Clinical Profile and Predictors of Mortality in H1N1 and Non-H1N1 Related ARDS in a Tertiary Care Center in South India
Mahendra M1, Jayaraj BS2, Chaya SK3, Mahesh PA4*
1 Assistant Professor in Pulmonary Medicine, Shimoga Institute of Medical Sciences, Shivamogga, Karnataka, India.
2 Professor of Pulmonary medicine, JSS Medical College, JSS University, Mysore, India.
3 Assistant Professor in Pulmonary Medicine, JSS Medical College, JSS University, Mysore, India.
4 Professor and Head of Pulmonary Medicine, JSS Medical College, JSS University, Mysore, India.
*Corresponding Author
Dr. Mahesh PA, DNB (TB and respiratory disease),
Professor and Head of Pulmonary Medicine,
JSS Medical College, JSS University, Mysore, India.
Tel: 9448044003
E-mail: mahesh1971in@yahoo.com
Received: May 29, 2018; Accepted: July 19, 2018; Published: July 27, 2018
Citation:Mahendra M, Jayaraj BS, Chaya SK, Mahesh PA. Clinical Profile and Predictors of Mortality in H1N1 and Non-H1N1 Related ARDS in a Tertiary Care Center in South India. Int J Resp Dis Care Med. 2018;3(2):54-61. doi:dx.doi.org/10.19070/2577-4409-180009
Copyright: Mahesh PA© 2018. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Introduction: H1N1 influenza has caused significant mortality across the world mostly due to ARDS. It is important to differentiate H1N1 ARDS from non-H1N1 ARDS for early treatment and also to study predictors of mortality in ARDS patients.
Methodology: Prospective study was done in patients with ARDS admitted to RICU. Clinical characteristics and outcomes were compared among H1N1 and non-H1N1 ARDS. Cox regression was done for survival analysis.
Results: 97 patients were admitted with ARDS. 59 were positive for H1N1. H1N1 ARDS patients had higher APACHE2 score, shorter duration of symptoms, worse hypoxemia compared to non-H1N1 ARDS. There was no difference in mortality between H1N1 and non-H1N1 ARDS. On multivariate Cox regression analysis, higher APACHE2 score, need for invasive ventilation and prolonged ICU were associated with increased mortality.
Conclusion: Acute respiratory symptoms with severe hypoxemia, lymphopenia can be helpful to suspect H1N1 influenza in periphery for early referral. On multivariate Cox regression, higher APACHE2 score, prolonged ICU and need for invasive ventilation were independently associated with higher mortality.
2.Abbreviations
3.Introduction
4.Methodology
4.1 Study Design
4.2 Data Collection
4.3 Sputum Collection
4.4 Induced Sputum
4.5 Bronchoalveolar Lavage (BAL)
4.6 Blood Culture
4.7 Ethics
4.8 Statistical Methods
5.Results
6.Discussion
7.Conclusion
8.References
Keywords
H1N1; non-H1N1; ARDS; Mortality; ICU.
Abbreviations
OGTT: Oral Glucose Tolerance Test; APACHE II: Acute Physiology and Chronic Health Evaluation II; CDC: Centers for Disease Control and Prevention; BAL: Bronchoalveolar Lavage; NIV: Noninvasive ventilation; FIO2: Fraction of Inspired Oxygen; ADA: American Diabetes Association; FPG: Fasting Plasma Glucose.
Introduction
H1N1 influenza pandemics have caused significant mortality and healthcare burden across the world mostly due to lower respiratory tract infections. In the recent pandemic caused by novel H1N1 influenza virus in 2009 resulted in 277,607 cases and 3205 deaths [1]. Although there were few sporadic cases in post pandemic phase, WHO had warned of possible localized outbreaks especially in India and New zealand [2]. In 2014, there was resurgence of H1N1 epidemic in India causing 33000 cases that claimed 2000 lives [3]. H1N1 influenza presents with a broad spectrum of clinical manifestations ranging from mild flu to fulminant pneumonia and acute respiratory distress syndrome (ARDS). Patients with H1N1 pneumonia often exhibit rapidly progressive refractory hypoxemia requiring invasive ventilation in around 80% of patients admitted to ICU [4].
Incidence of ARDS was found to be 6.6-19.7 per 100000 personyears in various studies done across world [5-8]. Worldwide, severe sepsis is most common trigger for ARDS. Other triggers include Pneumonia, polytrauma, severe burns, massive blood transfusion and Pancreatitis. Etiology of ARDS in India differs from western countries with higher incidence of ARDS due to tropical diseases like malaria, dengue, leptospirosis next to Pneumonia [9]. In developed countries mortality rates in these ARDS patients have been showing a declining trend which was 70% in 1990 to 51% in 1999 to 26% in 2005 mainly due to better management of ventilation strategies [10-12]. In India mortality rates are comparatively higher and vary between 44-57% [9-13].
It is observed that H1N1 pneumonia causes more severe disease with higher mortality compared to other community acquired pneumonia [14]. It is noteworthy that H1N1 affects relatively younger healthy population [15]. It is very important to differentiate clinically between H1N1 and non-H1N1 ARDS patients during flu outbreak in order to identify, triage and treat H1N1 pneumonia early with antiviral agents and also it is important to study predictors of mortality among patients with ARDS as there is scarce data in Indian population.
We conducted a prospective observational study of all patients presenting with acute onset of fever, cough and dyspnea with bilateral opacities on chest x-ray admitted to Respiratory ICU in a tertiary care teaching hospital during the period December- June 2016. All patients were subjected to throat swab for H1N1. Patients getting admitted to ICU with ARDS were included into study after obtaining informed consent. Clinical characteristics, Course of illness, radiological and laboratory investigations and survival were compared among patients with H1N1 and non-H1N1 ARDS patients. Patients who succumbed within 1 hour of admission and patients refusing to consent were excluded from the study.
We collected information on patient demographics, clinical symptoms and duration, comorbid conditions like diabetes, hypertension, COPD and IHD, risk factors such as smoking and alcohol consumption were recorded. Clinical parameters such as pulse rate, respiratory rate, blood pressure and oxygen saturation and laboratory parameters such as chest x-ray, complete hemogram, serum electrolytes, liver function test, renal function tests and arterial blood gas analysis were recorded. Severity of illness was assessed using Acute Physiology and Chronic Health Evaluation II (APACHE II) at admission.
Description of procedures and investigations undertaken: Throat swab for H1N1 was taken on the day of admission. Single sample of nasopharyngeal swab was taken under strict aseptic precautions from the posterior pharyngeal wall and bronchial-aspirate samples were obtained from patients on ventilator and put in a sterile container containing 3ml of viral transport media - HiViral (Himedia) and sent in cold chain, to Kasturba Medical College (KMC), Manipal, which is a government approved lab for detecting H1N1. Samples reached the lab within 24-48 hours. The samples were analyzed by Taqman Real-Time PCR for influenza-A, influenza-B, swine flu-A, swineflu-H1 in accordance with published guidelines from the U.S. Centers for Disease Control and Prevention (CDC). Results were obtained on 5th day from the day of sample dispatch.
A single sputum sample in a sterile wide mouthed container was obtained within 24 hours of admission to ICU, early morning sample whenever possible. At least 15 ml of sputum was collected and sample was considered adequate if the sample was mucopurulent on gross visual examination. Sample was sent to lab immediately. Specimen was considered satisfactory if the sample had than 10 squamous epithelial cells and ≥25 leukocytes per high power field, as well as presence of alveolar macrophages. Blood agar and Mac- Conkey agar was used for quantitative cultures and >106 CFU/ml on quantitative culture was considered pathological.
In patients who were conscious and unable to expectorate satisfactory sputum sample, induction of sputum was done using 20 ml of 3% saline nebulization in a well ventilated room for 5 minutes. Patient was asked to attempt forceful cough and sputum yield of 2ml was considered satisfactory. Procedure was terminated if patient complained of dyspnea, chest pain and wheeze. Patients with hemodynamic instability, asthma, hypoxemia (SPO2 <88% at room air) were excluded from this procedure [16].
BAL was done in selected patients to rule out malignancy and in whom microbiologic diagnosis was not established. Bronchoalveolar was performed as per ATS guidelines [17]. BAL was taken using a portable flexible bronchoscopy Olympus, 2.6mm diameter, around 100-120 ml of normal saline was instilled through bronchoscope and instilled saline was retrieved using negative suction pressure of 50-80 mm Hg adjusted to avoid visible airway collapse. Sample was considered adequate if the minimal total volume retrieved was more than or equal to 5% of the instilled volume. BAL samples were processed similar to sputum samples. BAL samples were considered satisfactory if there were ciliated cells, alveolar macrophages and <5% squamous epithelial cells. BAL quantitative culture >104 CFU/ml was considered pathological.
Blood culture was done in patients with suspected bacteremia and sepsis. Skin was cleaned with 70% isopropyl alcohol and two sets of blood cultures of 10 ml each were taken from each arm.
Patients requiring mechanical ventilation, requiring a fraction of inspired oxygen (FiO2) greater than or equal to 60%, receiving intravenous infusion of inotropes or vasopressors were admitted to ICU. A trial of non-invasive ventilation (NIV) was considered as per the recent guidelines for acute respiratory failure [18]. Severity of ARDS was assessed by PaO2/FiO2 ratio (200-300- mild, 100-200- moderate and <100 severe). Patients with ARDS were managed as per ARDS net protocol with low tidal volume and high PEEP [19].
Non invasive ventilation was tried in all patients with PaO2/FiO2 >150 mm Hg. Face mask (Philips respironics) was used to deliver NIV via ventilator in all patients. Size of face mask was selected based on measurement from eyebrows to bottom of the chin to ensure a tight but comfortable seal. NIV titrations of pressures were set according to standard protocol: inspiratory pressure was increased in increments of 2-3 cm H2O to achieve respiratory rate <25/min and disappearance of usage of accessory muscle of respiration. PEEP was increased in 2-3 cm H2O increments and aimed to achieve saturation >90% with FiO2 <60%. Patients with impending cardiorespiratory failure, Glascow coma score <8, Vomiting, tracheostomy, increased intracranial pressure and uncooperative patients were excluded from NIV trial. Vitals were closely monitored and if there was no improvements in vitals and if clinical symptoms worsen in half an hour of NIV trial, patients were switched to invasive ventilation.
Arterial Blood gas analysis was done in ABL FLEX 800 machine (Radiometer), Serum electrolytes in DIESTRO electrolyte analyzer, liver and renal function tests in TOSHIBA TBA 120 FR a fully automated chemistry analyzer. Complete hemogram was analyzed in NIHON KOHDEN (5 part differential cell counter) and SYSMEX (6 part differential cell counter). Hemoglobin was quantified by Cyanmeth hemoglobin method and platelet count and total count was quantified using Flow-cytometer.
We defined ARDS and severity of ARDS as per Berlin definition,” the acute onset of respiratory failure, bilateral infiltrates on chest radiograph, hypoxemia as defined by a PaO2/FiO2 ratio ≤300 mmHg, and no evidence of left atrial hypertension or a pulmonary capillary pressure <18 mmHg (if measured) to rule out cardiogenic edema” [20].
We defined lymphopenia as lymphocytes ≤ 20% of total leukocytes [21] and Leukopenia as WBC count <4.5X 109/ml [22].
We defined smoking as “ An adult who has smoked 100 cigarettes in life time and who currently smokes cigarettes” [23].
We defined Alcoholism as chronic alcohol use to the degree that interferes with physical and mental health, or with normal social or work behavior [24].
We defined Diabetes mellitus as per American Diabetes Association (ADA) as, A hemoglobin A1c (HbA1c) level of 6.5% or higher or A fasting plasma glucose (FPG) level of 126 mg/dL (7 mmol/L) or higher; fasting is defined as no caloric intake for at least 8 hours, or A 2-hour plasma glucose level of 200 mg/dL (11.1 mmol/L) or higher during a 75-g oral glucose tolerance test (OGTT), or A random plasma glucose of 200 mg/dL (11.1 mmol/L) or higher in a patient with classic symptoms of hyperglycemia (ie, polyuria, polydipsia, polyphagia, weight loss) or hyperglycemic crisis [25].
We defined Hypertension as per blood pressure >130 mm Hg systolic and >80mm Hg diastolic pressure.
The Institutional Ethics Committee approved the study IEC no-JSS/MC/IEC/05/5237/2015-16. Informed consent from patient/legal representative was taken prior to inclusion in the study.
Descriptive data are presented as frequencies (percentages) for discrete variables and as means (SDs) for continuous variables. For comparisons between two groups, Mann-Whitney U test was used or, when appropriate, the two-sample t-test. Chi-square test was used to evaluate categorical factors. Kaplan meier analysis and Cox regression analysis was done for survival in patients with ARDS. All statistical tests were 2-tailed, and factors were considered statistically significant at p <0.05. IBM SPSS version 22 and CDC Epi Info version 7 was used for analysis.
Results
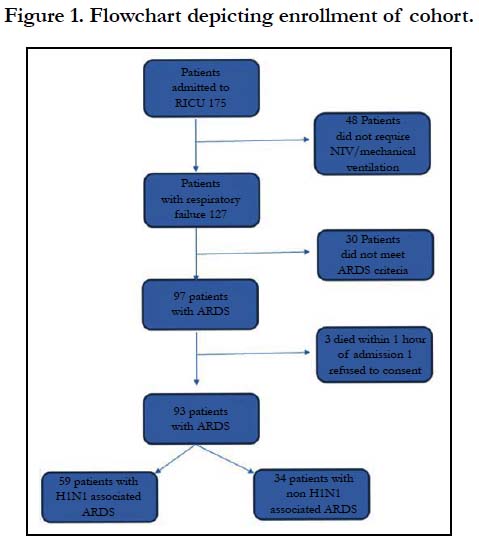
In our prospective study, we found 97 patients (55.4% of total) were admitted to respiratory ICU with diagnosis of ARDS (Figure 1). Four patients were excluded from the study (three patients succumbed within 1 hour of admission and 1 patient refused to consent). Fifty nine patients tested positive for H1N1 and 34 patients tested negative for H1N1. Patients with H1N1 ARDS had higher proportion of Cough (100%), Dyspnea (94.92%) and sore throat (42.37%) at presentation compared to non-H1N1 ARDS patients. Co-morbidities were noted in 42(45%) of patients and diabetes mellitus (27.96%) was the most common comorbidity. All patients were started on oseltamivir 75mg BD based on clinical symptoms as per guidelines given by WHO [26] and was stopped if throat swab for H1N1 was negative.
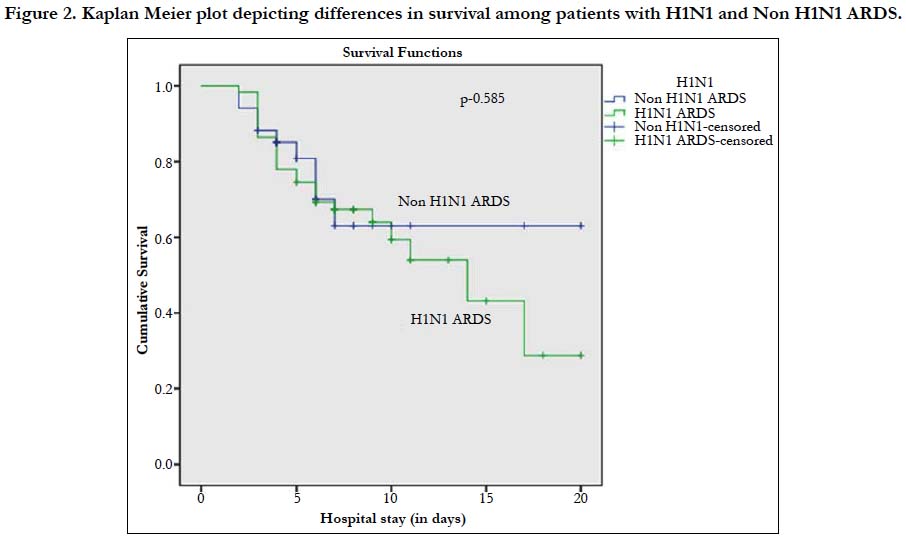
Forty four percent (26/59) of patients with H1N1 associated ARDS needed invasive ventilation and Forty seven percent (28/59) of patients needed NIV. Twenty six percent (9/34) of patients needed invasive ventilation and Seventeen percent (6/34) of patients were put on NIV among patients with non-H1N1 ARDS. We identified organisms other than H1N1 in 28% (26/93) of the patients. Isolation rate of organisms varied biological specimens: Blood culture (4/14, 28.5%), Endotracheal aspirate (12/35, 34.3%), Sputum culture (3/13, 23%) BAL (6/8, 75%), Urine culture (1/3,33%).Ventilator associated pneumonia was seen in 12 patients (36.36%). Acinetobacter baumanii was the most common organism isolated (9/26, 34.6%) followed by Streptococcus (6/26, 23%), Klebsiella pneumoniae (5/26, 19.2%). We did not observe barotrauma in patients on ventilator. Mortality rate among patients with H1N1 associated and non-H1N1 associated ARDS was 40.67% and 26.47% respectively (Figure 2).
Figure 2. Kaplan Meier plot depicting differences in survival among patients with H1N1 and non-H1N1 ARDS.
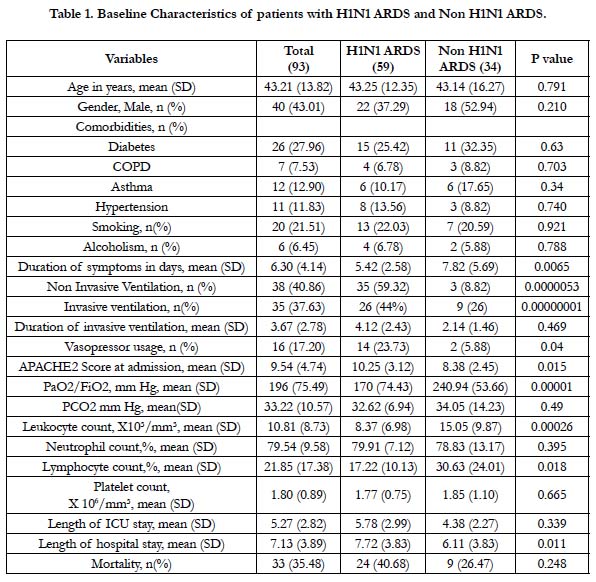
We found H1N1 associated ARDS patients had higher APACHE2 score and worse hypoxemia at admission compared to non-H1N1 ARDS (Table 1). Duration of hospitalization was more in patients with H1N1 associated ARDS (7.72 vs 6.11 days). There was significantly lower leukocyte count in H1N1 associated ARDS patients (8.37 vs 15.05 X10³/mm³).We found lower lymphocyte count in patients with H1N1 associated ARDS (34 % vs 59%). However we did not find any statistical difference in mortality rates among patients with and without H1N1 associated ARDS.
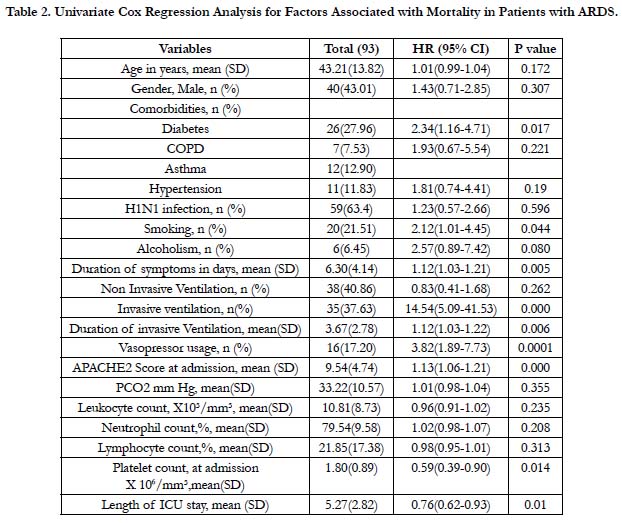
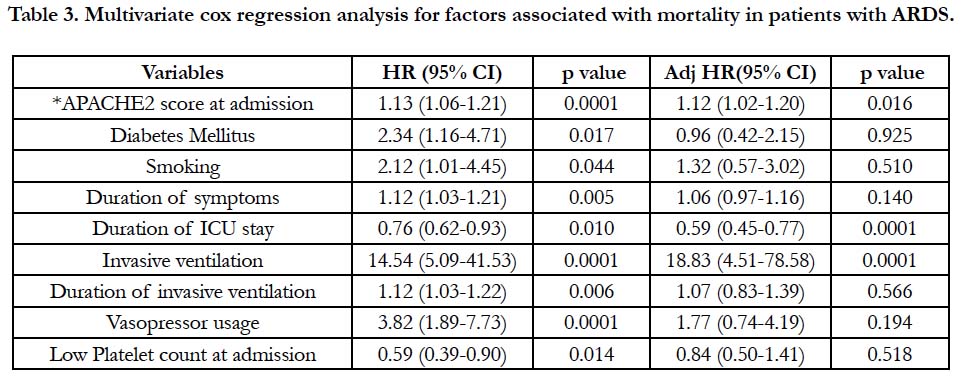
We found on univariate Cox regression analysis, patients with ARDS with higher APACHE2 score, presence of diabetes, smoking, prolonged ICU stay, use of invasive ventilation, prolonged duration of invasive ventilation, requiring vasopressors, longer duration of symptoms, low platelets were associated with increased risk for mortality (Table 2). On multivariate Cox regression analysis, we found patients with higher APACHE2 score, need for invasive ventilation and prolonged ICU stay were associated increased hazard for mortality (Table 3).
Table 2. Univariate Cox Regression Analysis for Factors Associated with Mortality in Patients with ARDS.
Table 3. Multivariate cox regression analysis for factors associated with mortality in patients with ARDS.
Discussion
The epidemic of H1N1 in 2015 claimed many lives in India mostly young and previously healthy. The global H1N1 2009 pandemic has been studied well, but the epidemic that occurred in India has sparse data. H1N1 patients have more severe ARDS than non-H1N1 ARDS patients requiring advanced rescue strategies [27, 28]. In the present study we observed that patients with H1N1 associated ARDS had shorter duration of symptoms, higher incidence of sorethroat, higher APACHE2 score at admission, need for invasive ventilation, vasopressor usage, relative lymphopenia, lower leukocyte count compared to non-H1N1 ARDS patients. Although nearly 46% of patients with H1N1 ARDS required invasive ventilation, there was no difference in mortality rates among H1N1 and non-H1N1 ARDS patients. On Multivariate cox regression analysis for mortality predictors among ARDS patients, we found higher APACHE2 score, need for mechanical ventilation and shorter duration of ICU stay were independently associated with increased hazard for mortality.
Factors that are more commonly observed in H1N1 ARDS patients than in non-H1N1 pneumonia patients include younger age, female gender, leucopenia, obesity, lesser comorbidties, lower Creactive protein, higher lactate dehydrogenase, lower PaO2/FiO2 ratio, bilateral radiological opacities [29-35]. A 5 point regression model was developed by Bewick et al., to identify clinical variables most predictive of H1N1 pneumonia which included age <65, WBC <12000/mm³, bilateral radiological opacities, oriented mental status, temperature >38°C [34]. We did not find any statistical difference in age and temperature between the cohort however we did find lower WBC counts and bilateral radiological opacities in patients with H1N1 ARDS patients which was statistically significant.
There are several noteworthy observations in patients with H1N1 ARDS compared to non-H1N1 ARDS. First, we found H1N1 associated ARDS patients had higher severity of illness as identified by APACHE2 score than non-H1N1 ARDS at admission. Higher APACHE 2 score was largely driven by severe hypoxemia and we noted less extra pulmonary organ failure in H1N1 ARDS patients. Development of severe ARDS in H1N1 influenza pneumonia may be due to induction of aberrant immune response to virulent viral infection, causing extensive lung damage [36]. It also been observed that patients with H1N1 pneumonia are more predisposed to pulmonary embolism there by accounting for refractory hypoxemia, although there is no sufficient data to substantiate it [37]. A retrospective study done in Delhi in 2009-10 found patients with H1N1 ARDS had severe hypoxemia similar to our study [38]|. Similarly an US study in 2009 also found severe hypoxemia as a major factor in higher severity of illness score in H1N1 associated ARDS patients [27]. An Indian study done in 2009 also found severe hypoxemia to be predominant feature in all patients with H1N1 patients [39].
Second, we found patients with H1N1 ARDS had lower lymphocyte and leukocyte count than non-H1N1 ARDS. Lower leukocyte count suggests low inflammatory response unlike in bacterial infections. Lymphocytes in blood participate in variety of host defense mechanisms against viral infections. It has been recognized that in-vivo influenza infection transiently depress the number of circulating lymphocytes and their response probably by interfering with ‘helper T cell’ lymphocyte function [40]. Relative lymphopenia has been observed as a surrogate marker for H1N1 influenza in many studies previously [41-43]. Criswell studied the lymphocyte response to influenza infection in human volunteers and found reduction of all subpopulation of lymphocytes during the illness after inoculation of influenza A virus to volunteers and reduction in B-lymphocytes occur on day 3 who exhibit infection but no changes occurred in uninfected volunteers. This implicates that relative lymphopenia is a reliable surrogate marker for H1N1 influenza infection [44].
Last, although patients with H1N1 ARDS have more severe disease we did not find any difference in mortality rates between H1N1 and non-H1N1 ARDS. Similar findings were noted in studies by Riscili [27] and Samra [38]. In contrast a retrospective Brasilian study done by Nardocci et al., found higher mortality in H1N1 patients compared non-H1N1 pneumonia patients (40% vs 20%) [29]. The possible explanation could be due to selection bias as each H1N1 patient were matched at a 2:1 ratio with consecutive cases of Community acquired pneumonia admitted to ICU.
Mortality in ARDS is approximately 34-58% [45]. The past few decades have not seen an improvement in ARDS mortality rates despite of availability of advanced ventilator strategies [46]. We found mortality rate of 35.48% in our study consistent with previous studies. ARDS etiology may affect the prognosis [47] but could not be confirmed in other studies [48, 49]. However we did not find any difference in mortality between different etiologies in our study. Various factors such as cardiac disease [32] cirrhosis [47], obesity [33], thrombocytopenia [50], elevated LDH [32], elevated creatine kinase [51], sepsis [52], old age, length of mechanical ventilation [47], need for invasive ventilation [8], APACHE II scores [53, 54], SAPS II score [47], vasopressor usage [55] were observed as independent predictors of mortality in different studies. APACHE2 score has been a reliable marker for mortality in patients with ARDS. A german study on ARDS patients found APACHE2 score to be the only clinical predictor for mortality [56], similar to our study. We found shorter ICU stay to be protective for mortality possibly due to early transfer out of patients from ICU with less severe disease.
There are several limitations in our study. First our results may not generalized because it was a single centre with a relatively small sample size. There may be many confounding variables which we have not included in our study like fluid management strategy, blood transfusions, changes in ventilator settings made during the course of illness which are known to influence oxygenation in ARDS patients. Our centre was not equipped with advanced rescue ventilation strategies like ECMO, HFOV. Second, we have not used SOFA score to sequentially monitor organ functions. Third, tests for detection of other viruses like respiratory syncytial virus, adenovirus and rhinovirus could not be done due to lack of resources. Last, we have taken single throat swab for H1N1 confirmation but yield of positive PCR for H1N1 with throat swab is around 68% and lower respiratory tract samples is 81% there by misclassifying few cases of H1N1 cases into non-H1N1 category [57].
Conclusion
Presence of acute clinical symptoms like presence of dyspnea, sore throat and evidence of severe hypoxemia, relative lymphopenia and leucopenia on lab parameters can be helpful to suspect H1N1 influenza patients in the primary care center as well as an emergency setting which aids the physician for early referral and treatment. On multivariate Cox regression analysis, higher APACHE2 score, shorter ICU stay and need for invasive ventilation was independently associated with higher mortality in patients with ARDS.
References
- WHO [Internet]. Pandemic (H1N1) 2009. [cited 2010 Aug 10]. Available from: http://www.who.int/csr/disease/swineflu/en/
- WHO [Internet]. What is post-pandemic?. [cited 2010 Aug 10]. Available from: http://www.who.int/csr/disease/swineflu/frequently_asked_questions/ post_pandemic/en/
- Indian swine flu outbreak [Internet]. India: [cited 2015 Jan 1]. Office of Wikimedia Foundation, Inc. Available from: https://en.wikipedia.org/ wiki/2015_Indian_swine_flu_outbreak
- Ramsey C, Kumar A. H1N1: viral pneumonia as a cause of acute respiratory distress syndrome. Curr Opin Crit Care. 2011 Feb;17(1):64-71. doi: 10.1097/MCC.0b013e3283427259. PubMed PMID: 21157318.
- Estenssoro E, Dubin A, Laffaire E, Canales H, Sáenz G, Moseinco M, et al. Incidence, clinical course, and outcome in 217 patients with acute respiratory distress syndrome. Crit Care Med. 2002 Nov;30(11):2450-6. PubMed PMID: 12441753.
- Roupie E, Lepage E, Wysocki M, Fagon JY, Chastre J, Dreyfuss D, et al. Prevalence, etiologies and outcome of the acute respiratory distress syndrome among hypoxemic ventilated patients. Intensive Care Med. 1999 Sep;25(9):920-9. PubMed PMID: 10501746.
- Sigurdsson MI, Sigvaldason K, Gunnarsson TS, Moller A, Sigurdsson GH. Acute respiratory distress syndrome: nationwide changes in incidence, treatment and mortality over 23 years. Acta Anaesthesiol Scand. 2013 Jan;57(1):37-45. doi: 10.1111/aas.12001. PubMed PMID: 23216361.
- Esteban A, Anzueto A, Alia I, Gordo F, Apezteguia C, Palizas F, et al. How is mechanical ventilation employed in the intensive care unit? An international utilization review. Am J Respir Crit Care Med. 2000 May;161(5):1450-8. PubMed PMID: 10806138.
- Magazine R, Rao S, Chogtu B, Venkateswaran R, Shahul HA, Goneppanavar U. Epidemiological profile of acute respiratory distress syndrome patients: A tertiary care experience. Lung India. 2017 Jan-Feb;34(1):38-42. doi: 10.4103/0970-2113.197097. PubMed PMID: 28144059.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, et al. Comparison of two fluid- management strategies in acute lung injury. N Engl J Med. 2006 Jun 15;354(24):2564-75. PubMed PMID: 16714767.
- Rocco Jr TR, Reinert SE, Cioffi W, Harrington D, Buczko G, Simms HH. A 9-year, single-institution, retrospective review of death rate and prognostic factors in adult respiratory distress syndrome. Ann Surg. 2001 Mar;233(3):414-22. PubMed PMID: 11224631.
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005 Oct 20;353(16):1685-93. PubMed PMID: 16236739.
- Singh G, Gladdy G, Chandy TT, Sen N. Incidence and outcome of acute lung injury and acute respiratory distress syndrome in the surgical intensive care unit. Indian J Crit Care Med. 2014 Oct;18(10):659-65. doi: 10.4103/0972-5229.142175. PubMed PMID: 25316976.
- Hernández-Cárdenas CM, Serna-Secundino H, García-Olazarán JG, Aguilar-Pérez CL, Rocha-Machado J, Campos-Calderón LF, et al. Acute Respiratory Distress Syndrome Secondary to Influenza A (H1N1) pdm09: Clinical Characteristics and Mortality Predictors. Rev Invest Clin. 2016 Sep-Oct;68(5):235-244. PubMed PMID: 27941959.
- Lai AR, Keet K, Yong CM, Diaz JV. Severe H1N1-associated acute respiratory distress syndrome: a case series. Am J Med. 2010 Mar;123(3):282-285. e2. doi: 10.1016/j.amjmed.2009.11.004. PubMed PMID: 20193840.
- NSW [Internet]. Sputum induction guidelines - Tuberculosis. [cited 2018 May 16]. Available from: http://www.health.nsw.gov.au/Infectious/tuberculosis/Pages/tb-sputum-induction-guidelines.aspx
- American Thoracic Society [Internet]. Bronchoalveolar Lavage. [cited 2018 Jan 04]. Available from: https://www.thoracic.org/professionals/clinical-resources/critical-care/clinical-education/critical-care-procedures/bronchoalveolar-lavage.php
- Antonelli M, Conti G, Rocco M, Bufi M, De Blasi RA, Vivino G, et al. A comparison of noninvasive positive-pressure ventilation and conventional mechanical ventilation in patients with acute respiratory failure. N Engl J Med. 1998 Aug 13;339(7):429-35. PubMed PMID: 9700176.
- Acute Respiratory Distress Syndrome Network, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000 May 4;342(18):1301-8. PubMed PMID: 10793162.
- ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012 Jun 20;307(23):2526-33. doi: 10.1001/jama.2012.5669. PubMed PMID: 22797452.
- GLOWM [Internet]. White Blood Cell Differential Count. [cited 2018 Jan 4]. Available from: http://www.glowm.com/lab_text/item/9
- MedlinePlus [Internet]. WBC count: MedlinePlus Medical Encyclopedia. [cited 2018 Jan 5]. Available from: https://medlineplus.gov/ency/article/ 003643.htm.
- NCHS [Internet]. NHIS - Adult Tobacco Use - Glossary. [cited 2016 Dec 4]. Available from: https://www.cdc.gov/nchs/nhis/tobacco/tobacco_glossary. htm.
- National Institute on Alcohol Abuse and Alcoholism (NIAAA) [Internet]. Drinking Levels Defined. [cited 2016 Dec 4]. Available from: https://www. niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderatebinge-drinking
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010 Jan;33 Suppl 1:S62-9. doi: 10.2337/dc10-S062. PubMed PMID: 20042775.
- WHO [Internet]. WHO guidelines for pharmacological management of pandemic (H1N1) 2009 influenza and other influenza viruses. [cited 2010 Feb 1]. Available from: http://www.who.int/csr/resources/publications/swineflu/h1n1_use_antivirals_20090820/en/
- Riscili BP, Anderson TB, Prescott HC, Exline MC, Sopirala MM, Phillips GS, et al. An assessment of H1N1 influenza-associated acute respiratory distress syndrome severity after adjustment for treatment characteristics. PLoS One. 2011 Mar 25;6(3):e18166. doi: 10.1371/journal.pone.0018166. PubMed PMID: 21464952.
- Töpfer L, Menk M, Weber-Carstens S, Spies C, Wernecke KD, Uhrig A, et al. Influenza A (H1N1) vs non-H1N1 ARDS: analysis of clinical course. J Crit Care. 2014 Jun;29(3):340-6. doi: 10.1016/j.jcrc.2013.12.013. PubMed PMID: 24508203.
- Nardocci P, Gullo CE, Lobo SM. Severe virus influenza A H1N1 related pneumonia and community-acquired pneumonia: differences in the evolution. Rev Bras Ter Intensiva. 2013 Apr-Jun;25(2):123-9. doi: 10.5935/0103-507X.20130023. PubMed PMID: 23917977.
- Miller III RR, Markewitz BA, Rolfs RT, Brown SM, Dascomb KK, Grissom CK, et al. Clinical findings and demographic factors associated with ICU admission in Utah due to novel 2009 influenza A (H1N1) infection. Chest. 2010 Apr;137(4):752-8. doi: 10.1378/chest.09-2517. PubMed PMID: 19933372.
- Ramakrishna K, Sampath S, Chacko J, Chacko B, Narahari DL, Veerendra HH, et al. Clinical profile and predictors of mortality of severe pandemic (H1N1) 2009 virus infection needing intensive care: a multi-centre prospective study from South India. J Glob Infect Dis. 2012 Jul;4(3):145-52. doi: 10.4103/0974-777X.100569. PubMed PMID: 23055645.
- Reyes S, Montull B, Martínez R, Córdoba J, Molina JM, Martí V, et al. Risk factors of A/H1N1 etiology in pneumonia and its impact on mortality. Respir Med. 2011 Sep;105(9):1404-11. doi: 10.1016/j.rmed.2011.04.011. PubMed PMID: 21561754.
- Tsatsanis C, Margioris AN, Kontoyiannis DP. Association between H1N1 infection severity and obesity-adiponectin as a potential etiologic factor. J Infect Dis. 2010 Aug 15;202(3):459-60. doi: 10.1086/653842. PubMed PMID: 20557238.
- Bewick T, Myles P, Greenwood S, Nguyen-Van-Tam JS, Brett SJ, Semple MG, et al. Clinical and laboratory features distinguishing pandemic H1N1 influenza-related pneumonia from interpandemic community-acquired pneumonia in adults. Thorax. 2011 Mar;66(3):247-52. doi: 10.1136/thx.2010.151522. PubMed PMID: 21252388.
- Bjarnason A, Thorleifsdottir G, Löve A, Gudnason JF, Asgeirsson H, Hallgrimsson KL, et al. Severity of influenza A 2009 (H1N1) pneumonia is underestimated by routine prediction rules. Results from a prospective, population-based study. PLoS One. 2012;7(10):e46816. doi: 10.1371/journal. pone.0046816. PubMed PMID: 23071646.
- Homsi S, Milojkovic N, Homsi Y. Clinical pathological characteristics and management of acute respiratory distress syndrome resulting from influenza A (H1N1) virus. South Med J. 2010 Aug;103(8):786-90. doi: 10.1097/ SMJ.0b013e3181e6ca0c. PubMed PMID: 20622733.
- Agarwal PP, Cinti S, Kazerooni EA. Chest radiographic and CT findings in novel swine-origin influenza A (H1N1) virus (S-OIV) infection. AJR Am J Roentgenol. 2009 Dec;193(6):1488-93. doi: 10.2214/AJR.09.3599. PubMed PMID: 19933638.
- Samra T, Pawar M, Yadav A. Comparative evaluation of acute respiratory distress syndrome in patients with and without H1N1 infection at a tertiary care referral center. Indian J Anaesth. 2011 Jan;55(1):47-51. doi: 10.4103/0019-5049.76602. PubMed PMID: 21431053.
- Chacko J, Gagan B, Ashok E, Radha M, Hemanth HV. Critically ill patients with 2009 H1N1 infection in an Indian ICU. Indian J Crit Care Med. 2010 Apr;14(2):77-82. doi: 10.4103/0972-5229.68220. PubMed PMID: 20859491.
- Denman AM. Lymphocyte function and virus infections. J Clin Pathol Suppl (R Coll Pathol). 1979;13:39-47. PubMed PMID: 230207.
- Coșkun Ö, Avci IY, Sener K, Yaman H, Ogur R, Bodur H, et al. Relative lymphopenia and monocytosis may be considered as a surrogate marker of pandemic influenza a (H1N1). J Clin Virol. 2010 Apr;47(4):388-9. doi: 10.1016/j.jcv.2010.01.007. PubMed PMID: 20133186.
- Cunha BA, Pherez FM, Schoch P. Diagnostic importance of relative lymphopenia as a marker of swine influenza (H1N1) in adults. Clin Infect Dis. 2009 Nov 1;49(9):1454-6. doi: 10.1086/644496. PubMed PMID: 19824851.
- Merekoulias G, Alexopoulos EC, Belezos T, Panagiotopoulou E, Jelastopulu DM. Lymphocyte to monocyte ratio as a screening tool for influenza. PLoS Curr. 2010 Mar 29;2:RRN1154. PubMed PMID: 20383263.
- Criswell BS, Couch RB, Greenberg SB, Kimzey SL. The lymphocyte response to influenza in humans. Am Rev Respir Dis. 1979 Sep;120(3):700-4. PubMed PMID: 314763.
- MacCallum NS, Evans TW. Epidemiology of acute lung injury. Curr Opin Crit Care. 2005 Feb;11(1):43-9. PubMed PMID: 15659944.
- Phua J, Badia JR, Adhikari NK, Friedrich JO, Fowler RA, Singh JM, et al. Has mortality from acute respiratory distress syndrome decreased over time? A systematic review. Am J Respir Crit Care Med. 2009 Feb 1;179(3):220-7. doi: 10.1164/rccm.200805-722OC. PubMed PMID: 19011152.
- Monchi M, Bellenfant F, Cariou A, Joly LM, Thebert D, et al. Early predictive factors of survival in the acute respiratory distress syndrome: a multivariate analysis. Am J Respir Crit Care Med. 1998 Oct;158(4):1076-81. PubMed PMID: 9769263.
- Agarwal R, Aggarwal AN, Gupta D, Behera D, Jindal SK. Etiology and outcomes of pulmonary and extrapulmonary acute lung injury/ARDS in a respiratory ICU in North India. Chest. 2006 Sep;130(3):724-9. PubMed PMID: 16963669.
- Agarwal R, Srinivas R, Nath A, Jindal SK. Is the mortality higher in the pulmonary vs the extrapulmonary ARDS?: A metaanalysis. Chest. 2008 Jun;133(6):1463-1473. doi: 10.1378/chest.07-2182. PubMed PMID: 17989150.
- Lopez-Delgado JC, Rovira A, Esteve F, Rico N, Manez Mendiluce R, Ballus Noguera J, et al. Thrombocytopenia as a mortality risk factor in acute respiratory failure in H1N1 influenza. Swiss Med Wkly. 2013 Apr 18;143:w13788. doi: 10.4414/smw.2013.13788. PubMed PMID: 23739994.
- Domínguez-Cherit G, Lapinsky SE, Macias AE, Pinto R, Espinosa-Perez L, et al. Critically ill patients with 2009 influenza A (H1N1) in Mexico. JAMA. 2009 Nov 4;302(17):1880-7. doi: 10.1001/jama.2009. PubMed PMID: 19822626.
- Doyle RL, Szaflarski N, Modin GW, Wiener-Kronish JP, Matthay MA. Identification of patients with acute lung injury. Predictors of mortality. Am J Respir Crit Care Med. 1995 Dec;152(6 Pt 1):1818-24. PubMed PMID: 8520742.
- Estenssoro E, Ríos FG, Apezteguía C, Reina R, Neira J, Ceraso DH, et al. Pandemic 2009 influenza A in Argentina: a study of 337 patients on mechanical ventilation. Am J Respir Crit Care Med. 2010 Jul 1;182(1):41-8. doi: 10.1164/201001-0037OC. PubMed PMID: 20203241.
- Koegelenberg CF, Irusen EM, Cooper R, Diacon AH, Taljaard JJ, Mowlana A, et al. High mortality from respiratory failure secondary to swine-origin influenza A (H1N1) in South Africa. QJM. 2010 May;103(5):319-25. doi: 10.1093/qjmed/hcq022. PubMed PMID: 20219780.
- Vieillard-Baron A, Girou E, Valente E, Brun-Buisson C, Jardin F, Lemaire F, et al. Predictors of mortality in acute respiratory distress syndrome. Focus On the role of right heart catheterization. Am J Respir Crit Care Med. 2000 May;161(5):1597-601. PubMed PMID: 10806161.
- Luecke T, Muench E, Roth H, Friess U, Paul T, Kleinhuber K,et al. Predictors of mortality in ARDS patients referred to a tertiary care centre: a pilot study. Eur J Anaesthesiol. 2006 May;23(5):403-10. PubMed PMID: 16469204.
- Sehgal N, Woodhead M. Predicting the unpredictable: is it possible clinically to separate H1N1 from non-H1N1 community-acquired pneumonia?. Thorax. 2011 Mar;66(3):187-8. doi: 10.1136/thx.2010.157404. PubMed PMID: 21335459.