The Effect of Salmeterol, Tiotropium Bromide and its Combination in Patients with Acute Exacerbation Of Chronic Obstructive Pulmonary Disease
Omid Otroshi1, Mohammad Ali Saba1*, Abdulhossein DavodAbadi1, Azam Zilochian1
Autoimmune Diseases Research Center, Kashan University Of Medical Sciences, Kashan, Iran.
*Corresponding Author
Dr. Mohammad Ali Saba,
Autoimmune Diseases Research Center, Kashan University of Medical Sciences, Ghotb Ravandi Highway, Kashan, Iran.
Tel: 00989133613523
E-mail: drmasaba@gmail.com
Received: July 15, 2020; Accepted: August 06, 2020; Published: August 08, 2020
Citation:Omid Otroshi, Mohammad Ali Saba, Abdulhossein DavodAbadi, Azam Zilochian. The Effect of Salmeterol, Tiotropium Bromide and its Combination in Patients with Acute Exacerbation Of Chronic Obstructive Pulmonary Disease. Int J Resp Dis Care Med. 2020;4(1):54-58. doi:dx.doi.org/10.19070/2577-4409-2000010
Copyright: Mohammad Ali Saba© 2020. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: In order to managing patients with acute exacerbated COPD, long-acting anticholinergic drugs and long-acting
beta-agonists are used alone. Therefore, the purpose of this study was to investigate the effect of salmeterol, tiotropium bromide
and its combination in patients with acute exacerbation of chronic obstructive pulmonary disease.
Materials and Methods: This tripartite clinical trial study was performed on patients with acute exacerbated COPD referred
to the Emergency Department of Shahid Beheshti Hospital of Kashan in 2019 (135 people). Patients were randomly divided
into three groups. For each of the three groups, salmeterol sprays, tiotropium bromide and combination were administered
separately during the admission. FEV1 and FVC measures were evaluated before and after the intervention for all three
groups via spirometry. Data were analyzed using chi-square test, ANOVA, paired t-test and covariance analysis.
Results: The results of covariance analysis (age variables and pre-intervention as covet) indicated a significant difference
between the three groups of salmeterol (60/137), tiova (98/98) and combination 0f both medications(167.64) in terms of
FEV1 variable (P <0.001) and between the three groups of salmeterol (146.41), tiova (12.51) and combination (29.22) in terms
of FVC variable (p <0.001).
Conclusion: The findings of this study confirm the significant impact of combination of two long-acting bronchodilators
with different pharmacological mechanisms in patients who need two drug classes for control of their disease is effective for
them.
2.Introduction
3.Methods
4.Discussion
5.Conclusion
6.Acknowledgement
7.References
Keywords
Acute Exacerbated COPD; Salmeterol; Tiotropium Bromide; FEV1; FVC.
Introduction
Acute exacerbation of chronic pulmonary obstructive pulmonary
disease (AECOPD) is a condition that is characterized by progressive
airway obstruction in the lungs [1]. AECOPD is a disease
that is diagnosed with three symptoms of coughing, phlegm, and
shortness of breath [2]. The World Health Organization (WHO)
estimates in 2008 that 210 million people in the world are suffering
from COPD and that the overall COPD mortality rate will
increase by more than 30% over the next 10 years [3].
COPD is the fourth leading cause of death in the world, affecting
10% of adults over 40 years of age [4]. According to a study
in Tehran, in people aged 18 years and older, the incidence of
COPD was 9.2% in 2015 [5]. AECOPD is usually associated
with reduced lung function. These patients experience symptoms
such as cough with sputum, reduced exercise tolerance, wheezing,
shortness of breath and prolonged exhalation [6]. Patients
usually complain of decreased activity levels due to shortness of
breath or fatigue, or both of them. These symptoms affect the
daily activity of patients [7]. Cough is also an important symptom
in these patients, as it causes discomfort and poor quality
of life [8]. Therefore, in order to control symptoms, improve the
health status and reduce the incidence of COPD, the necessity for
care and treatment in these patients is highly necessary [9]. Tiotropium
bromide is a long-acting inhaled drug that helps to dilate
airways and is used to manage AECOPD. Based on the evidence
from the trials, continuous therapy with tiotropium bromide has
significantly reduced AECOPD and hospitalization risk due to
exacerbation of the disease [11, 12]. Another inhaled bronchodi-lator drug used in patients with COPD is salmeterol. Salmeterol is
one of the direct-acting sympathetic mimic drugs that stimulates
beta-adrenoceptor activity and optionally selects beta-2 receptors
(a beta 2 agonist) [13]. In addition to bronchodilator properties,
it has anti-inflammatory activity [14] and decreased bio sorbent
reactivity and mucosal clarity [15]. Several controlled trials have
reported the efficacy and safety of these drug agents (tiotropium
bromide and salmeterol) as a single treatment in COPD [16-19].
Several clinical trials have been conducted on the combination
of anticholinergics and adrenergic agonist drugs as a long-term
bronchodilator, and the potential benefits of administering daily
tiotropium bromide with adrenoceptor agonists twice daily
have been reported to improve lung function and clinical positive
outcomes [20-22]. In general, considering the single efficacy
of tiotropium bromide inhaler capsules and salmeterol spray in
COPD, and regarding that most studies conducted evaluating the
combination of tiotropium bromide with Formoterol. Therefore,
the aim of this study was to investigate the effect of salmeterol,
tiotropium bromide and their combination in patients with acute
exacerbated COPD.
This study was a single-blind randomized clinical trial in 3 groups.
This study was conducted from April to March 2013 on 135 patients
with acute exacerbated COPD referred to the Emergency
Department of Shahid Beheshti Hospital in Kashan, Iran. In order
to estimate the sample size, according to the previous study
[23], with 95% confidence and statistical power of 90%, the minimum
required sample size was calculated for 38 persons in each
group, and with 20% drop, 45 persons were considered for each
group.
Individuals willing to participate in
the study, Adults over the age of 45 years with AECOPD due to
the severity of symptoms defined on the basis of the GOLD criteria
(cough, sputum production, dyspnea, or history of exposure
to risk factors) present to the hospital, Patients reporting a history
of at least 10 cigarette pocket a day , patients with documented
medical records that shows had an airway obstruction (FEV1/
FVC ratio less than 0.7 and FEV1 after treatment bronchodilator
less than 0.65), and Patients diagnosed with COPD by spirometry.
patients with allergic rhinitis,
atopy, positive skin test, patients with eosinophil count of more
than 400 mm3, patients with other pulmonary disorders or cardiopulmonary
disorders, patients with recent history of myocardial
infarction, previous failure, or arrhythmia required medication,
prostatic hypertrophy and glaucoma with closed angle [24].
Exclusion criteria included: patient's decision to withdraw, use of
other bronchodilator drugs other than salmeterol spray or tiotropium
bromide (thiourea) capsule during the study, irregular use of
salmeterol spray and tiotropium capsule in terms of the amount
and time of use and death of the patient.
Patients entered the study if they had the criteria for entering and providing comprehensive explanations by the researcher if they
were satisfied and completed a written informed consent form.
After selecting patients in a continuous manner, using a randomized
computer table, through a triple and six-member blocking
with a 1: 1: 1 assignment ratio, divided into three groups of 45
(salmeterol spray receptor group, capsule recipient group Inhaled
tiotropium bromide and the combination salmeterol and tiotropium
bromide spray group). Patients are voluntarily and free of
charge and can be removed at any stage of the study without any
restrictions.
For each of the three groups, salmeterol sprays, tiotropium bromide
and combination were administered separately during the
admission. Salmeterol spray, which was manufactured by Sina
Drug, was given three times a day at a dose of 25 micrograms.
Tiotropium Bromide Capsules Imported by Shafiabat Gostar
Pharmaceuticals using oral Handihaler was given as an inhaler
twice daily with a dose of 18 μg. The combined use of salmeterol
spray and tiotropium bromide capsules by simultaneous administration
of salmeterol spray with 25 micrograms twice daily and
tiotropium bromide, 18 micrograms once daily. Spray and inhalation
capsules were used by the nurses who were selected as research
fellow. Patients during the study period were evaluated by a
researcher and expert in the lung and were evaluated clinically as
the patient was clinically free of symptoms.
This study was conducted according to
guidelines published in the Helsinki Statement. To conduct this
study, the Ethics Committee of Kashan University of Medical
Sciences was licensed (IR.KAUMS.MEDNT.REC.1397.32) and
the study was registered on the website of the Iranian Clinical
Trials (IRCTID: IRCT20190123042466N1).
At the beginning of the study, after completing the personal information
questionnaire by self-report and with the help of the
researcher, FEV1 and FVC by Spirometry (Elite DL, MedGraphicss,
St Paul, MN, USA) Immediately before receiving Spray or
Inhaler capsules and then 0, 30 minutes, 1, 2, 4, 6, 8, 10, 12, 14, 16,
18, 20, 22 and 24 hours after medication AUCs FVE1 and AUCs
FVC through the trapezoidal rule Calculated.
Considering the fact that this study was performed on patients
with AECOPD and according to the hemodynamic condition of
the patients, 5 patients from each group were excluded from the
study because of their unwillingness to continue co-operation
and concurrent use of other bronchodilator drugs, so the analysis
Data analysis was performed on 120 patients.
The Kolmogorov-Smirnov test was used to verify normality and
confirmed. Using descriptive and inferential statistics such as Chisquare
and one-way ANOVA (to compare the status of demographic
variables in the three study groups), paired t-test was used
to evaluate the mean FEV1 AUCs and FVC AUCs 2 times before
and after the intervention in three The covariance test was used
to remove the confounding factors and Bonferron's post hoc test
to compare two drugs. Data were analyzed by SPSS software (Version 16.0, Chicago, USA). In all cases, the significance level was
considered to be 0.05.
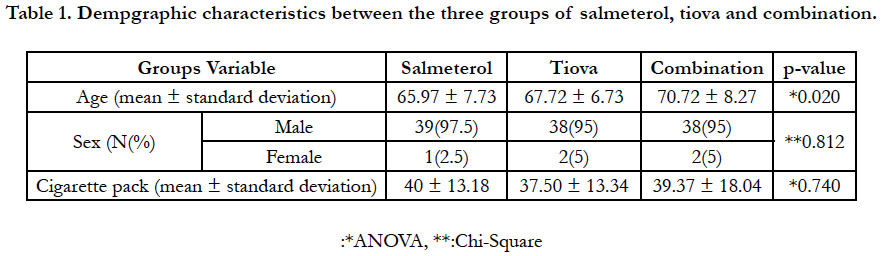
In this study, 120 people entered the final analysis stage (Figure
2). There was no significant difference between the two groups
in terms of demographic characteristics. However, there was a
significant difference in age (P = 0.02) between the three groups
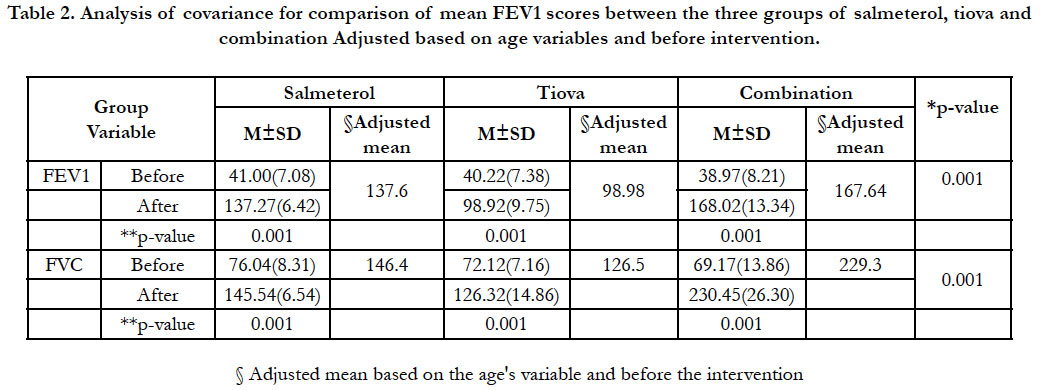
(Table 1). The results of covariance analysis (age, FVC, and
FEV1 variables before intervention as covet) indicated a significant
difference between the three groups of salmeterol (60.137),
tiova (98/98) and combination (167.64) in terms of FEV1 variable
AUCs, as well as between the three groups of salmeterol
(144/40), tiova (12/50) and combination (292,230) in terms of
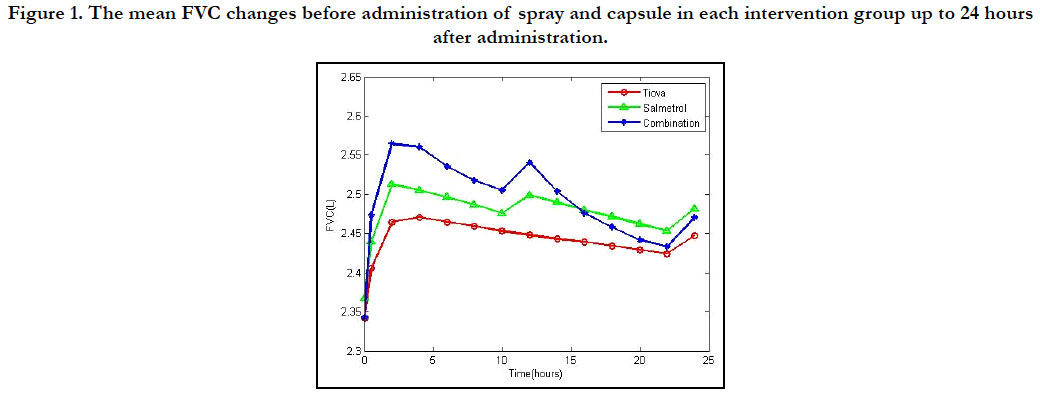
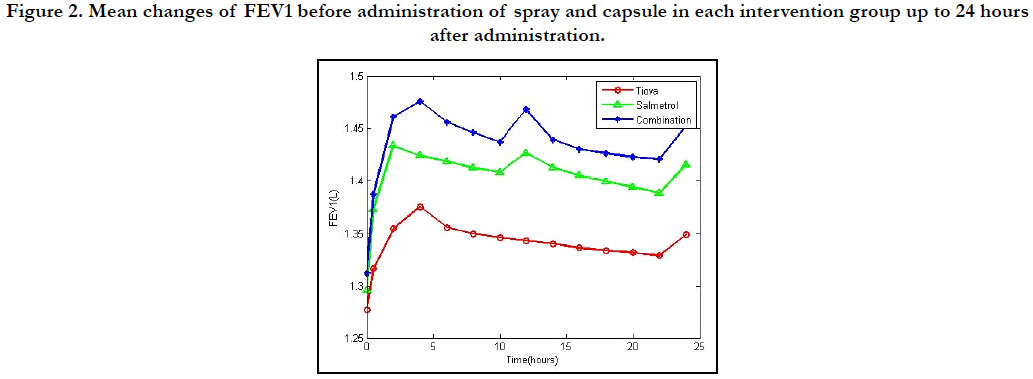
variables AUCs FVC (p < 0.001) (Figures 1 and 2). Before and after
intervention, the FEV1 AUCs and FVC AUCs were observed
in each of the three groups (p < 0.001) (Table 2). Following the
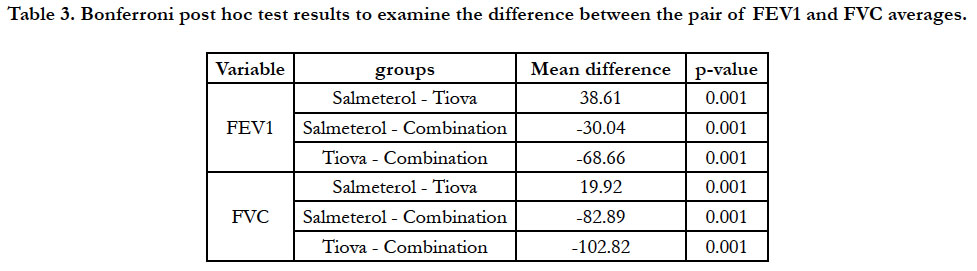
significant difference between the three groups, a follow-up test
was used to examine the pair of meanings, the results of which
are presented in Table 3.
Figure 1. The mean FVC changes before administration of spray and capsule in each intervention group up to 24 hours after administration.
Figure 2. Mean changes of FEV1 before administration of spray and capsule in each intervention group up to 24 hours after administration.
Table 2. Analysis of covariance for comparison of mean FEV1 scores between the three groups of salmeterol, tiova and combination Adjusted based on age variables and before intervention.
Table 3. Bonferroni post hoc test results to examine the difference between the pair of FEV1 and FVC averages.
Discussion
The aim of this study was to investigate the effect of salmeterol,
tiotropium bromide and their combination in patients with acute
exacerbation of chronic obstructive pulmonary disease. The findings
of the study showed that the average of the AUCs of FEV1
and AUCs of FVC in the combined group of both groups and
in the salmeterol group was significantly higher than the tiotropium
bromide group. Together with this finding, Oba et al., (2018)
found that the combination of long-acting muscarinic antagonist
(LAMA) and long-acting beta-agonist (LABA) with the highest
therapeutic effect has been exacerbated by COPD reduction [25].
Noord et al., (2010) also in another study found that, 24 hours after treatment, the combined effect of Salmeterol-Tiova on average
of FEV1 and FVC was significantly higher than that of the two
drugs separately [26]. Pasco et al., (2009) found that, compared
to placebo, Tiova with salmeterol and pulmonary rehabilitation
were associated with improved pulmonary function (improved
FEV1 and FVC) [27]. Aaron et al., (2007) also reported that the
combination of Tiova, Salmeterol and Fluticasone significantly
improved pulmonary function and quality of life, also reduced
the number of hospitalization days associated with exacerbated
COPD compared to placebo and Tiova [28].
In confirmation of this finding, Marco et al., (2006) found in
their study that a significant improvement of FEV1 and FVC was
achieved after 0-12 hours after the combination of Formetroline
and Tiova compared to the separate doses of the two drugs [23].
Villar et al. (2005) also found that the use of the Fluticasone
-salmeterol-Tiova combination significantly improved the lung
function (FEV1) in patients with moderate to severe COPD
compared with the combination of Fluticasone -Tiova-placebo
and the combination of Fluticasone - Tiova-placebo [29]. Other
study findings (2004), additionally revealed that using Formetrol
(as a long-acting beta-agonist) in combination with Tiova initiated
a fast and maximal bronchodilation effect compared to single use
of Tiova [30]. Harrison (2018), cited that prescription of both
inhaled beta-agonist and muscarinic antagonist therapy, caused
improvement in lung function, so that the effect of combination
therapy is more effective than separate doses in reducing exacerbations
[31].
Bronchodilation can be achieved by stimulating adrenergic betaagonist
receptors or by inhibiting acetylcholine action in muscarinic
receptors with anticholinergic agents [32]. Due to the fact that beta-agonists and anticholinergics have different mechanisms
of action that can involve different airway locations, and both
drugs have long-term binding to the recipients, it provides the
possibility of sustained bronchodilation. It is expected to observe
additional effects due to potential beta-agonists which directly affect
the cholinergic system.
Conclusion
The findings of this study confirm the significant impact of combination
of two long-acting bronchodilators with different pharmacological
mechanisms in patients who need two drug classes
for control of their disease is effective for them.
Acknowledgement
This study has been approved by ethical committee of Kashan
University of Medical Sciences with the code of IR.KAUMS.
REC.1397.085 The authors are grateful to all the patients who
participated in the study and the supervisors of Shahid Beheshti
hospital.
References
- Cheyne L, Irvin-Sellers MJ, White J. Tiotropium versus ipratropium bromide for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013; 9. PMID: 24043433.
- Nazir SA, Erbland ML. Chronic obstructive pulmonary disease. Drugs & aging. 2009; 26(10): 813-31.
- Statistics - COPD Foundation. https://www.copdfoundation.org/What-is- COPD/Understanding HYPERLINK "http://www.copdfoundation.org/ What-is-COPD/Understanding-COPD/Statistics.aspx"-COPD/Statistics. aspx 2017 [cited 47 1]. 207-14.
- Varmaghani M, Farzadfar F, Sharifi F, Rashidian A, Moin M, Moradi-Lakeh M, et al. Prevalence of asthma, COPD, and chronic bronchitis in Iran: A systematic review and meta-analysis. Iranian Journal of Allergy, Asthma and Immunology. 2016; 15(2): 93-104. PMID: 27090362.
- Sharifi H, Masjedi MR, Emami H, Ghanei M, Eslaminejad A, Radmand G, et al. Burden of obstructive lung disease study in Tehran: Prevalence and risk factors of chronic obstructive pulmonary disease. Lung India: official organ of Indian Chest Society. 2015; 32(6): 572-577. PMID: 26664162.
- Brunner LS. Brunner & Suddarth's textbook of medical-surgical nursing: Lippincott Williams & Wilkins; 2010.
- Meek PM, Lareau SC. Critical outcomes in pulmonary rehabilitation: assessment and evaluation of dyspnea and fatigue. Journal of rehabilitation Research and Development. 2003; 40(5): 13 - 24. PMID: 15074450.
- McGarvey L, Morice A. Clinical cough and its mechanisms. Respiratory physiology & neurobiology. 2006; 152(3): 363-71. PMID: 16406741.
- O’donnell DE, Hernandez P, Kaplan A, Aaron S, Bourbeau J, Marciniuk D, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease–2008 update–highlights for primary care. Canadian Respiratory Journal. 2008; 15(Suppl A): 1A-8A. PMID: 18292855.
- Gerald LB, Bailey WC. Global initiative for chronic obstructive lung disease. Journal of Cardiopulmonary Rehabilitation and Prevention. 2002; 22(4): 234-44.
- Farne HA, Cates CJ. Long‐acting beta2‐agonist in addition to tiotropium versus either tiotropium or long‐acting beta2‐agonist alone for chronic obstructive pulmonary disease. The Cochrane Library. 2015. PMID: 26490945.
- Barr RG, Bourbeau J, Camargo CA, Ram FS. Tiotropium for stable chronic obstructive pulmonary disease: a meta-analysis. Thorax. 2006; 61(10): 854- 62. PMID: 16844726.
- Chowdhury BA, Dal Pan G. The FDA and safe use of long-acting beta-agonists in the treatment of asthma. New England Journal of Medicine. 2010; 362(13): 1169-71. PMID: 20181964.
- Anderson R, Feldman C, Theron A, Ramafi G, Cole P, Wilson R. Antiinflammatory, membrane‐stabilizing interactions of salmeterol with human neutrophils in vitro. British journal of pharmacology. 1996; 117(7): 1387- 94. PMID: 8730730.
- Johnson M, Rennard S. Alternative mechanisms for long-acting β2- adrenergic agonists in COPD. Chest. 2001; 120(1): 258-70. PMID: 11451847.
- Dransfield MT, Bailey WC. Maintenance pharmacotherapy of chronic obstructive pulmonary disease: an evidence-based approach. Expert opinion on pharmacotherapy. 2005; 6(1): 13-25. PMID: 15709879.
- Cooper C, Tashkin D. Recent developments in inhaled therapy in stable chronic obstructive pulmonary disease. BMJ: British Medical Journal. 2005; 330(7492): 640-644. PMID: 15774995.
- Chen AM, Bollmeier SG, Finnegan PM. Long-acting bronchodilator therapy for the treatment of chronic obstructive pulmonary disease. Annals of Pharmacotherapy. 2008; 42(12): 1832-42. PMID: 18957624.
- Friedman M, Cioppa GD, Kottakis J. Formoterol therapy for chronic obstructive pulmonary disease: a review of the literature. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2002; 22(9): 1129-39. PMID: 12222549.
- Van Noord J, Aumann J, Janssens E, Smeets J, Verhaert J, Disse B, et al. Comparison of tiotropium once daily, formoterol twice daily and both combined once daily in patients with COPD. European Respiratory Journal. 2005; 26(2): 214-22. PMID: 16055868.
- Van Noord JA, Aumann JL, Janssens E, Verhaert J, Smeets JJ, Mueller A, et al. Effects of tiotropium with and without formoterol on airflow obstruction and resting hyperinflation in patients with COPD. Chest. 2006; 129(3): 509-17. PMID: 16537846.
- Tashkin DP, Littner M, Andrews CP, Tomlinson L, Rinehart M, Denis- Mize K. Concomitant treatment with nebulized formoterol and tiotropium in subjects with COPD: a placebo-controlled trial. Respiratory medicine. 2008; 102(4): 479-87. PMID: 18258423.
- Di Marco F, Verga M, Santus P, Morelli N, Cazzola M, Centanni S. Effect of formoterol, tiotropium, and their combination in patients with acute exacerbation of chronic obstructive pulmonary disease: a pilot study. Respiratory medicine. 2006; 100(11): 1925-32. PMID: 16626956.
- Cazzola M, Centanni S, Santus P, Verga M, Mondoni M, Di Marco F, et al. The functional impact of adding salmeterol and tiotropium in patients with stable COPD. Respiratory medicine. 2004; 98(12): 1214-21. PMID: 15588043.
- Oba Y, Keeney E, Ghatehorde N, Dias S. Dual combination therapy versus long‐acting bronchodilators alone for chronic obstructive pulmonary disease (COPD): a systematic review and network meta‐analysis. Cochrane Database of Systematic Reviews. 2018; 12(12). PMID: 30521694.
- Van Noord J, Aumann J-L, Janssens E, Smeets J, Zaagsma J, Mueller A, et al. Combining tiotropium and salmeterol in COPD: effects on airflow obstruction and symptoms. Respiratory medicine. 2010; 104(7): 995-1004. PMID: 20303247.
- Pasqua F, Biscione G, Crigna G, Auciello L, Cazzola M. Combining triple therapy and pulmonary rehabilitation in patients with advanced COPD: a pilot study. Respiratory medicine. 2010; 104(3): 412-7. PMID: 19892540.
- Aaron SD, Vandemheen KL, Fergusson D, Maltais F, Bourbeau J, Goldstein R, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone– salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Annals of internal medicine. 2007;146(8):545-55. PMID: 17310045.
- Villar AB, Pombo CV. Bronchodilator efficacy of combined salmeterol and tiotropium in patients with chronic obstructive pulmonary disease. Archivos de Bronconeumología. 2005;41(3):130-4. PMID: 15766465.
- Cazzola M, Di Marco F, Santus P, Boveri B, Verga M, Matera MG, et al. The pharmacodynamic effects of single inhaled doses of formoterol, tiotropium and their combination in patients with COPD. Pulmonary pharmacology & therapeutics. 2004;17(1):35-9. PMID: 14643169.
- Malani PN. Harrison’s principles of internal medicine. JAMA. 2018;308(17):1813-4.
- Cazzola M, Centanni S, Santus P, Verga M, Mondoni M, Di Marco F, et al. The functional impact of adding salmeterol and tiotropium in patients with stable COPD. Respiratory medicine. 2004; 98(12):1214-21. PMID: 15588043.
- Vestbo J, Agustí A, Anzueto A, Decramer M, Fabbri L, Jones P. Global strategy for the diagnosis, management and prevention of COPD, Global Initiative for chronic Obstructive Lung Disease (GOLD) 2015. 2015.
- Plankeel JF, McMullen B, MacIntyre NR. Exercise outcomes after pulmonary rehabilitation depend on the initial mechanism of exercise limitation among non-oxygen-dependent COPD patients. Chest. 2005; 127(1):110-6. PMID: 15653970.