Influence of Anti-Mullerian Hormone on ART Outcomes in Infertility Patients of Different Ages
JO Sohn1,2, MJ Kim1, JH Jeon1, YM Jo1, SH Kim1, JM Lim2,3*, HJ Song1*
1 Fertility Medical Center, Seoul Women’s Hospital, Bucheon, Korea.
2 Department of Agricultural Biotechnology, Seoul National University, Seoul, Korea.
3 Institute of Agriculture and Life Sciences, Seoul National University, Seoul, Korea.
*Corresponding Author
Hyun Jin Song,
Fertility Medical Center, Seoul Women’s Hospital, Bucheon, 14544, Korea.
Tel/Fax: +8232-230-7010
E-mail: shyunjin@chol.com
Jeong Mook Lim,
Department of Agricultural Biotechnology, Seoul National University, Seoul, 08826, Korea.
Institute of Agriculture and Life Sciences, Seoul National University, Seoul, 08826, Korea.
Tel/Fax : +822-874-2555
E-mail: limjm@snu.ac.kr
Received: November 27, 2018; Accepted: December 28, 2018; Published: December 31, 2018
Citation: MJ Kim, JH Jeon, YM Jo, SH Kim, JM Lim, HJ Song, et al., Influence of Anti-Mullerian Hormone on ART Outcomes in Infertility Patients of Different Ages. Int J Reprod Fertil Sex Health. 2018;5(3):126-130. doi:dx.doi.org/10.19070/2377-1887-1800023
Copyright: JM Lim, HJ Song© 2018. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Aging-related intervention by anti-mullerian hormone (AMH) on ART profile were retrospectively examined, and ART outcome of different AMH categories (<1.0, 1.0-3.5, >3.5) was compared. Overall, increased AMH accompanying with endogenous FSH decrement improved oocyte retrieval and maturation. Of those 112 cycles recruited, 77% had ≥1 ng/ml AMH, which accompanied with a significant (p<0.05) reduction in endogenous FSH and age. Improved oocyte retrieval and maturation resulted in better pregnancies than did cycles with <1 ng/ml AMH. A significant decrease in AMH was detected in patients ≥35 years old and great increase in the number of ET embryos in patients maintaining high AMH levels resulted marked increase of the pregnancy rate (11% vs. 69%). However, most AMH effects were disappeared in the 30-34 years group. These results demonstrated that endogenous AMH influenced ART outcomes, and the effect was prominent in patients aged beyond 35 years old, which can predict ART outcomes in middle-aged, infertile patients.
2.Abbreviations
3.Inrtoduction
4.Materials and Methods
4.1 Patients
4.2 IVF Regimen
4.3 Measures
4.4 Statistics Analysis
5.Results and Discussions
5.1 Overall Profiles of Patients
5.2 The Comparison between Ages
6.Discussion
7.Conclusion
8.Acknowledgement & Declarations
9.References
Keywords
AMH; Infertility; Oocyte Maturation; Fecundity; Age.
Abbreviations
ART: Applied Reproductive Technology; AMH: Anti-Mullerian Hormone; FSH: Follicle Stimulating Hormone; ICSI/ET: Intracytoplasmic Sperm Injection/Embryo Transfer; PCOS: Polycystic Ovary Syndrome.
Inrtoduction
Changes in life patterns in most developed countries have resulted in marriage at a later age. The mean age of couples has increased gradually, which may expose them to an age-related fertility disorder. Such social and clinical cases are considered a serious problem that leads to population decline. Aging accompanies diminished ovarian reserves and further understanding of reproductive physiology at middle age is critical for mobilizing developmentally competent oocytes during infertility treatment in aging women. Thus, establishing new parameters to precisely predict fecundity of a patient contributes to developing an efficient applied reproductive technology (ART) program for couples that marry late.
Aging induces a gradual decrease in the level of endogenous anti-Mullerian hormone (AMH), which directly influences the number of primordial follicles [1-3] and the sensitivity of the ovarian follicles to the follicle stimulating hormone (FSH)-dependent follicular wave [4] and folliculogenesis [5, 6]. Due to its relationship with endogenous FSH levels, AMH has been used as a parameter to evaluate the status of the controlled ovarian hyperstimulation cycle [7-13]. Based on previous reports, we evaluated whether measuring blood AMH levels would be useful to predict ART outcomes in middle-aged, infertile patients. Two major factors, the AMH level and patient age, were used to categorize the patients, and various patient profiles and ART outcomes including oocyte maturity and intracytoplasmic sperm injection/embryo transfer (ICSI/ET) results were employed for the evaluation. A retrospective cohort study was conducted for the analysis.
Collection of data was made from the total 113 cases undergoing ICSI-ET undergoing at Fertility Medical Center of Seoul Women’s Hospital between September 2012 and July 2014. The study was approved by the Institutional Review Board of the Seoul Women’s Hospital. Serum AMH level of each patient was measured without relating of cyclicity. According to AMH level and ages, the cycles examined were classified into three or four groups. The selection criteria were (i) within the range of 25-48 years old, (ii) only ICSI attempt, (iii) no evidence of endocrinological disorders, (iv) no evidence of polycystic ovary syndrome (PCOS) and (v) regular menstrual cycles. Informed consent was retrieved from all the patients participating in this study.
All women underwent controlled ovarian hyperstimulation with recombinant FSH or highly purified hMG with preventing of premature ovulation by GnRH agonist down-regulation or antagonist suppression. hCG (Ovidrel, 250ng/0.5ml; Merck Serono, UK) was administered when there were at least three follicles measuring 18 mm or more in diameter and transvaginal ultrasound- guided oocyte retrieval performed 36 hours later. ICSI was performed by using described techniques and instruments [14]. Embryo transfer was performed 2 days later using embryo transfer catheter under transvaginal ultrasound. The luteal phase was supported by progesterone (90 mg of Crinone Gel; Merck Serono, UK) beginning on the day of embryo transfer. Pregnancy was determined by the increase of plasma β-hCG more than 50 mIU/ mL 14 days after embryo transfer. An ultrasound scan 3 weeks after a positive pregnancy test confirmed a clinical pregnancy.
Serum was separated from blood samples within 2 hours and was frozen in aliquots at -20°C until used. AMH was measured with Immunotech Enzyme Immunoassay kit (Bechman-Caulter, France). All samples were concomitantly assessed for minimizing intra assay variation. The analytical sensitivity for AMH was 0.09 ng/mL, and the intra- and inter assay coefficients of variation with serum controls were approximately 4.5%–5.6% and 3.6%–5.4%, respectively.
Statistical analyses were performed with Statistical Analysis Software (SAS Institute, Cary, NC) using general linear models with analysis of variance. When a significant model effect was detected, each treatment effect was compared using the least-squares method. The level of significance was determined when P value was less than 0.05.
Total 112 cycles comprised of 111 patients were employed for the analysis, which was undertaken during the period of September 2012 to July 2014. Mean age of patients were 36.7 years old and average level of AMH was 3.1 ng/ml. A mean of 6.6 oocytes were retrieved from a patient and ET of an average of 2.6 embryos per patient resulted the pregnancy rate of 37%.
Two categories of ART cycles according to AMH levels (less or more than 1 ng/ml AMH) were initially designed without considering of age factors. 77% of the cycles had the patients of more than 1 ng/ml AMH (49 cases consisting of 1-3.4 and 37 cases of >3.5 ng/ml). In the cycles of >1 ng/ml AMH, a significant difference was detected in the number of cycle (26 cases vs. 86 cases; p<0.0001), age (39.2 years old vs. 35.9 years old; p=0.0034), FSH level (10.9 ng/ml vs. 6 ng/ml; p=0.0006), oocyte retrieval (2.7 oocytes vs. 7.8 oocytes per patient; p<0.0001), maturation (oocytes provided for ICSI: 2.3 oocytes vs. 6.2 oocytes per patient; p<0.0001), mean number of ET embryos (1.8 embryos vs. 2.8 embryos; p=0.0006), endometrial thickness (8.8 mm vs. 10 mm; p=0.04) and pregnancy rate (18% vs. 41%; p=0.0226) compared with the cycles of <1 ng/ml AMH. The patients with high AMH of >1 ng/ml yielded better pregnancy rate without increasing of offspring number (1.3 to 1.4 babies per delivery) than the patients with low AMH.
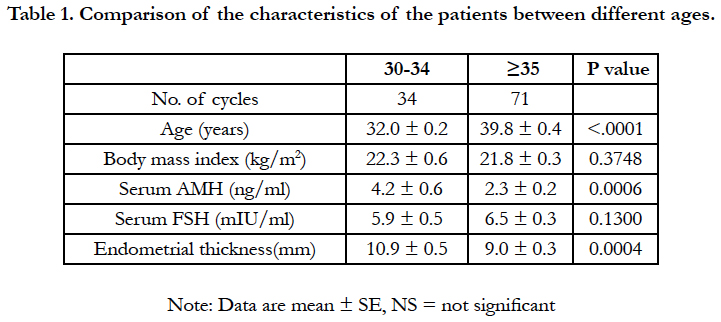
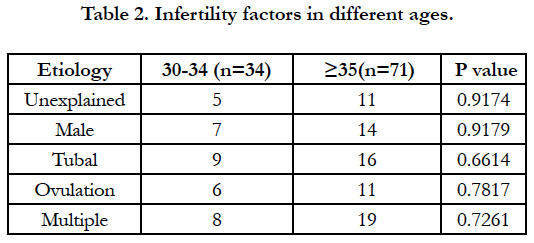
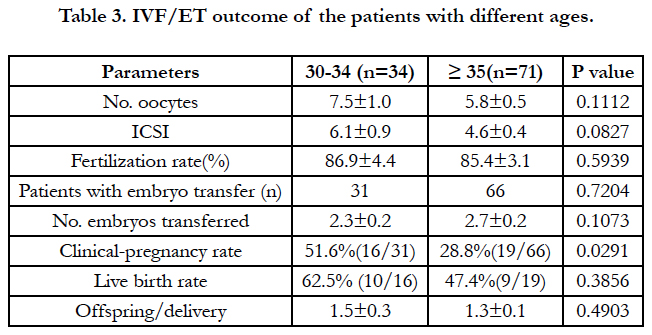
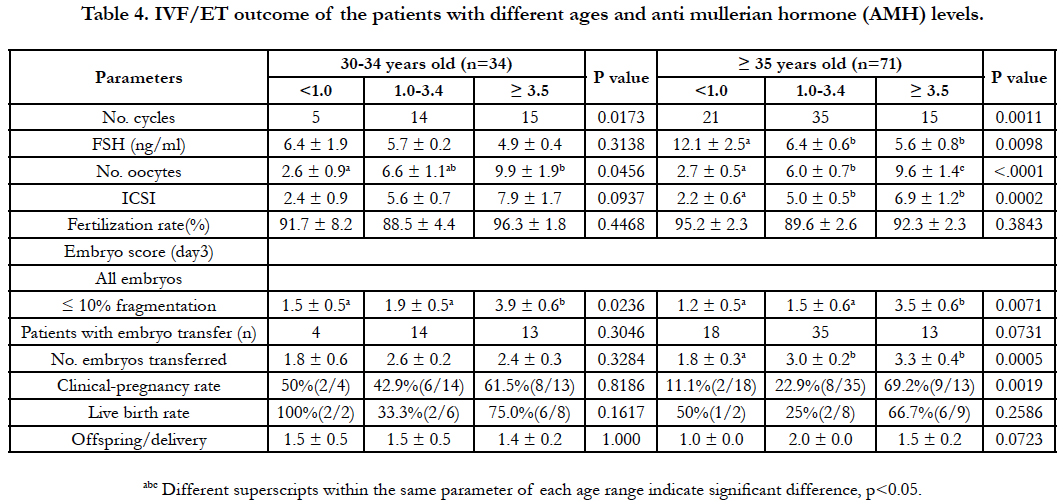
As shown in Table 1, total cases were divided into two groups according to age (30-34 vs. over 35) to search the effective AMH levels on IVF-ET outcomes. BMI and levels of serum FSH were not difference in two groups, however, significant difference was detected in endometrial thickness and levels of serum AMH. No significant difference among infertility factors was detected in two groups (Table 2). Considering IVF-ET outcomes in two groups (Table 3), no significant difference was detected without clinical pregnancy rate (51.6% vs. 28.8%; p=0.0291).
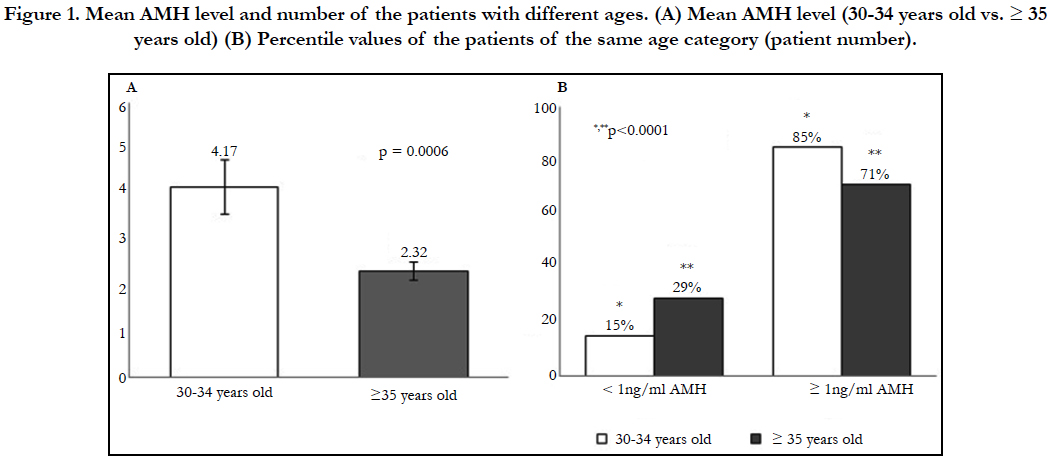
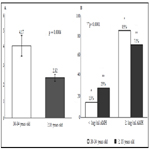
As shown in Figure 1, the cycles consisting of the patients of 30-34 years old had higher AMH level (4.17 ng/ml vs. 2.32; p=0.0006) than the cycles consisting of the patients of >35 years old. The percentile value of the number of the cycles of >1 ng/ml AMH significantly increased in the cycles of the ≥ 35 years old (15 to 29%) compared with the cycles of the 30-34 years old. As shown in Table 4, the comparison among three AMH levels (<1, 1-3.4 and ≥ 3.5) was undertaken separately within two groups. In the cycle of ≥ 35 years old, a significant difference was detected in the parameters of cycle number (21 cases vs. 15-35 cases; p=0.0011), FSH levels (12.1 ng/ml vs. 5.6-6.4 ng/ml; p=0.0098), oocyte retrieval (2.7 oocytes vs. 6-9.6 oocytes; p<0.0001), maturation (oocytes provided for ICSI: 2.2 oocytes vs. 5-6.9 oocytes; p=0.0002), mean number of ET embryos (1.8 embryos vs. 3-3.3 embryos; p=0.0005) and pregnancy rate (11% vs. 23-69%; p=0.019). In the cycles consisting of the patients aged ≥ 35 years old, the cycles consisting of the patients maintaining AMH level of 1 to 3.4 was predominant (p < 0.05). Significant decrease in FSH level (12.1 ng/ml to 5.6 ng/ml; p=0.0098) and increase in oocyte retrieval (2.7 to 9.6 oocytes per patient; p<0.0001), maturation (2.2 to 6.9 oocytes per patient: p=0.0002), ET embryos (1.8 to 3.3 embryos per cycle; p=0.005) and pregnancy rate (11 to 69%; p=0.0019) was detected in the cycles of the patients with increased AMH level. However, no significant difference among AMH level was detected in mean number of offsprings per delivery (1 to 2 offsprings). In the 30-34 years old group, significant model effect was detected only in the parameter of cycle number (5 cases vs. 14-15 cases; p=0.0173) and the >1 ng/ml AMH group was predominant. Although the tendency was similar to the cycles consisting of the patients of ≥35 years olds, no significant difference was detected in all comparisons among AMH groups except for oocyte retrieval (2.6 to 9.9 oocytes per patient). In vitro-development of embryos prior to ET was subsequently assessed (Table 4) and regardless of age and AMH categories, no significant difference was detected among comparisons.
Figure 1. Mean AMH level and number of the patients with different ages. (A) Mean AMH level (30-34 years old vs. ≥ 35 years old) (B) Percentile values of the patients of the same age category (patient number).
Table 4. IVF/ET outcome of the patients with different ages and anti mullerian hormone (AMH) levels.
In addition, embryo score at the day 3 showed better scores in ≥ 3.5 category (p=0.0236, p=0.0071) than other categories of two groups.
Discussion
Our results strongly suggest that measuring serum AMH levels is a powerful parameter for predicting ART outcomes in middleaged, infertile patients. Feasibility of the AMH measurement is based on its positive relationship with the retrieval of developmentally competent oocytes, which directly improve clinical pregnancy rates. Maintenance of endogenous FSH levels within a presumptively optimal range is one of the principal roles of AMH, which is particularly critical for patients aged > 35 years.
AMH is a dimeric glycoprotein expressed at the beginning of the perinatal period [15]. AMH expression continues throughout sexual maturity, but endogenous AMH levels begin to decline near the end of reproductive life [16] and completely disappear after menopause [17]. The major function of AMH is to inhibit the initiation of primordial follicle recruitment and to maintain the FSH-dependent follicular wave [4, 18-20]. AMH antagonizes FSH function in pre-antral and early antral follicles [21]. Our results confirmed the relationship between FSH and AMH and further suggest that this relationship is critical for maintaining ART-based fecundity in middle-aged women with declining AMH levels. Accordingly, concomitantly measuring AMH and FSH is an efficient strategy for predicting the ovarian response and ART outcome in aging patients, although further studies are necessary to elucidate the complications of AMH-related physiological events.
Conclusion
Our study demonstrated endogenous AMH levels influenced ART outcomes, and the effect was prominent in patients >35 years of age. Measuring endogenous AMH may be a sensitive parameter for predicting ART outcomes in middle-aged, infertile patients.
Acknowledgement & Declarations
The authors report no declarations of interest. The authors are responsible for the content and writing of the paper.
References
- Durlinger AL, Gruijters MJ, Kramer P, Karels B, Ingraham HA, Nachtigal MW, et al. Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology. 2002 Mar;143(3):1076-84. PubMed PMID: 11861535.
- Hansen KR, Hodnett GM, Knowlton N, Craig LB. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril. 2011 Jan;95(1):170-5. doi: 10.1016/j.fertnstert.2010.04.006. PubMed PMID: 20522327.
- Seifer DB, MacLaughlin DT. Mullerian inhibiting substance is an ovarian growth factor of emerging clinical significance. Fertil Steril. 2007 Sep;88(3):539-46. PubMed PMID: 17559842.
- Visser JA, Themmen AP. Anti-Müllerian hormone and folliculogenesis. Mol Cell Endocrinol. 2005 Apr 29;234(1-2):81-6. PubMed PMID: 15836956.
- Fanchin R, Schonäuer LM, Righini C, Frydman N, Frydman R, Taieb J. Serum anti‐Müllerian hormone dynamics during controlled ovarian hyperstimulation. Hum Reprod. 2003 Feb;18(2):328-32. PubMed PMID: 12571169.
- Van Rooij IA, Broekmans FJ, Te Velde ER, Fauser BC, Bancsi LF, De Jong FH, et al. Serum anti-Müllerian hormone levels: a novel measure of ovarian reserve. Hum Reprod. 2002 Dec;17(12):3065-71. PubMed PMID: 12456604.
- Fanchin R, Schonauer LM, Righini C, Guibourdenche J, Frydman R, Taieb J. Serum anti-Mullerian hormone is more strongly related to ovarian follicular status than serum inhibin B, estradiol, FSH and LH on day 3. Hum Reprod. 2003 Feb;18(2):323-7. PubMed PMID: 12571168.
- Feyereisen E, Lozano DH, Taieb J, Hesters L, Frydman R, Fanchin R. Anti- Müllerian hormone: clinical insights into a promising biomarker of ovarian follicular status. Reprod Biomed Online. 2006 Jun;12(6):695-703. PubMed PMID: 16792844.
- Hazout A, Bouchard P, Seifer DB, Aussage P, Junca AM, Cohen-Bacrie P. Serum antimüllerian hormone/müllerian-inhibiting substance appears to be a more discriminatory marker of assisted reproductive technology outcome than follicle-stimulating hormone, inhibin B, or estradiol. Fertil Steril. 2004 Nov;82(5):1323-9. PubMed PMID: 15533354.
- Lee TH, Liu CH, Huang CC, Hsieh KC, Lin PM, Lee MS. Impact of female age and male infertility on ovarian reserve markers to predict outcome of assisted reproduction technology cycles. Reprod Biol Endocrinol. 2009 Sep 17;7:100. doi: 10.1186/1477-7827-7-100. PubMed PMID: 19761617.
- Nardo LG, Gelbaya TA, Wilkinson H, Roberts SA, Yates A, Pemberton P, et al. Circulating basal anti-Müllerian hormone levels as predictor of ovarian response in women undergoing ovarian stimulation for in vitro fertilization. Fertil Steril. 2009 Nov;92(5):1586-93. doi: 10.1016/j.fertnstert. 2008.08.127. PubMed PMID: 18930213.
- Seifer DB, MacLaughlin DT, Christian BP, Feng B, Shelden RM. Early follicular serum müllerian-inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertil Steril. 2002 Mar;77(3):468-71. PubMed PMID: 11872196.
- Visser JA, de Jong FH, Laven JS, Themmen AP. Anti-Müllerian hormone: a new marker for ovarian function. Reproduction. 2006 Jan;131(1):1-9. Pub- Med PMID: 16388003.
- Tesarik J, Sousa M. Key elements of a highly efficient intracytoplasmic sperm injection technique: Ca2+ fluxes and oocyte cytoplasmic dislocation. Fertil Steril. 1995 Oct;64(4):770-6. PubMed PMID: 7672149.
- Ueno S, Kuroda T, Maclaughlin DT, Ragin RC, Manganaro TF, Donahoe PK. Mullerian inhibiting substance in the adult-rat ovary during various stages of the esrous-cycle. Endocrinology. 1989 Aug;125(2):1060-6. PubMed PMID: 2752965.
- van Rooij IA, Broekmans FJ, Scheffer GJ, Looman CW, Habbema JD, de Jong FH, et al. Serum antimüllerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril. 2005 Apr;83(4):979-87. PubMed PMID: 15820810.
- La Marca A, De Leo V, Giulini S, Orvieto R, Malmusi S, Giannella L, et al. Anti-Mullerian hormone in premenopausal women and after spontaneous or surgically induced menopause. J Soc Gynecol Investig. 2005 Oct;12(7):545-8. PubMed PMID: 16046154.
- Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, et al. Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology. 1999 Dec;140(12):5789-96. PubMed PMID: 10579345.
- Gruijters MJ, Visser JA, Durlinger AL, Themmen AP. Anti-Müllerian hormone and its role in ovarian function. Mol Cell Endocrinol. 2003 Dec 15;211(1-2):85-90. PubMed PMID: 14656480.
- Knight PG, Glister C. TGF-β superfamily members and ovarian follicle development. Reproduction. 2006 Aug;132(2):191-206. PubMed PMID: 16885529.
- Baarends WM, Hoogerbrugge JW, Post MI, Visser JA, De Rooij DG, Parvinen MA, et al. Anti-müllerian hormone and anti-müllerian hormone type II receptor messenger ribonucleic acid expression during postnatal testis development and in the adult testis of the rat. Endocrinology. 1995 Dec;136(12):5614-22. PubMed PMID: 7588316.