Real-Life Clinical Effectiveness of Razumab® (World’s First Biosimilar Ranibizumab) in Wet Age-Related Macular Degeneration, Diabetic Macular Edema, and Retinal Vein Occlusion: A Retrospective Pooled Analysis
(Major) Sharma S1*, RE-ENACT Study Investigators Group2, Khan MA3, Chaturvedi A3
1 Medical Lead - Ophthalmology, Medical Services, Intas Pharmaceuticals Ltd., Ahmedabad, Gujarat, India.
2 RE-ENACT Study Investigators Group.
3 Medical Services, Intas Pharmaceuticals Ltd., Ahmedabad, Gujarat, India.
*Corresponding Author
Dr. (Major) Shashikant Sharma, MD,
Medical Lead, Ophthalmology, Medical Services,
Intas Pharmaceuticals Ltd. Ahmedabad, Gujarat, 380054, India.
E-mail: shashikant_sharma@intaspharma.com
Received: May 17, 2018; Accepted: June 12, 2018; Published: June 13, 2018
Citation: (Major) Sharma S, RE-ENACT Study Investigators Group, Khan MA, Chaturvedi A. Real-Life Clinical Effectiveness of Razumab® (World’s First Biosimilar Ranibizumab) in Wet Age-Related Macular Degeneration, Diabetic Macular Edema, and Retinal Vein Occlusion: A Retrospective Pooled Analysis Int J Ophthalmol Eye Res. 2018;6(4):377-383. doi: dx.doi.org/10.19070/2332-290X-1800076
Copyright: (Major) Sharma S© 2018. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Purpose: To evaluate the effectiveness of Razumab® (world’s first biosimilar ranibizumab; Intas Pharmaceuticals Ltd., India) in Indian patients with wet age-related macular degeneration (wet AMD), diabetic macular edema (DME) and retinal vein occlusion (RVO).
Methods: RE-ENACT, a retrospective, multicenter study, analyzed pooled data of patients with wet AMD, DME, and RVO. Patients who had received ≥3 injections of Razumab® between January and August 2016, were included. Endpoints were: improvement in best corrected visual acuity (BCVA, measured by logMAR/Snellen’s chart), decrease in central macular thickness (CMT, measured by Spectral Domain Optical Coherence Tomography), and proportion of patients with intraretinal fluid (IRF) and subretinal fluid (SRF) at Weeks 4, 8 and 12.
Results: Of 561 patients included, 348 (62.04%) were men. Mean ± SE BCVA improved from baseline (0.75 ± 0.01) to Week 4 (0.72 ± 0.01, p = 0.0318), attained significance at Week 8 (0.59 ± 0.01, p < 0.0001), which was maintained at Week 12 (0.49 ± 0.01, p < 0.0001). Mean ± SE CMT significantly (p < 0.0001) decreased from baseline (418.47 ± 4.78μm) to Weeks 4 (407.35 ± 4.65μm), 8 (342.10 ± 3.66μm), and 12 (301.17 ± 2.82μm). Proportion of patients with IRF and SRF significantly (p < 0.0001) decreased from baseline to Weeks 4, 8 and 12 (67.02% vs. 48.48%, 42.60%, and 34.22%, respectively for IRF; and 72.37% vs. 48.48%, 37.97%, 31.37%, respectively for SRF). No new safety concerns with biosimilar ranibizumab were observed.
Conclusions: Razumab® is effective in reducing macular thickness and improving visual acuity in patients with wet agerelated macular degeneration, diabetic macular edema, and retinal vein occlusion in routine clinical practice. Razumab® demonstrated considerable effectiveness with no new safety concerns.
2.Introduction
3.Methods
3.1 Study Design and Population
3.2 Statistical Analysis
4.Results
4.1 Patients Disposition and Demographics
5.Discussion
6.Limitations
7.Acknowledgments
8.Competing Interests
9.Funding
10.RE-ENACT Study Investigators Group
11.References
Keywords
Razumab; Wet Age-Related Macular Degeneration; Diabetic Macular Edema; Retinal Vein Occlusion; Wet AMD; DME; RVO.
Introduction
Ranibizumab is a recombinant, humanized fragment of a murine monoclonal antibody that binds to the vascular endothelial growth factor (VEGF)-A and subsequently improves the visual acuity (VA). It has become the preferred treatment for various retinal disorders, including wet age-related macular degeneration (wet AMD), diabetic macular edema (DME), and retinal vein occlusion (RVO) [1-3].
Neovascular or wet AMD is one of the major causes of vision impairment worldwide [4]. Intravitreal anti-VEGF drugs including ranibizumab, are the current gold standard for wet AMD management [5]. Several studies have shown the efficacy and safety of ranibizumab in the treatment of wet AMD including pivotal long-term Phase III MARINA [2] and ANCHOR [1] studies. Diabetic macular edema is the most common complication of diabetic retinopathy [6] and is a major cause of vision loss in the working population [7]. The treatment of DME has evolved with anti-VEGF agents; amongst which ranibizumab is the first approved agent for DME treatment [6]. Several short- and long term clinical studies including the READ [8], RESOLVE [9], RESTORE [10], RETAIN [11], RISE and RIDE [12], RELIGHT [13], and TREX studies [14] have confirmed the efficacy and safety of ranibizumab in the treatment of DME. Retinal vein occlusion (RVO) is a common cause of unilateral, sudden and painless loss of vision. Increase in the VEGF levels are observed in the ocular fluid of RVO (BRVO or CRVO) patients, which is proportional to the severity of macular edema [15]. The approval of ranibizumab for the treatment of macular edema secondary to RVO was based on the results of the Phase III BRAVO [16] and CRUISE [17] studies.
Ranibizumab is approved for the treatment of several choroidal vascular complications i.e., wet AMD, DME, RVO, and mCNV. The innovator ranibizumab is quite an expensive treatment option [18]; hence, the world’s first biosimilar of ranibizumab - Razumab ® was developed by Intas Pharmaceuticals Ltd., India, as a cost-effective alternative, and is approved by the Drug Controller General of India.
Razumab® has been approved for the treatment of wet age-related macular degeneration (wet AMD), diabetic macular edema (DME), macular edema following retinal vein occlusion (RVO), and visual impairment due to choroidal neovascularization (CNV) secondary to pathological myopia. The efficacy and tolerability of Razumab® has been documented in the treatment of wet AMD, RVO, and DME in Indian patients in a prospective study [18]. However, these data may not completely reflect the conditions found in routine clinical settings due to their restrictive design and strict inclusion and exclusion criteria. Hence, the effectiveness of Razumab® in Indian patients was evaluated in the RE-ENACT study. This was a retrospective, multicenter, observational study in patients with wet AMD, DME and RVO. The subgroup analyses from the RE-ENACT study for wet AMD and RVO populations have been published elsewhere [19, 20]. This report presents the effectiveness of Razumab® in Indian patients from the pooled data of patients with wet AMD, DME, and RVO.
The complete methodology of this study has been published earlier with the results of the subgroup analyses [19, 20] and briefly described here. This observational, organized study analyzed the data of patients who had received treatment with Razumab® for the treatment of wet AMD, DME or RVO at different centers across India between January and August 2016. Adult patients who received at least three injections (4-weekly) of Razumab® were included in the study. Endpoints included the mean change in the best corrected visual acuity (BCVA) and central macular thickness (CMT), and proportion of patients with no intraretinal fluid (IRF) and subretinal fluid (SRF) from baseline to Weeks 4, 8 and 12.
The BCVA is defined as the measurement of clarity of central vision which was measured by Snellen’s chart and converted to logarithm of the minimal angle of resolution (logMAR) scale for statistical analysis. The CMT is the distance between the inner limiting membrane (ILM) and the inner boundary of retinal pigment epithelium (RPE), which was measured using spectral domain optical coherence tomography. Subretinal fluid corresponds to the accumulation of clear or lipid-rich exudates (serous fluid) in the subretinal space, i.e., between the neurosensory retina (NSR) and the underlying retinal pigment epithelium (RPE), in the absence of retinal breaks, tears, or traction. Intraretinal fluid consists of contiguous fluid-filled spaces containing columns of retinal tissue.
Best corrected visual acuity and central macular thickness data were analyzed using two-tailed paired t-test. Intraretinal fluid and subretinal fluid data were analyzed using χ2 test. Mean % change in BCVA and CMT was calculated as an average value of % change from baseline to a particular visit. All statistical analyses were done using SAS 9.3 or higher.
Data of 561 patients (348 [62.04%] men and 213 [37.96%] women) was analyzed. The spectrum of indications included wet AMD in 194 (34.59%) patients, DME in 207 (36.89%) and RVO in 160 (28.52%) patients. The majority (335, 59.71%) of the patients were treatment naïve and 217 (38.68%) patients were previously treated with other anti-VEGF/steroids/laser treatment. A total of 66.84% (375/561) of the patients had diabetes and 56.32% (316/561) had hypertension; history of smoking was present in 10.16% (57/561) patients. At baseline, IRF was present in: left eye, 40.1% (225/561) patients and right eye, 31.01% (174/561) patients; SRF in: left eye, 41.17% (231/561) patients and right eye, 35.82% (201/561) patients. The baseline characteristics of the patients are summarized in Table 1.
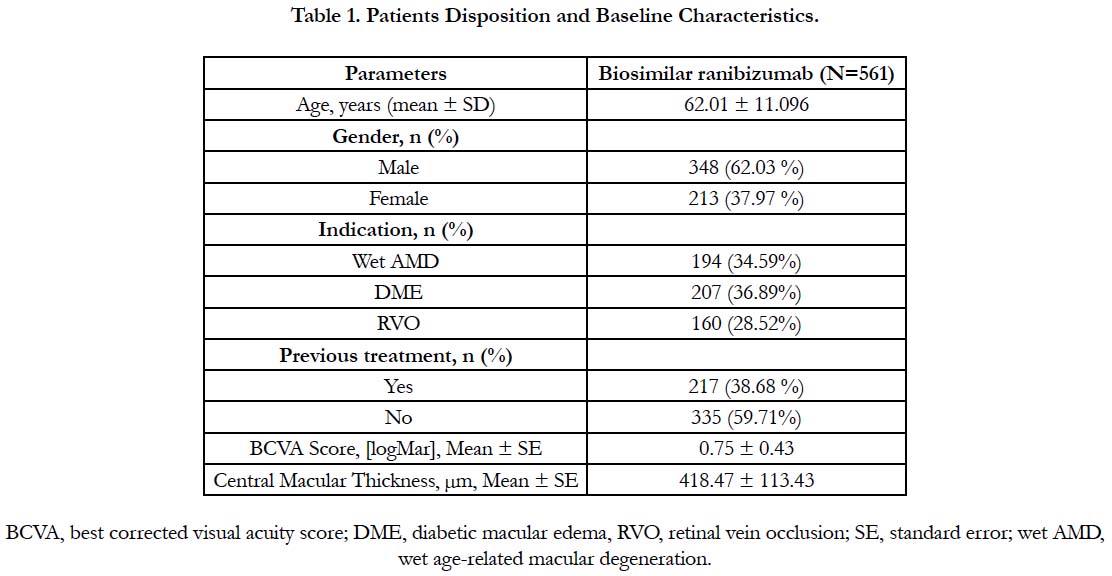
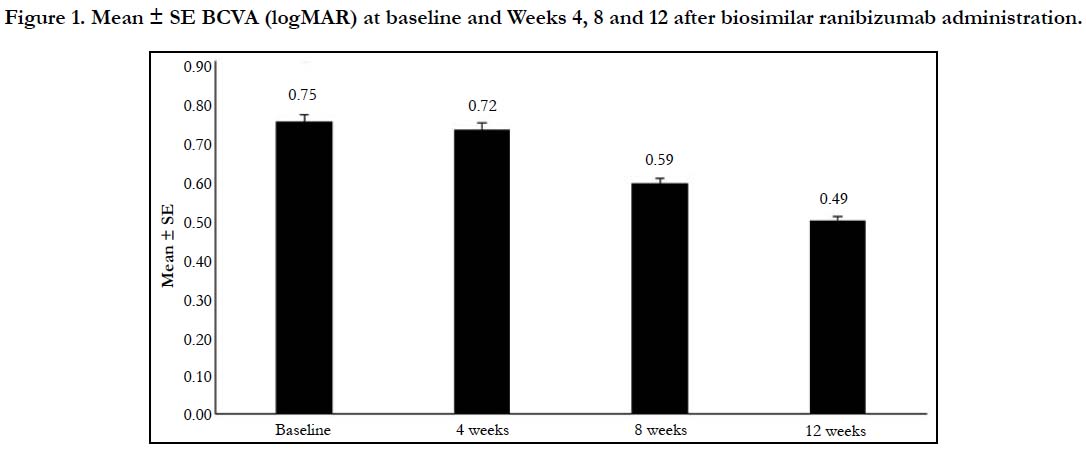
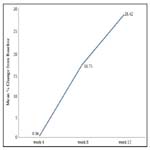
An improvement in the mean ± SE logMar BCVA scores indicating improved visual acuity was observed from baseline (0.75 ± 0.01) to Week 4 (0.72 ± 0.01; p = 0.0318), which attained significance at Week 8 (0.59 ± 0.01) and maintained till Week 12 (0.49 ± 0.01; p < 0.0001 for both time points). Biosimilar ranibizumab led to a rapid and continuous improvement in visual acuity, with benefits observed as early as Week 4 (Figure 1). From baseline, the mean % change in the BCVA was 0.36% at Week 4, 16.75% at Week 8, and 28.42% at Week 12 (Figure 2).
Figure 1. Mean ± SE BCVA (logMAR) at baseline and Weeks 4, 8 and 12 after biosimilar ranibizumab administration.
Figure 2. Mean % change in BCVA from baseline to Weeks 4, 8 and 12 after biosimilar ranibizumab administration.
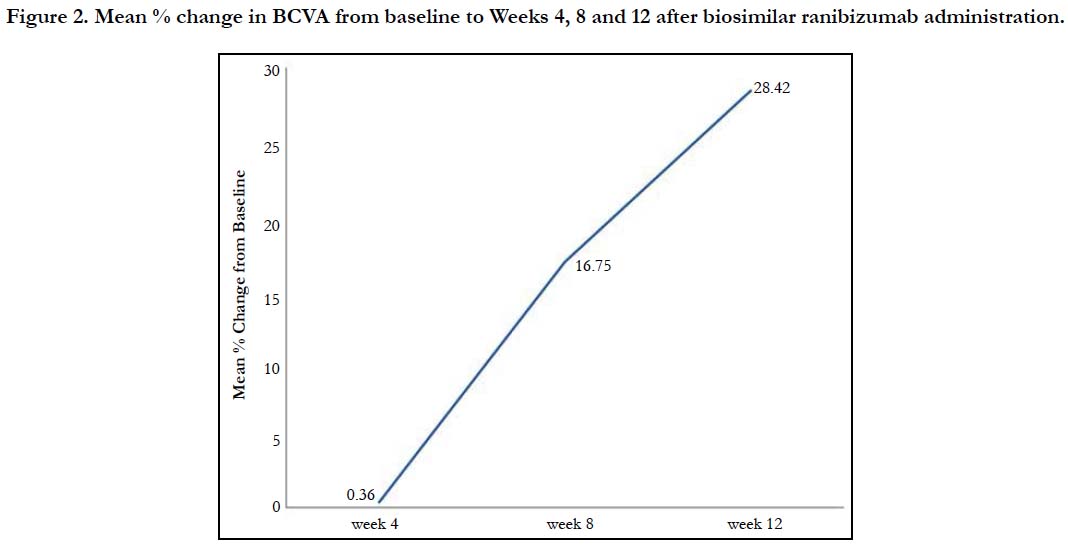
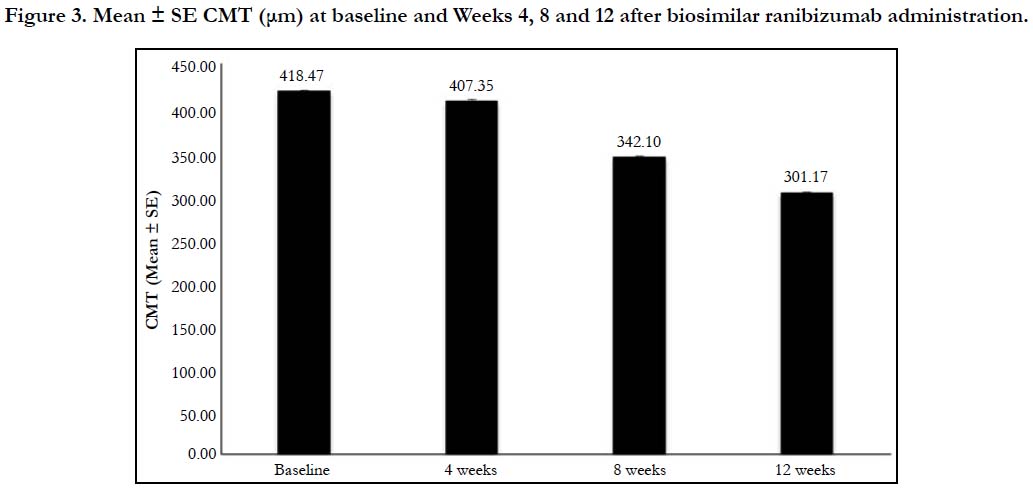
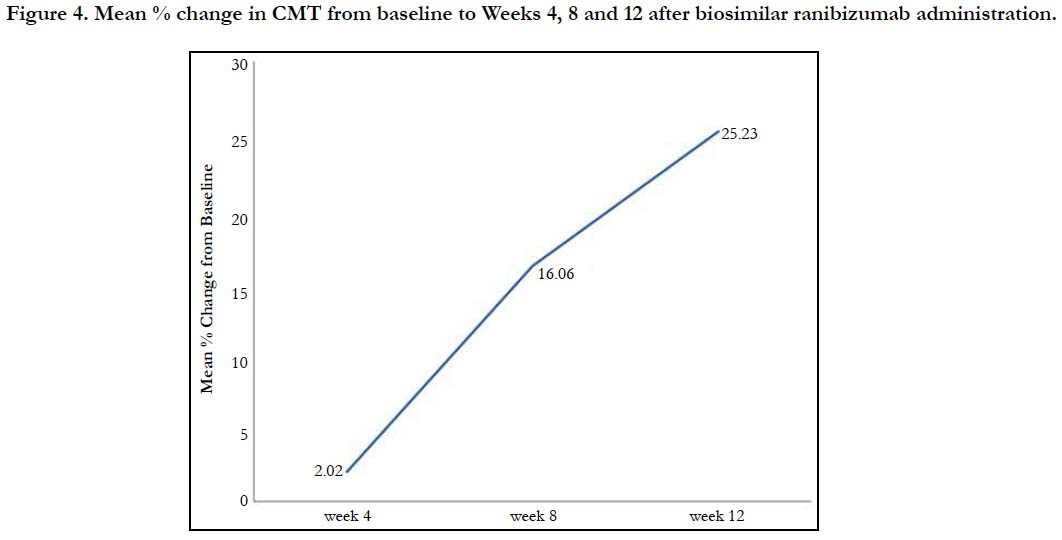
Similarly, significant improvement in disease condition as measured by a decrease in mean ± SE CMT scores was observed from baseline (418.47 ± 4.78μm) to Weeks 4 (407.35 ± 4.65μm), 8 (342.10 ± 3.66μm) and 12 (301.17 ± 2.82μm); P < 0.0001 for all time points (Figure 3). From baseline, the mean % change in CMT was 2.02% at Week 4, 16.06% at Week 8 and 25.23% at Week 12 (Figure 4).
Figure 3. Mean ± SE CMT (μm) at baseline and Weeks 4, 8 and 12 after biosimilar ranibizumab administration.
Figure 4. Mean % change in CMT from baseline to Weeks 4, 8 and 12 after biosimilar ranibizumab administration.
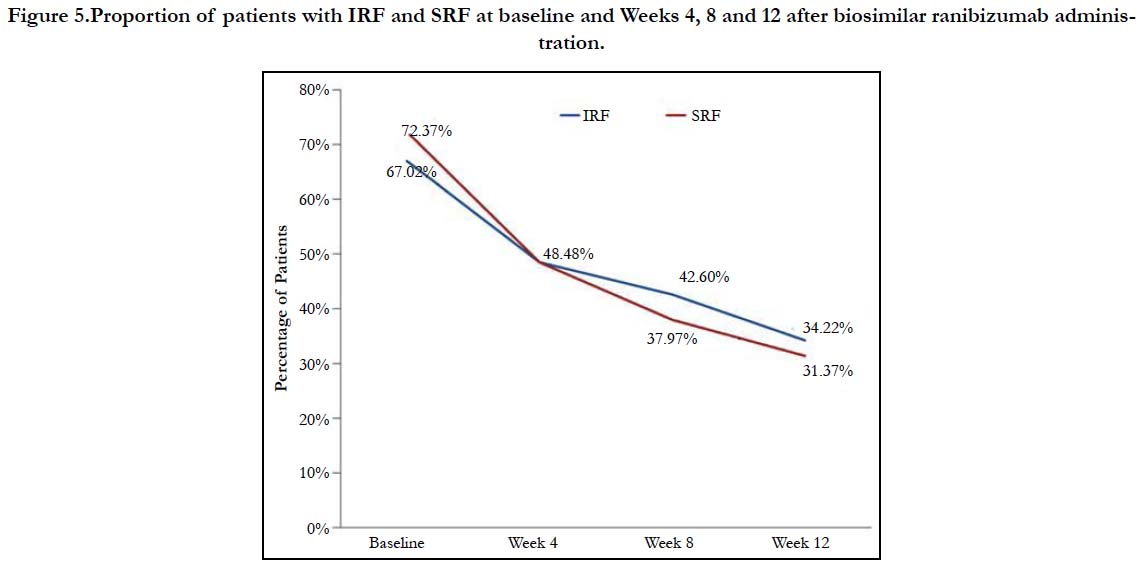
A significant reduction in the proportion of patients having IRF or SRF from baseline to all the time points (Weeks 4, 8 and 12) were observed, indicating improved disease condition. The percentage of patients having IRF at baseline vs. Weeks 4, 8 and 12 were 67.02% vs. 48.48%, 42.60%, and 34.22%, respectively (P < 0.0001 for all). The percentage of patients having SRF at baseline vs. Weeks 4, 8 and 12 were 72.37% vs. 48.48%, 37.97%, 31.37% respectively (P < 0.0001 for all) (Figure 5).
Figure 5.Proportion of patients with IRF and SRF at baseline and Weeks 4, 8 and 12 after biosimilar ranibizumab administration.
Discussion
Anti-VEGF agents have dramatically changed the treatment of retinal diseases which may progress to blindness. These agents provide a great treatment option for patients to preserve their sight as they are known to improve vision along with the prevention of vision loss leading to significantly improved prognosis and outcomes, and eventually improved quality of life [21-25].
The efficacy and tolerability of ranibizumab have been demonstrated in several multi-center, randomized, prospective studies, and it has become the first-choice treatment for various retinal disorders including wet AMD, DME, and RVO. Differences in the treatment outcomes have been observed with innovator ranibizumab in real-world clinical practice than those seen in its controlled clinical trials [26, 27]. Hence, it was necessary to have the real-world clinical data with ranibizumab. RE-ENACT, a retrospective data collection study sought to investigate the ‘real-world’ clinical effectiveness of Razumab® in the treatment of patients with wet AMD, DME, and RVO with the results pooled from all the 3 indications. Pooled data are scarce in the public domain and this is one of the few studies to provide pooled analysis of the data to understand the effectiveness of ranibizumab. The pooled results showed that BCVA improved from baseline indicating improvement in the visual acuity as early as after the 1st injection and attained significance after the 2nd injection and maintained after the 3rd injection of biosimilar ranibizumab. The CMT values decreased showing significant improvement in the disease condition after the 1st biosimilar ranibizumab injection (Week 4), that was maintained till Weeks 8 and 12. Furthermore, improvements in the subretinal fluid (SRF) and intraretinal fluid (IRF) were also seen at all time points. The results for reduction in macular thickness and improvement in visual acuity for pooled data are in consistence with the subgroups analyses of the RE-ENACT study on wet AMD [19] and RVO [20] populations published earlier. Similarly, in a study conducted in 100 Indian patients with wet AMD, DME, and RVO, ranibizumab treatment showed a significant improvement in the visual acuity (measured by Snellen’s chart) in the majority of the patients [22]. Furthermore, a recent study conducted in Indian patients (n=95) with wet AMD, DME, and RVO demonstrated that biosimilar ranibizumab treatment improved the visual acuity and disease outcomes (measured by BCVA and CMT). The treatment was well tolerated over a month and there was no ocular and systemic toxicity detected [18].
These ophthalmological indications are generally chronic, requiring long-term administration of anti-VEGF treatment, with few studies evaluating the effects up to 5-years [28]. Ranibizumab has shown to be effective for improvement in visual functions, and the reduction of intraretinal and subretinal fluids [23]. The current study, which was only for a duration of 3 months, demonstrated marked improvements in BCVA, CMT, SRF and IRF as early as 4 weeks of biosimilar ranibizumab administration with lasting benefits till 12 weeks follow-up.
Ranibizumab has shown to be effective for not only preventing vision loss, but also for significant gains in visual activity, and has been considered as the standard of care [1, 2, 29]. In clinical studies (including ANCHOR, MARINA, RESTORE, BRAVO, and CRUISE), patients with wet AMD, DME, and RVO treated with ranibizumab had a marked improvement in their visual acuity scores. In the current study, the visual acuity improved (as measured by BCVA) in patients with wet AMD, DME, and RVO at each time point. The results are consistent with the findings from earlier studies in wet AMD patients in which improvement in the visual acuity was observed as early as after the first dose, which was maintained at 3 months [30]. Similar results have been demonstrated by other studies in real-world settings including retrospective [31], and a multi-centre, prospective, observational post-marketing surveillance study [32].
Similar to our study, previous reports in DME patients have also demonstrated improvement in the BCVA scores starting at 1 month of ranibizumab, which was maintained till 3 months [33-35]. For patients with RVO, studies have demonstrated a significant improvement in BCVA, sustaining over 12 months with ranibizumab treatment [15, 16, 36]. Another prospective randomized study (RABAMES) showed improvements in the logMAR BCVA at Weeks 4, 8 and 12 after ranibizumab treatment in 30 patients with BRVO [37]. Ranibizumab was associated with improved BCVA till 6 months in macular edema after BRVO. The short-term effects of ranibizumab were positively correlated with long-term improvements [38].
The visual acuity is worsened with very thin or thick retinas, thick subretinal tissue, atrophy, and scar [39]. A greater central macular thickness is indicative of ganglion cell damage due to traction in the ganglion, and a pharmacological treatment is expected to decrease the CMT with an accompanying increase in the visual acuity [39, 40]. In our study, a significant decrease in the CMT values were seen with biosimilar ranibizumab treatment, which are consistent with the findings from earlier studies in patients with wet AMD [41, 42], DME [43] and RVO [44].
The presence of IRF and SRF is a predictor of a worse visual acuity [45, 46]. Biosimilar ranibizumab has shown a consistent decrease in both the IRF and SRF values from baseline to Weeks 4, 8 and 12 endpoints in the current analysis. Previously reported studies with innovator ranibizumab have also demonstrated a decrease in IRF and SRF after ranibizumab administration in wet AMD [45], DME [47], and RVO patients [48]. There was a reduction in the proportion of patients having IRF or SRF after biosimilar ranibizumab treatment, similar to that seen in other prospective studies with innovator ranibizumab [45].
Limitations
Details pertaining to the bilaterality, previous treatments, ischemia and severity of the disease were not captured in the medical records and therefore could not be included in this study due to its retrospective nature. Another limitation is the short duration of the study at 3 months but still, the study results provide a clear picture about the effectiveness of the biosimilar ranibizumab in the real-world setting. Furthermore, in this study, visual acuity measurement was done by logMAR BCVA/Snellen’s values, which is considered inferior to ETDRS [49].
Since this study is retrospective in nature, the complete information on adverse events was not captured in the medical records and hence, not used in the analysis. However, there were no new safety concerns compared to what is known for the innovator product as per published information or no AEs were reported that were severe in nature.
Overrall, the intravitreal injection of Razumab® improved the visual acuity and decreased the macular thickness without new safety concerns in patients with wet age-related macular degeneration, diabetic macular edema, and retinal vein occlusion in the real-world setting.
Acknowledgments
The authors thank Mr. Shreekant Sharma (Lambda Therapeutic Research Ltd.) for providing writing assistance and Dr. Venugopal Madhusudhana (Lambda Therapeutic Research Ltd.) for additional editorial assistance for the development of this manuscript. The authors also thank the clinical data management and biostatistics department of Lambda Therapeutic Research Ltd. for their contribution in the preparation of statistical analysis report in this retrospective data collation and analysis.The authors are thankful to Dr. Ramandeep Sharma (Intas Pharmaceuticals Ltd.) for the conceptualization of the RE-ENACT study. The authors also thank Mr Tanishq Sharma (Shree Krishna Hospital and Pramukhswami Medical College, Karamsad, Anand, Gujarat, India) for conceptualization of the RE-ENACT study and also providing support for data collection and analysis.
Competing Interests
Drs. Shashikant Sharma, Mujtaba Khan, and Alok Chaturvedi are employees of Intas Pharmaceuticals Ltd, Ahmedabad.
Funding
RE-ENACT Study Investigator Group received an honorarium from Intas Pharmaceuticals Ltd. for their patient data contribution.
RE-ENACT Study Investigators Group
1. Dr. Manjunath Bhaskar Anandkumar, MS; Ganesh Netralaya, Sirsi, Karnataka, India, 581401. Email:manj009@gmail.com
2. Dr. Naresh Yadav, FRCS; Narayana Nethralaya Bengaluru, Karnataka, India, 560010. Email: vasudha.naresh@gmail.com
3. Dr. Vaibhav Shrivastava, MS; Shradha Eye Care, Kolkata, West Bengal, India, 700038. Email: vaibhav.shrivastava.28@gmail.com
4. Dr. Pasupathy Elankumaran, MD; Navasakthi Netralaya Bengaluru, Karnataka, India, 560043. Email: drelankumaran@gmail.com
5. Dr. Shrinivas Joshi, MS; MM Joshi Eye Institute Hubli, Karnataka, India, 580031. Email: shrinivasmjoshi@gmail.com
6. Dr. Subijay Sinha, MD; Susrut Eye Hospital Kolkata, West Bengal, India, 700106. Email: subijay.sinha@gmail.com
7. Dr. Ananyabrata Das, DOMS; Spectra Eye Hospital Kolkata, West Bengal, India, 700136. Email: drananyabrata@yahoo.com
8. Dr. Naveenam Srinivasa Muralidhar, MS; Retina Institute of Karnataka, Bengaluru, Karnataka, India, 560018. Email: retina. nsm@gmail.com
9. Dr. Sangita Jain, MS; Dev Bhumi Superspeciality Hospital, Dehradun, Uttarakhand, India, 248001. Email: sangitavrs@yahoo.co.uk
10. Dr. Prosenjit Mondal, MS; Susrut Eye Hospital Kolkata, West Bengal, India, 700106. Email: drprosenjit2007@gmail.com
11. Dr. Hemanth Murthy, MS; Retina Institute of Karnataka Bengaluru, Karnataka, India, 560018. Email: hemanthmurthy@yahoo.com
12. Dr. Alay Banker, MS; Bankers Retina Clinic Ahmedabad, Gujarat, India, 380014. Email: alay.banker@gmail.com
13. Dr. Nishikant Borse, MS;Insight Eye Clinic Mumbai, Maharashtra, India, 400014. Email: nishikantborse@yahoo.com
14. Dr. Girish Rao, MS; Sri Ganpati Netralaya, Jalna; Maharashtra, India, 431203. Email:drgr1968@gmail.com
15. Dr. Rajender Pal Singh, MD; Visitech Eye Centre Delhi, India, 110025. Email: rp@visitech.org
16. 16. Dr.Gunjan Prakash, MD; SPG Medicare & Diagnostics Agra, Uttar Pradesh, India, 282005. Email:gunjanprakash@gmail.com
References
- Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY,et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006 Oct 5;355(14):1432-44. PubMed PMID: 17021319.
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006 Oct 5;355(14):1419-31. PubMed PMID: 17021318.
- Cha DM, Kim TW, Heo JW, Woo SJ, Park KH, et al. Comparison of 1-year therapeutic effect of ranibizumab and bevacizumab for myopic choroidal neovascularization: a retrospective, multicenter, comparative study. BMC ophthalmology. 2014 Dec;14(1):69. PubMed PMID: 24884970.
- Danyliv A, Glanville J, McCool R, Ferreira A, Skelly A, Jacob RP. The clinical effectiveness of ranibizumab treat and extend regimen in nAMD: systematic review and network meta-analysis. Adv Ther. 2017 Mar;34(3):611-619. PubMed PMID: 28188433.
- García-Layana A, Figueroa MS, Arias L, Araiz J, Ruiz-Moreno JM, et al. Individualized therapy with ranibizumab in wet age-related macular degeneration. J Ophthalmol. 2015;2015:412903. PubMed PMID: 26491550.
- Dervenis N, Mikropoulou AM, Tranos P, Dervenis P. Ranibizumab in the Treatment of Diabetic Macular Edema: A Review of the Current Status, Unmet Needs, and Emerging Challenges. Adv Ther. 2017 Jun;34(6):1270-1282. PubMed PMID: 28484955.
- Ciulla TA, Amador AG, Zinman B. Diabetic retinopathy and diabetic macular edema: pathophysiology, screening, and novel therapies. Diabetes Care. 2003 Sep;26(9):2653-64. PubMed PMID: 12941734.
- Channa R, Sophie R, Khwaja AA, Do DV, Hafiz G, et al. Factors affecting visual outcomes in patients with diabetic macular edema treated with ranibizumab. Eye (Lond). 2014 Mar;28(3):269-78. PubMed PMID: 24263379.
- Massin P, Bandello F, Garweg JG, Hansen LL, Harding SP, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010 Nov;33(11):2399-405. doi: 10.2337/dc10-0493. PubMed PMID: 20980427.
- Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011 Apr;118(4):615-25. PubMed PMID: 21459215.
- Prünte C, Fajnkuchen F, Mahmood S, Ricci F, Hatz K, et al. Ranibizumab 0.5 mg treat-and-extend regimen for diabetic macular oedema: the RETAIN study. Br J Ophthalmol. 2016 Jun;100(6):787-95. PubMed PMID: 26453639.
- Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012 Apr;119(4):789-801. PubMed PMID: 22330964.
- Pearce I, Banerjee S, Burton BJ, Chakravarthy U, Downey L, et al. Ranibizumab 0.5 mg for diabetic macular edema with bimonthly monitoring after a phase of initial treatment: 18-month, multicenter, phase IIIB RELIGHT study. Ophthalmology. 2015 Sep;122(9):1811-9. PubMed PMID: 26150052.
- Payne JF, Wykoff CC, Clark WL, Bruce BB, Boyer DS, Brown DM. Randomized trial of treat and extend ranibizumab with and without navigated laser for diabetic macular edema: TREX-DME 1 year outcomes. Ophthalmology. 2017 Jan;124(1):74-81. Pubmed PMID: 27836430.
- Gerding H, Monés J, Tadayoni R, Boscia F, Pearce I, Priglinger S. Ranibizumab in retinal vein occlusion: treatment recommendations by an expert panel. Br J Ophthalmol. 2015 Mar;99(3):297-304. PubMed PMID: 25075121.
- Campochiaro PA, Heier JS, Feiner L, Gray S, Saroj N, et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010 Jun;117(6):1102-1112.e1. PubMed PMID: 20398941.
- Suñer IJ, Bressler NM, Varma R, Lee P, Dolan CM, et al. Reading speed improvements in retinal vein occlusion after ranibizumab treatment. JAMA Ophthalmol. 2013 Jul;131(7):851-6. PubMed PMID: 23699977.
- Sameera VV, Apoorva AG, Joshi S, Guruprasad AS. Safety and efficacy of Razumab-The new biosimilar in India:Our experience. Kerala J Ophthalmol. 2016 Sep 1;28(3):180.
- (Major) Sharma S, RE-ENACT Study Investigators Group, Khan MA, Chaturvedi A. Real Life Clinical Effectiveness of Razumab® (World’s First Biosimilar Ranibizumab) in Wet Age-Related Macular Degeneration: A Subgroup Analysis of Pooled Retrospective RE-ENACT Study. Int J Oph thalmol Eye Res. 2018;6(2):368-373.
- (Major) Sharma S, RE-ENACT Study Investigators Group, Khan MA, Chaturvedi A. Real Life Clinical Effectiveness of Razumab® (World’s First Biosimilar Ranibizumab) in Retinal Vein Occlusion: A Subgroup Analysis of Pooled Retrospective RE-ENACT Study. Ophthalmologica. DOI: 10.1159/000488602
- CADTH. Treating Retinal Diseases in the Era of Anti-VEGF Therapies. A Position Paper Regarding the CADTH Therapeutic Review. Anti-Vascular Endothelial Growth Factor Drugs for the Treatment of Retinal Conditions – Recommendations Report. September 2016. PubMed PMID: 28825785.
- Siddiqui PA, Warkhede P. Role of Ranibizumab in various retinal disorders. IOSR Journal of Dental and Medical Sciences. 2017 Mar;16(3):142-147.
- Bolz M, Simader C, Ritter M, Ahlers C, Benesch T, et al. Morphological and functional analysis of the loading regimen with intravitreal ranibizumab in neovascular age-related macular degeneration. Br J Ophthalmol. 2010 Feb;94(2):185-9. PubMed PMID: 19692384.
- Ercalik NY, Imamoglu S, Kumral ET, Yenerel NM, Bardak H, Bardak Y. Influence of the epiretinal membrane on ranibizumab therapy outcomes in patients with diabetic macular edema. Arq Bras Oftalmol. 2016 Nov-Dec;79(6):373-375. PubMed PMID: 28076563.
- Lucentis® (ranibizumab) ABBREVIATED UK PRESCRIBING INFORMATION. Eye (Lond). 2017 Aug;31(S2):S18-S20. doi: 10.1038/eye.2017.149. PubMed PMID: 28845845.
- Harrison L. Real-World Anti-VEGF Data Disappointing. Medscape. 2016 Aug 11.
- Harrison L. Real-World Anti-VEGF Results Fall Short in Macular Edema. Medscape. 2017 Aug 13.
- Wecker T, Ehlken C, Bühler A, Lange C, Agostini H, Böhringer D, Stahl A. Five-year visual acuity outcomes and injection patterns in patients with pro-re-nata treatments for AMD, DME, RVO and myopic CNV. Br J Ophthalmol. 2017 Mar 1;101(3):353-9.
- Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology. 2009 Jan 1;116(1):57-65. PubMed PMID: 19118696.
- Figurska M, Stankiewicz A. Effectiveness of ranibizumab intravitreal injections for exudative age-related macular degeneration treatment: 12-month outcomes. Med Sci Monit. 2011 Sep;17(9):CR485-90. PubMed PMID: 21873944.
- Querques G, Azrya S, Martinelli D, Berboucha E, Feldman A, et al. Ranibizumab for exudative age-related macular degeneration: 24-month outcomes from a single-centre institutional setting. Br J Ophthalmol. 2010 Mar;94(3):292-6. PubMed PMID: 19951942.
- Cho G, Cho J, Woo S. Short-term efficacy and safety of ranibizumab for neovascular age-related macular degeneration in the real world using the regulatory post-marketing surveillance study in South Korea. 17th EURETINA Congress, Barcelona, Spain. 2017.
- Gonzalez VH, Campbell J, Holekamp NM, Kiss S, Loewenstein A, et al. Early and Long-Term Responses to Anti–Vascular Endothelial Growth Factor Therapy in Diabetic Macular Edema: Analysis of Protocol I Data. Am J Ophthalmol. 2016 Dec;172:72-79. PubMed PMID: 27644589.
- Lai IA, Hsu WC, Yang CM, Hsieh YT. Prognostic factors of short-term outcomes of intravitreal ranibizumab in diabetic macular edema. Int J Ophthalmol. 2017 May 18;10(5):765-771. PubMed PMID: 28546935.
- Minami Y, Nagaoka T, Ishibazawa A, Yoshida A. Short-term effects of intravitreal ranibizumab therapy on diabetic macular edema. BMC Ophthalmol. 2017 Mar 14;17(1):28. doi: 10.1186/s12886-017-0420-8. PubMed PMID: 28292270.
- Brown DM, Campochiaro PA, Singh RP, Li Z, Gray S, et al. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010 Jun;117(6):1124-1133.e1. PubMed PMID: 20381871.
- Pielen A, Mirshahi A, Feltgen N, Lorenz K, Korb C, et al. Ranibizumab for Branch Retinal Vein Occlusion Associated Macular Edema Study (RABAMES): six‐month results of a prospective randomized clinical trial. Acta Ophthalmol. 2015 Feb;93(1):e29-37. PubMed PMID: 25042729.
- Minami Y, Nagaoka T, Ishibazawa A, Yoshida A. Correlation between shortand long-term effects of intravitreal ranibizumab therapy on macular edema after branch retinal vein occlusion: a prospective observational study. BMC Ophthalmol. 2017 Jun 13;17(1):90. doi: 10.1186/s12886-017-0485-4. PubMed PMID: 28610573.
- Jaffe GJ, Martin DF, Toth CA, Daniel E, Maguire MG, et al. Macular morphology and visual acuity in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2013 Sep 1;120(9):1860-70. PubMed PMID: 23642377.
- Bong A, Doughty MJ, Button NF, Mansfield DC. On the relationship between visual acuity and central retinal (macular) thickness after interventions for macular oedema in diabetics: a review. Clin Exp Optom. 2016 Nov;99(6):491-497. PubMed PMID: 27320640.
- Castiblanco CP, Adelman RA. Visual Acuity and Central Macular Thickness in Patients Treated With Anti-Angiogenic Intravitreal Injections for Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci. 2008 May 14;49(13):267.
- Suthar T, Lieberman RM, Rhee DY. Effect of Intravitreal Ranibizumab (Lucentis ®) on the Foveal and Macular Thickness in the Contralateral Eye in Patients with Neovascular Age Related Macular Degeneration. Invest Ophthalmol Vis Sci. 2012 Mar 26;53(14):5160.
- Fouda SM, Bahgat AM. Intravitreal aflibercept versus intravitreal ranibizumab for the treatment of diabetic macular edema. Clin Ophthalmol. 2017 Mar 23;11:567-571. PubMed PMID: 28356711.
- Brown DM, Wykoff CC, Wong TP, Mariani AF, et al. Ranibizumab in preproliferative (ischemic) central retinal vein occlusion: the rubeosis anti- VEGF (RAVE) trial. Retina. 2014 Sep;34(9):1728-35. doi: 10.1097/IAE.0000000000000191. PubMed PMID: 24914476.
- Sharma S, Toth CA, Daniel E, Grunwald JE, Maguire MG, Ying GS, et al. Macular morphology and visual acuity in the second year of the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2016 Apr;123(4):865-75. PubMed PMID: 26783095.
- Arnold JJ, Markey CM, Kurstjens NP, Guymer RH. The role of sub-retinal fluid in determining treatment outcomes in patients with neovascular agerelated macular degeneration-a phase IV randomised clinical trial with ranibizumab: the FLUID study. BMC Ophthalmol. 2016 Mar 24;16:31. doi: 10.1186/s12886-016-0207-3. PubMed PMID: 27009515.
- Heng LZ, Pefianaki M, Hykin P, Patel PJ. Interobserver agreement in detecting spectral-domain optical coherence tomography features of diabetic macular edema. PLoS One. 2015 May 21;10(5):e0126557. PubMed PMID: 25996150.
- Tadayoni R, Waldstein SM, Boscia F, Gerding H, Pearce I, et al. Individualized Stabilization Criteria–Driven Ranibizumab versus Laser in Branch Retinal Vein Occlusion: Six-Month Results of BRIGHTER. Ophthalmology. 2016 Jun;123(6):1332-44. PubMed PMID: 27039022.
- Kaiser PK. Prospective evaluation of visual acuity assessment: a comparison of snellen versus ETDRS charts in clinical practice (An AOS Thesis). Trans Am Ophthalmol Soc. 2009 Dec;107:311-24. PubMed PMID: 20126505.