Acrodermatitis Enteropathica: Case Report and Review of the Literature
Elmahi H*, Gallouj S, Mernissi FZ
Department of dermatology .university center Hassan II, Fes, Morocco.
*Corresponding Author
Hakima Elmahi,
Department of Dermatology,
University Center Hassan II, Fes, Morocco.
E-mail: Elmahi.hakima@gmail.com
Received: March 04, 2017; Accepted: April 24, 2017; Published: April 27, 2017
Citation: Elmahi H, Gallouj S, Mernissi FZ (2017) Acrodermatitis Enteropathica: Case Report and Review of the Literature. Int J Pediat Health Care Adv. 4(3), 34-36. doi: dx.doi.org/10.19070/2572-7354-1700011
Copyright: Elmahi H© 2017. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Acrodermatitis enteropathica (AE) is a rare hereditary disorder caused by impaired absorption of zinc from the gastrointestinal tract. It is characterized by acral and periorificial dermatitis, alopecia, and diarrhea. Early recognition and treatment of the disease will decrease morbidity and mortality. We report a breast-fed infant with the classic signs of AE, had been a fatal evolution. After reviewing the literature we emphasize the important role of zinc in human metabolism and the difference between AE and acquired zinc deficiencies.
2.Introduction
3.Case Report
4.Discussion
5.Conclusion
6.References
Keywords
Acrodermatitis Enteropathica; Autosomal Recessive Disease; Zinc.
Introduction
Acrodermatitis enteropathica (AE) is a rare autosomal recessive disease with an estimated incidence of 1 per 500,000 children and is characterized by Zn deficiency secondary to the inability to absorb Zn intestinally, which leads to varying degrees of decreased Zn concentration in serum [1, 2]. This condition was first described in 1936 by Brandt [3]. Symptoms of AE occur within the first few months after birth and tend to appear shortly after discontinuation of breast-feeding [4]. It’s rare in exclusively breast fed infant and it was essentially reported in premature babies. Characterized by skin lesions distributed predominantly in acral and periorificial sites, alopecia, and diarrhea; Retardation of growth and secondary infections are frequently observed [4, 5]. Without zinc therapy, AE is fatal [4]. We report a breast-fed infant with the classic signs of AE, had been a fatal evolution.
Case Report
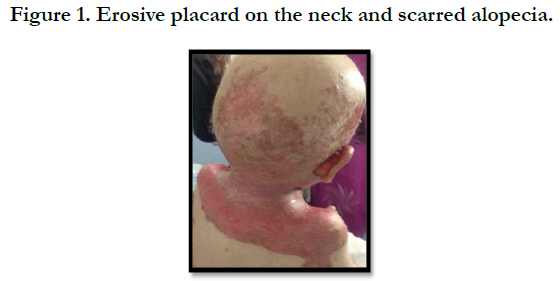
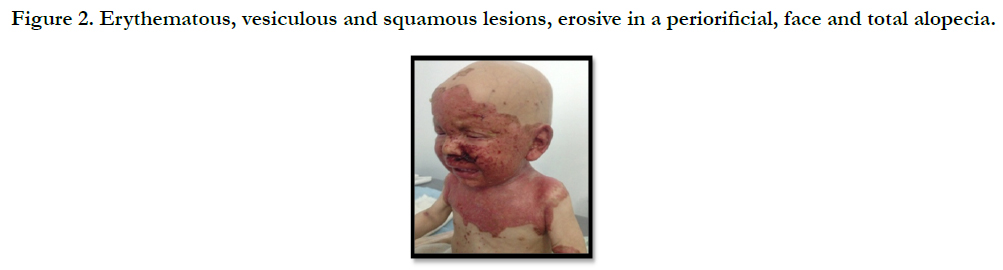
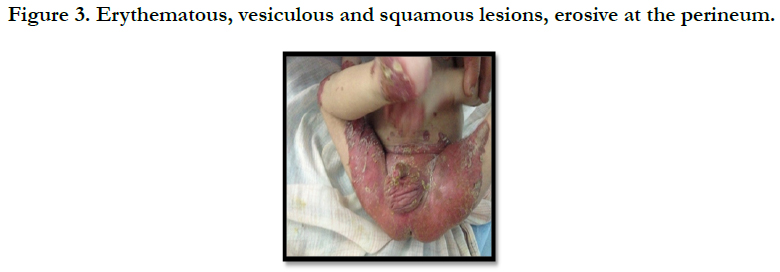
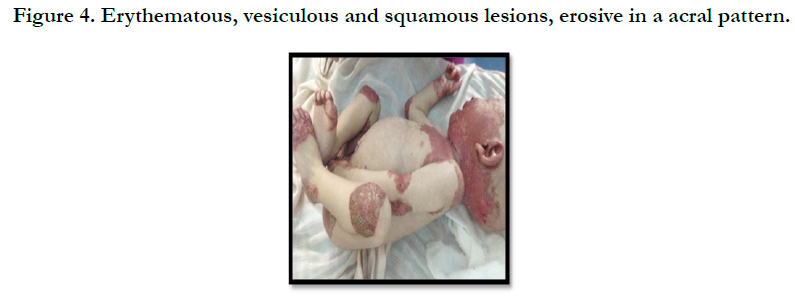
A full term, 12 month-old boy consulted in dermatology department for persistent and refractory anogenital lesions since the age of 2 month, with progressive erythematous, vesiculous and squamous lesions, erosive in a periorificial, face and acral pattern, diarrhea, total alopecia, growth failure, and irritability emotional disturbances [Figures: 1, 2, 3, 4]. Pregnancy and delivery were normal; the child was exclusively breast feed up to 4 months of age. His parents were consanguineous (second cousins), and stated that his sister had died at the age of 6 months with similar cutaneous manifestations and without diagnosis. He had been treated for his cutaneous lesions with topical antimycotic agents and steroids, but had shown no improvement. He had a previous history of upper airway infections and otitis media, treated in the outpatient clinic, but his skin lesions remained unchanged. The diagnosis of AE was confirmed by decreased plasma zinc level (2, 8 micro mol/l (normal value: 66.0~110.0 μg/dl). He was given zinc sulphate. After 48h, he had died after a bacterial infection.
Figure 2. Erythematous, vesiculous and squamous lesions, erosive in a peri orificial, face and total alopecia.
Discussion
Zinc is an essential trace element required for the proper functioning of all cells, and plays an important role in the metabolism of protein, carbohydrate, and vitamin A [6, 7]. It is a cofactor of numerous metal enzymes such as alkaline phosphatases, alcohol dehydrogenase, RNA polymerase, and numerous digestive enzymes [6, 7]. Of all tissues, the skin has the third highest abundance of zinc in the body [2]. In the skin, the zinc concentration is higher in the epidermis than in the dermis, owing to a zinc requirement for the active proliferation and differentiation of epidermal keratinocytes [2]. Zinc can be found in several components of a regular diet, mostly the animal origin [8]. It is also present in human breast milk and pancreatic secretions [6]. The recommended daily dose varies with age, and of the ingested zinc, 30% is absorbed [6]. Absorption of zinc occurs mainly in the duodenum and proximal small intestine and its excretion is primarily intestinal, with a small amount being excreted in sweat [1, 6-8]. Zinc deficiency can be acquired or inherited. There are many causes of acquired zinc deficiency: premature infants, low birth weight, zinc deficiency in maternal milk, exclusive parenteral nutrition, malabsorption syndromes such as Crohn disease and celiac disease, alcoholism, low calcium and phytate diet, and kwashiorkor [5]. The hereditary deficiency of zinc is classically known as “acrodermatitis enteropathica”. It is caused by an autosomal recessive mutation of SLC39A4 (solute carrier family 39 member 4) gene on chromosome 8q24.3, which determines a congenital partial or total deficiency of the zinc transporter protein zinc-ligand binding protein 4 (ZIP 4) [9].
The clinical manifestations of acquired zinc deficiency and AE are similar and consist of 3 essential symptoms: periorificial dermatitis, alopecia, and diarrhea [1, 4]. This clinical triad is complete in only 20% of patients with AE [4]. The disease begins with symmetrical erythematous, squamous or eczematous lesions, sometimes vesiculobullous or pustular lesions, located around perioral, anogenital, and acral areas. The severity of the skin lesions is variable. Without treatment, skin damage becomes erosive and spreads to other periorificial areas of the face (eyes, nose, and ears), neck, lower abdomen, back, inguinal area, and thighs [4, 5]. In some instances, the skin lesions may appear psoriasiform. Other mucous and cutaneous signs consist of diffuse alopecia, loss of eyelashes and eyebrows, glossitis, gingivitis, stomatitis, onychodystrophy, onycholysis, and pachyonychia [5]. Diarrhea is the predominant extracutaneous symptom. Unfortunately, it is also the most variable, and it can be intermittent or totally absent. In children with watery diarrhea, general symptoms and neuropsychological disorders are common (irritability, lethargy, depression, anorexia), and also growth retardation, weight loss, anemia, and ophthalmic involvement (photophobia, blepharitis, conjunctivitis) [4]. Secondary bacterial infections (with Grampositive and sometimes Gram-negative) or candidiasis (Candida albicans) are common and they can modify the clinical picture. [5, 10] So, clinical findings in AE described in the literature encompass a broad spectrum, making the diagnosis challenging. Indeed, the rarity of the disease does not make the diagnosis easy for practitioners. Thus, our patient is diagnosed late, examined by several doctors or received ineffective treatments during several months before being referred to our department. Consequently, a death of our patient. Diagnosis of AE is made primarily by clinical manifestations and a low serum zinc level is confirmative [5]. The differential diagnoses to consider are psoriasis, atopic dermatitis, seborrheic dermatitis, contact dermatitis, skin infections with candida, Langerhans cell histiocytosis, and cystic fibrosis [4]. Once the disease has been diagnosed, the treatment consists of oral zinc supplementation given daily, which leads to a dramatic disappearance of the symptoms within a few days [5]. There is no consensus on the doses of zinc sulfate. Most authors recommend an initial dose of 5-10 mg/kg/d [11] with maintenance doses of 1-2 mg/kg/d [5, 6]. Such doses should be increased with the accelerated stages of growth, and during pregnancy and lactation; Treatment must be initiated as soon as possible; if not, zinc deficiency eventually leads to a widespread organ failure and, ultimately, to the demise of the patient, as with our case. With treatment, the survival rate is 100% [3, 4]. AE must be treated and monitored throughout the entire life. In the event of treatment interruption, relapses are inevitable and the cutaneous signs are the first to recur. The long-term prognosis is excellent.
Conclusion
Acrodermatitis enteropathica is a rare disease which diagnosis should be considered in case of symmetric periorificial and acral skin lesions in the infant. Its biological confirmation is made on a simple dosage of zincemia. Treatment with zinc is obligatory for the life time. However, probably because of the rarity of the disease, the diagnosis is not always suggested, and is unfortunately made late.
References
- Sehgal VN, Jain S (2000) Acrodermatitis enteropathica. Clin Dermatol. 18(6): 745–748.
- Ogawa Y, Kawamura T, Shimada S (2016) Zinc and skin biology. Arch Biochem Biophys. 611: 113-119.
- Danbolt N (1979) Acrodermatitis enteropathica. Br J Dermatol. 100(1): 37-40.
- Nistor N, Ciontu L, Frasinariu OE, Lupu VV, Ignat A, et al., (2016) Acrodermatitis Enteropathica: A Case Report. Medicine (Baltimore). 95(20): e3553.
- Perafán-Riveros C, França LF, Alves AC, Sanches JA (2002) Acrodermatitis enteropathica: case report and review of the literature. Pediatr Dermatol. 19(5): 426–431.
- Lucky AW (1995) Cutaneous manifestations of endocrine, metabolic, and nutritional disorders. Pediatric dermatology. NewYork: Churchill Livingstone. 1077–1080.
- Aggett PJ, Harries JT (1979) The current status of zinc in health and disease states. Arch Dis Child. 54(12): 909–917.
- Prasad A (1984) Discovery and importance of zinc in human nutrition. Fed Proc. 43(13): 2829–2834.
- Küry S, Dréno B, Bézieau S, Kharfi M, Kamoun R, et al., (2002) Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat Genet. 31(3): 239–240.
- Scrivener Y, Bessis D (2012) Dermatoses carentielles. Manifestations dermatologiques des maladies d’organes. Springer-Verlag. 4: 45–62.
- Mancini J, Tunnessen WW (1998) Acrodermatitis enteropathica-like rash in a breast-fed full term infant with zinc deficiency. Arch Pediatr Adolesc Med 152(12): 1240-1240.