PB-contained ration.
Table 1. Chemical composition of experimental diets containing pine bark (PB) and wheat straw (WS).
1 The total mixed ration (% DM) in control and pine bark (PB) contained diets consisted of ground PB (0, 30), ground wheat straw (30, 0), corn (20, 20), soy bean meal (19, 21), soy hulls (4, 4), alfalfa meal (5, 3), molasses (6, 6), vitamins and mineral mix (0.5, 0.5), salt (0.5, 0.5), ammonium chloride (0.5, 0.5), and bermuda grass hay (15, 15) on an as-fed basis, respectively. Calcium (Ca); Phosphorous (P); Magnesium (Mg); Potassium (K); Sulfur (S); Sodium (Na); Copper (Cu); Manganese (Mn); Zinc (Zn); Iron (Fe).
2 Non fibrous carbohydrates.
3 Condensed tannins (CT) are relative to a purified Quebracho condensed tannins standard (on DM basis).
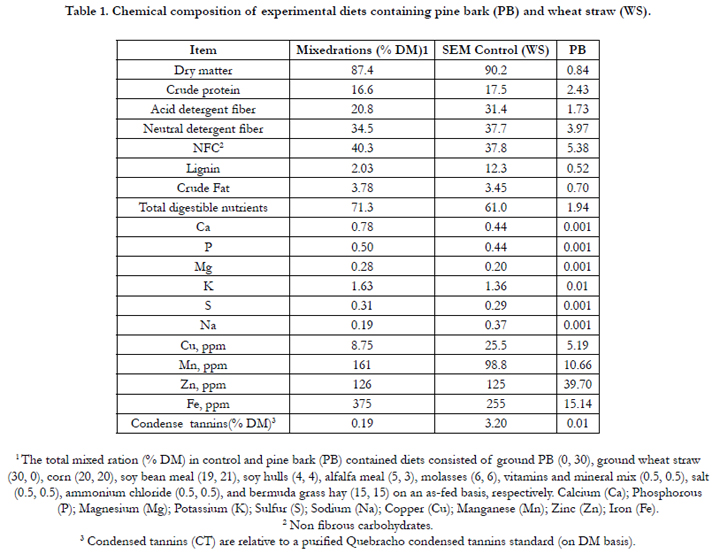
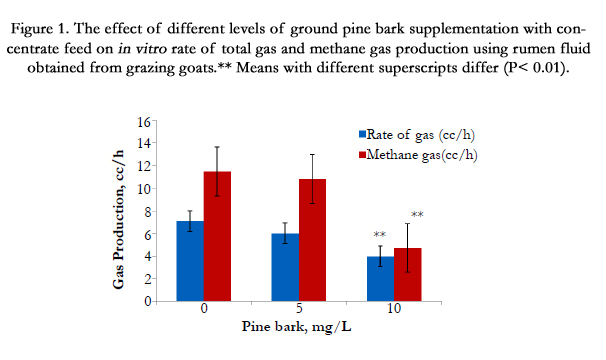
We were concern about the rumen fermentation of ground PB due to a high level of CT as well as dry form of PB. Our in vitro data in Figure. 1 shows that addition of ground pine bark up to 10 mg PB/mL with concentrate mixture, incubated with rumen fluid obtained from grazing goats, had significantly reduced (P < 0.01) for total gas and methane gas production (Figure. 1), indicating that minimum levels of ground PB supplementation to impact rumen fermentation was started at >10 mg PB/mL
Figure 1. The effect of different levels of ground pine bark supplementation with concentrate feed on in vitro rate of total gas and methane gas production using rumen fluid obtained from grazing goats.** Means with different superscripts differ (P< 0.01).
The summary of dry matter intake (DMI), growth performance and gain: feed (G: F) ratio were presented in Table 2. There were no differences between animals in diet groups in any of the variables shown in Table 2.
Table 2. Effects of condensed tannin-containing pine bark (PB) supplementation on animal performance in Kiko-cross goat.
As shown in Table 3, carcass characteristics of animals fed experimental diets are presented. Of variables fasting BW, HCW, CCW, carcass shrink, DP, 12th rib fat thickness and KPF, there was no significant difference in the characteristics of carcass between the dietary treatment groups.
Table 3. Effects of condensed tannin-containing pine bark (PB) supplementation on selected carcass characteristics of LM in Kiko-cross goat.
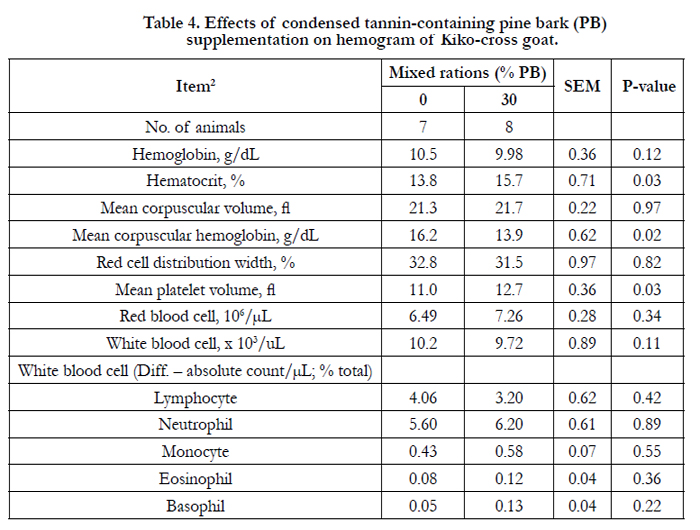
Hemogram and blood serum chemistry of goats consuming the separate diets of PB and WS are presented in Tables 4 and 5, correspondingly. In the current study, of the hemogram and serum chemistry showed no significant difference except for the mean corpuscular hemoglobin concentration (P < 0.02), mean platelet volume (P<0.03), cholesterol (P<0.03), creatine kinase (P<0.001), and blood urea (P< 0.001). Mean corpuscular hemoglobin concentration, cholesterol, triglyceride (P< 0.03), and blood mineral P (P< 0.001) were lower in PB diet, but mean platelet volume, creatine kinase, and blood urea N for PB diet was higher (P<0.001) than for those WS diet.
Table 4. Effects of condensed tannin-containing pine bark (PB) supplementation on hemogram of Kiko-cross goat.
Table 5. Effects of condensed tannin-containing pine bark (PB) supplementation on blood serum chemistry in Kiko-cross goat.
Data presented in Figure 2, displays the effects of PB versus WS diets fed to inoculated animals on FEC. During the performance period, FEC fluctuated between diet treatments. On day 84, control group had greater (P< 0.01) FEC than PB diet group.
Figure 2. Fecal egg counts of goats fed different diets throughout the experimental period.
** Means with different superscripts differ (P< 0.01).Experimental goats were dewormed on day 10 and inoculated on
day 20.
Table 6. Effects of condensed tannin-containing pine bark (PB) supplementation on total adult worm count.
Experimentally infected GI parasites in PB and WS diets are presented in Table 6. GI infected parasites animals that received PB had lower total worm burdens (P<0.05) included H. Contortus than WS mixed diet. Feeding PB diet reduced both male and female worm counts compared to WS diet.
Discussion
The most significant findings of this study were that experimentally parasite infected animals reduced H. contortus load in the presence of PB diet with no changes in DMI and animal performance. The increase efficacy of anti-parasites activity was due to decreases fecundity at both fecal egg shedding and adult worm burden.
Plant tannins may have substantial effects on all phases of in vitro rumen fermentation and metabolism. In vitro ruminal fermentation incubated with PB supplementation reduced as evidenced by decreasing the rate of gas and methane gas concentration. The results indicate that the effect of CT-containing PB in this expression is a dose dependent. These findings agree with the data of Bento et al. [21] reported that the reduced gas production from mimosa tannin extracts during in vitro incubation reflects inhibition of cell walls, and its components, by mimosa tannins. As a result, mimosa tannins reduced microbial degradation of carbohydrates, and subsequently gas production [22, 23]. It has been shown that plant tannins or commercial tannin extracts modified microbial population in the rumen, reduced microbial numbers and/or enzyme production from the rumen microorganisms available to ferment substrates [24-26]. A decrease in rate of gas and methane gas production by PB tannins addition was consistent with the result of other in vitro studies [27, 1, 6].
In the present study, there were no changes observed in DMI, ADG and carcass measurements. However, it has been reported that DMI was increased in the diets in goats fed PB (3.2% CT in DM) along with that of grain intake [4]. Similarly, Solaiman et al. [34] reported that total DMI of growing goats increased when sericea lespedeza (Lespedeza cuneata) ground hay (6.5% CT in DM) replaced alfalfa meal in the grain mixes, and Turner et al. (2005) reported that goats receiving the CT-containing sericea lespedeza hay (2.31 % CT/g soluble protein) had higher DMI than those fed the alfalfa hay based diet. Although this study was similar to the present experiment, variables such as inoculated animals may be the reason for DMI and animal performance difference. Other assumptions have been made such as, the feed intake by animals is usually reduced for diets with tannins in high concentrations due to a reduction of palatability, decreased rate of digestion in the rumen and the development of conditioned aversion [28-30].
Previous research reported that animals grazing on CT-containing forage sulla (Hedysarum coronarium; 5-7 % CT DM) and birds foot trefoil (Lotus corniculatus; 3.4% DM CT) had greater animal performance and carcass production compared to those grazing on alfalfa (Medicargo sativa) [31, 32]. In the present study shown that there was no difference in HCW, CCW, transport shrink, DP, 12th rib fat thickness, and KPF, except LM area. According to the experiment conducted by Priolo et al. [33], twenty-four male Comisana lambs were assigned to one of three treatments; control, CT-containing sulla and polyethylene glycol drench group to eliminate condensed tannin effects. The carcass yield in the animals given sulla without polyethylene glycol was decreased. Solaiman et al. [34] reported similar results in carcass characteristics. There was no difference in HCW, CCW, shrink or dressing % of goats fed different dietary treatments of sericea lespedeza 0%, 10%, 20% and 30% for 63 days. Carcass characteristics are known to respond gradually to changes in nutrition. However, it is not fully understood how CT affects carcass traits, and so this area needs further investigation.
In this study, visual inspection according to USDA regulations [14] standards indicated no anatomical lesions on liver and kidney organs. In correspondence with the data of Silanikove et al. [35] who reported using CT-containing diets that ranged from 5.0% CT (DM basis) in carob (Ceratonicasiliqua) to 9.5% in oak (Quercuscalliprinos) and 20.5% in pistacia (Pistacialentiscus) did not exhibit toxic symptoms in goats. The concentration of CT fed in current study was 0.19, and 3.2 % CT DM in 0 and 30% PB diets, which was lower than the concentration used in the study of Silanikove et al. [35]. It is apparent that experimental animals were able to tolerate the amount equaling or less than 30% of CT content in diets fed. In the current study, of the hemogram and serum chemistry showed no significant difference except for mean corpuscular hemoglobin concentration, mean platelet volume, blood urea nitrogen, triglycerides, phosphorus, cholesterol, and creatine kinase; however, all values fell within the normal range for goats, suggesting that no damage in the liver occurred. This has been confirmed by post-mortem necropsy and dissecting the liver and kidney in this study that indicated no anatomical lesions on liver and kidney organs. These parameters were used as a diagnostic tool for screening animal health problems and abnormality.
Creatine kinase helps convert creatine into phosphocreatine by converting ATP into ADP in muscles. This is a reversible reaction where phosphocreatine acts as an energy reservoir used for rapid regeneration of ATP in muscles [36]. Previous study has been showed that goats received diet containing 15 and 30% PB inclusion had no changed in blood creatine kinase enzyme. In the present study, however, creatine kinase level in PB diet was higher than control diet, indicating that animals received PB diet may associated with red blood cells increment that contribute to the enzymatic reaction for creatine kinase, artifactually increasing values [37].
Blood urea nitrogen is waste from the liver, processed by the kidneys. According to Solaiman et al. [34], blood urea nitrogen level is not correlated with kidney dysfunction. However, blood urea nitrogen level increases in the present study may be due to the changes of protein metabolism in gastrointestinal tract as affected by CT containing PB or gastrointestinal parasites infection. Chaichisemsariet al. [38] reported that parasitized sheep had a higher plasma blood urea nitrogen concentration than non-parasitized animal which attributed to the rate of irreversible loss and the rate of urinary excretion of urea.
Alternative parasites control strategies have recently been suggested based on using CT-containing forages [39, 40]. Min and Hart [39] reported that there was a direct effect of tannin-containing forages on adult worms, with significantly lower numbers of both abomasal (H. contortus, Teladorsagia circumcincta) and small intestinal (T.colubriformis) nematodes compared with goats fed bermuda grass hay (0% CT) [1,6]. The present study strongly supports this view, showing that goats consuming CT-containing PB diet reduced both male and female worm burdens and fecundity as measured by FEC compared to those receiving CT-free diets. Of the common protein binding and precipitate characteristic of tannin, binding to parasitic worms are directly related [41]. These parasitic worms, H. contortus, are composed of protein, allowing for a binding and catabolism of the structure of the parasite’s mouth after the initial attachment to the intestinal wall. To compare and agree with an experiment conducted by Hur et al. [42], the effects of feeding pine (Pinusdensifora) needles, oak (Quercusacutissima) leaves and lucerne chaff on coccidia egg yield were studied for a period of 10 days post-feeding. The results indicated that feeding fresh pine needles (40 g CT DM/day/goat) and oak leaves (40 g CT DM/day/goat) in blend with lucerne chaff had rapid anticoccidial activities in goats. This may begin to explain the results seen in this study although more research should be conducted for certain justifications. Regardless of the CT-parasite interactions, CT-containing diets may hold promise as a practical means of reducing contamination of pastures with parasites eggs and of decreasing exclusive reliance on commercial anthelmintics.
Conclusion
Pine bark powder supplementation up to 30% reduced fecal egg counts when fed for proper length of time. Feeding ground pine bark supplement reduced both male and female worm counts compared to wheat straw mixed diet. The results indicate that ground pine bark as a feed ingredient has potential to maintain animal performance while decreasing internal parasites infection. Thus, developing plant-based alternatives such as pine bark and other natural resources for gastrointestinal parasites control would be expected to have a greater impact on the goat and sheep industries. This will allow development of best management practices to prevent or treat gastrointestinal parasites in ruminant livestock.
Acknowledgement
This project was supported by the Sustainable Agriculture Research and Education (SARE) program, which is funded by the U.S. Department of Agriculture- National Institute of Food and Agriculture (USDA-NIFA), the USDA/NIFA Evans-Allen Research Program and Tuskegee University, George Washington Carver Agricultural Research Station.
References
- Min BR, Hart SP, Miller D, Tomita GM, Loetz E, et al. (2005a) The effect of grazing forage containing condensed tannins on gastro-intestinal parasite infection and milk composition in Angora does. Veterinary Parasitology 130:105-113.
- Shaik SA, Terrill TH, Miller JE, Kouakou B, Kannan G, et al. (2004) Effects of feeding sericea lespedeza hay to goats infected with Haemonchuscontortus. South African Journal of Animal Science 34: 248-250.
- Lange KC, Olcott DD, Miller JE, Mosjidis JA, Terrill TH, et al. (2006) Effect of sericea lespedeza, fed as hay, on natural and xperimental Haemonchuscontortus infections in lambs. Veterinary Parasitology 14: 273-278.
- Min BR, Solaiman S, Gurung N, Behrends J, Eun JS, et al. (2012) Effects of pine bark supplementation on performance, rumen fermentation, and carcass characteristics of Kiko crossbred male goats. Journal of Animal Science 90: 3556-3567.
- Min BR, Pinchak WE, Anderson RC, Fulford JD, Puchala R (2006) Effect of condensed tannins supplementation level on weight gain and in vitro and in vivo bloat precursors in steers grazing winter wheat. Journal of Animal Science 84: 2546-2554.
- Min BR, Pinchak WE, Fulford JD, Puchala R (2005) Wheat pasture bloat dynamics, in vitro ruminal gas production and potential bloat mitigation with condensed tannins. Journal of Animal Science 83:322-1331.
- Puchala R, Min BR, Goetsch AL, Sahlu T (2005) The effect of a condensed tannin-containing forage on methane emission by goats. Journal of Animal Science 83:182-186.
- AOAC (1984) Official Methods of Analysis Association of official Analytical Chemists (14th Edtn). Arlington VA, Sidney.
- Van Soest PJ, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent fiber and non-starch polysaccharides in relation to animal nutrition. Journal of Dairy Science 74:3583–3597.
- Terrill TH, Rowan AM, Douglas GB, Barry TN (1992) Determination of extractable and bound condensed tannin concentrations in forage plants, protein concentrate meals and cereal grains. Journal of the Science Food and Agriculture 58: 321-329.
- Blum JW, Kunz P, Eunberger G, Keller N (1983) Thyroid hormones, blood plasma metabolites and haematological parameters in relationship to milk yield in dairy cows. Animal Production 36: 93-104.
- Natt MP, Herrick CA (1952) A new blood diluent for counting the erythrocytes and leucocytes of the chicken. Poultry Science 31:735-738.
- Mukkur TKS, Bradley RE (1974) Determination of the absolute numbers of leukocyte cell types in chicken. Poultry Science 53:221-223.
- USDA (2001) Institutional meat purchased specifications for fresh goat. Series 11. USDA, MRP, AMF, Livestock and Seed Program, Washington DC.
- Nietfeld JC (2010) Field necropsy techniques and proper specimen submission for investigation of merging infectious diseases of food animals. Veterinary Clinical North American Food Animal Practice 26: 1–13.
- Stafford KJ, West DM, Pomroy WE (1994) Nematode worm egg output by ewes. Veterinary Journal 42:30–42.
- The Merck Veterinary Manual (2006) Polioencephalomalacia www.merckvetmanual. com.
- Zajac AM (1994) Fecal examination in the diagnosis of parasitism. In Veterinary clinical parasitology (6th Edtn) Iowa State University Press, Ames,Iowa. 3–88.
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD (1996) SAS system for mixed models. SAS Institute Inc., Cary, North Carolina,
- Φrskov ER, McDonald I (1979) The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. Journal of Agriculture Science 92:499-503.
- Bento MHL, Makkar HPS, Acamovic T (2005) Effect of mimosa tannin and pectin on microbial protein synthesis and gas production during in vitro fermentation of 15N-labelled maize shoots. Animal Feed Science and Technology 123-124:365-377.
- Akin DE, Rigsby LL, Theodorou MK, Hartley RD (1988) Population changes of fibrolytic rumen bacteria in the presence of phenolic acids and plant extracts. Animal Feed Science and Technology 19:261-275.
- Markkar HPS, Becker K, Abel HJ, Szegletti C (1995) Degradation of condensed tannins by rumen microbes exposed to quebracho tannins (QT) in rumen simulation technique (RUSTIC) and effects of QT on fermentation processes in the RUSTIC. Journal of the Science Food and Agriculture 69:495-500.
- Min BR, Attwood GT, Reilly C, Sun W, Peters JS, et al. (2002) Lotus corniculatus condensed tannins decrease in vivo populations of proteolytic bacteria and affect nitrogen metabolism in the rumen of sheep. Canadian Journal of Microbiology 48:911-921.
- Min BR, Pinchak WE, Anderson RC, Fulford JD, PuchalaR (2006a) Effects of condensed tannins supplementation level on weight gain and in vitro and in vivo bloat precursors in steers grazing winter wheat. Journal of Animal Science 94:2546-2554.
- Min BR, Pinchak WE, Anderson RC, Hume ME (2006b) In vitro bacterial growth and in vivo ruminal microbiota populations associated with bloat in steers grazing wheat forage. Journal of Animal Science 84:2873-2882.
- Hervas G, Frutos P, Giraldez FJ, Mantecon AR, Alvarez Del Pino MC (2003) Effect of different doses of quebracho tannins extract on rumen fermentation in ewes. Animal Feed Science and Technology 109:65-78.
- Turner KE, Wildeus S, Collins JR (2005) Intake, performance, and blood parameters in young goats offered high forage diets of lespedeza or alfalfa hay. Small Ruminant Research 59:15-23
- Patra AK, Saxena J (2010) A new perspective on the use of plant secondary metabolites to inhibit methanogenesis in ruminants. Phytochemistry 71:1198-1222.
- Vargas-Magaña JJ, Aguilar-Caballero AJ, Torres-Acosta JFJ (2013) Tropical tannin-rich fodder intake modifies saliva-binding capacity in growing sheep. Animal 7:1921-1924
- Hoskin SO, Barry TN, Wilson PR, Charleston WAG, Hodgson J(1999) Effects of reducing anthelmintic input upon growth and faecal egg and larval counts in young farmed deer grazing chicory (Cichoriumintybus) and perennial ryegrass (Loliumperenne)/white clover (Trifoliumrepens) pasture. Journal of Agricultural Science (Camb.)132:335–345.
- Wang Y, Douglas GB, Waghorn GC, Barry TN, Foote AG, et al (1996) Effect of condensed tannins upon the performance of lambs grazingLotus comiculatusand lucerne (Medicago sativa). Journal of Agricultural Science (Camb.) 126:87–98.
- Priolo A, Waghorn GC, Lanza M, Biondi L, PennisiP (2000) Polyethylene glycol as a means for reducing the impact of condensed tannins in carob pulp: effects on lamb growth performance and meat quality. Journal of Animal Science l78:810-816.
- Solaiman S, ThomasJ, Dupre Y, Min BR, Gurung N, et al (2010) Effect of feeding sericea lespedeza hay on growth performance, blood metabolites, and carcass characteristics of Kiko crossbred male kids. Small Ruminant Research 93:149-156.
- Silanikove N, Gilboa N, Perevolotsky A, Nitsan Z (1996) Goats fed tannincontaining leaves do not exhibit toxic syndromes. Small Ruminant Research 21:195-201.
- Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM (1992) Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochemistry Journal 281:21-40.
- Cornell University Veterinary Clinics (2013) Https://ahdc.vet.cornell.edu/
- Chaichisemsari MB, Eshratkhah B, Maherisis N, SadaghianM, Hassanpour S (2011) Evaluation of total protein, albumin, globulin and blood urea nitrogen concentration in gastrointestinal nematodes infected sheep. Global Veterinary 6:433-437.
- Min BR, Hart SP (2003) Tannins for suppression of internal parasites Journal of Animal Science 81 (E. Suppl. 2): E102-E109.
- Shaik SA, Terrill TH, Miller JE, Kouakou B, Kannan G, et al (2006) Sericea lespedeza hay as a natural deworming agent against gastrointestinal nematode infection in goats. Veterinary Parasitology 139:150-157.
- Reed JD (1995) Nutritional toxicology of tannins and related polyphenols in forage legumes. Journal of Animal Science 73:1516-28.
- Hur SN, Molan AL, Cha JO (2005) Effects of Feeding Condensed Tannincontaining Plants on Natural Coccidian Infection in Goats. Asian-Aust. Journal of Animal Science 18:1262-1266.