Novel Retinal Imaging Technologies
Li Y1,2, Xia X1, Paulus YM2,3*
1 Department of Ophthalmology, Xiangya Hospital, Central South University, Changsha, China.
2 Department of Ophthalmology and Visual Sciences, University of Michigan, Ann Arbor, MI, USA.
3 Department of Biomedical Engineering, University of Michigan, Ann Arbor, MI, USA..
*Corresponding Author
Yannis M. Paulus, M.D,
Department of Biomedical Engineering,
University of Michigan, Kellogg Eye Center,
1000 Wall Street, Ann Arbor, MI 48105, USA.
Tel: +1-734-232-8105
Fax: +1-734-936-3815
E-mail: ypaulus@med.umich.edu
Received: June 26, 2017; Published: July 27, 2017
Citation: Li Y, Xia X, Paulus YM (2017) Novel Retinal Imaging Technologies. Int J Ophthalmol Eye Res. 5(7), 1-5. doi: dx.doi.org/10.19070/2332-290X-1700010e
Copyright: Paulus YM© 2017. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Newly-developed imaging techniques show extensive promise and potential to improve early detection, accurate diagnosis, and management of retinal diseases. Optical coherernce tomography angiography (OCTA), photoacoustic imaging (PAI), and molecular imaging (MI) are all new and promising imaging modalities. As these imaging instruments have advanced, they have enabled visualization of the retina at an unprecedented resolution. Published studies have established the efficacy of these modalities in the assessment of common retinal diseases, such as age-related macular degeneration, diabetic retinopathy, and retinal vascular occlusions. Each of these systems is built upon different principles and all have different limitations. In addition, the three imaging modalities have complementary features and thus can be integrated in to a multimodal imaging system, which will be more powerful in future.
2.Introduction
3.Optical Coherence Tomography Angiography (OCTA)
3.1 Principles Of OCTA
3.2 Applications Of OCTA
3.3 Limitation And Prospects
4.Photoacoustic Imaging (PAI)
4.1 Principle Of PAI
4.2 Applications Of PAI
4.3 Limitation And Prospects
5.Molecular Imaging
5.1 Principle Of MI
5.2 Applications Of MI
5.3 Limitation And Prospects
6.References
Keywords
Retina; Optical Coherence Tomography Angiography (OCTA); Photoacoustic Imaging (PAI); Molecular Imaging (MI).
Introduction
The retina is a critical part of the eye that contains abundant blood vessels and a variety of cellular structures. Retinal manifestations of disease can have serious consequences and lead to significant vision loss and blindness. Imaging of the retina plays an important role in the discovery and evaluation of retinal pathophysiology in health and disease. With disease progression, imaging plays a critical role in disease diagnosis, monitoring, and assessing response to therapy. This review focuses on three novel, emerging retinal imaging technologies: optical coherence tomography angiography (OCTA), photoacoustic imaging (PAI), and molecular imaging (MI). It will be introduced from the following aspects: their fundamentals and basic principles, current clinical applications in retina, and their limitations and future prospects.
Optical Coherence Tomography Angiography (OCTA)
Optical coherence tomography angiography (OCTA) is a novel, non-invasive imaging modality. It can simultaneously obtain detailed structural and blood flow data within retinal and choroidal vessels. OCTA imaging occurs by motion contrast processing of decorrelation signals. In the past, optical coherence tomography (OCT), fluorescein angiography (FA), and indocyanine green angiography (ICGA) have been established as essential imaging modalities in the diagnosis and management of retinal diseases. OCT can non-invasively image intraocular structures in vivo with a high resolution approaching that of histological sections. OCT has advantages for the diagnosis of macular diseases such as macular hole, macular edema, and epiretinal membrane. It does not require invasive injections, and it is easier and faster to acquire. But since the similar reflectivities, OCT may not distinguish choroidal neovascularization (CNV) from subretinal fibrosis, fibrotic pigment epithelial detachments (PED), hemorrhage, or adjacent retinal tissue, such as retinal pigment epithelium (RPE) and Bruch’s membrane. FA originated in the 1960s and is the gold standard for retinal vascular imaging. FA uses several fluorescent patterns, such as leakage, pooling, staining, blockage, window defects, and the dye transit time to show vascular dynamic changes [1]. Whereas the choroidal vasculature wall is permeable to fluorescein, the choroidal vascular is not freely permeable to ICG, so ICGA can be used to image the choroid, neovascular membranes beneath the RPE, and polypoidal choroidal vasculopathy (PCV). However, both FA and ICGA involve invasive injections of exogenous dyes, and the dyes pose risks ranging from nausea and vomiting to allergic reactions, including anaphylaxis and rarely death. The three kinds of imaging methods have their drawbacks that will limit their application. For the patients that can not tolerate intravenous dye injection or those who require frequent examinations, it would be beneficial to use a non-invasive technique to visualize retinal and choroidal vessels. OCTA is the technique that acquires volumetric angiographic information without using exogenous dye.
OCTA provides vascular imaging via motion contrast processing of decorrelation signals. The variable signal intensities and amplitudes, which obtained from sequential B scans in the posterior segment area, generate decorrelation signals. Through software removal of bulk axial movement, the remaining difference in signal amplitude between sequential B scans is due to erythrocyte flow in the vasculature. Therefore, the retina and choroid functional vascular information is produced by the difference of signals and amplitudes, which can be processed by multiple algorithms.
Three main classes of imaging algorithms exist: phase based, amplitude based, and complex algorithm based. Nowadays, the split-spectrum amplitude decorrelation angiography (SSADA) is the most common algorithm [2], which is used with the spectral domain OCTA (SD-OCTA) machine. Using the SSADA OCTA algorithm, images will be provided with a higher quality via optimization of the signal-to-noise ratio of flow detection.
Since the retina is a laminar structure with corresponding stratification of blood supply, en-face visualization of the corresponding vascular supply for the layer provides the best visualization of the vascular layers. Based on different software processes, enface zones was been divided into 3 or 4 parts. The 3 parts include: the inter retina, which is defined from the internal limiting membrance (ILM) to the outer boundary of the outer plexiform layer (OPL); the outer retina, that is defined from the outer OPL to the Bruch’s membrane (BM); and the choroidal layer is defined as below BM. The 4 enface zones contain: the superficial plexus, the deep plexus, the outer retina, and the choroicapillaries.
Spaide et al.,[3] demonstrated that with detection of 12 eyes, fluorescein angiography couldn’t visualize the radial peripapillary capillary network around the optic nerve head completely, whereas the network was visualized by the SSADA scans. Yali Jia et al.,[4] used a highspeed (100,000 A-scans/sec) 1050 nm wavelength sweptsource optical coherence tomography (SS-OCT) to scanned five neovascular AMD and five normal eyes. Flow was detected by the SSADA algorithm. They determined that OCTA provided more distinct vascular network patterns that were less obscured by subretinal hemorrhage. OCTA acquires depth-resolved information and detailed images of CNV in neovascular AMD. Waheed et al.,[5] compared visualization of CNV using SD-OCTA (operating at 840nm wavelength) vs SS-OCTA (operating at 1050 nm wavelength). They both used the two systems to measure type 1, type 2, and mixed type CNV in exudative age-related macular degeneration (AMD). Due to the longer wavelength of SS-OCTA, it yielded significantly larger CNV areas than SD-OCTA. SS-OCTA may be able to overcome signal attenuation, such as from the RPE, media opacities, and any overlying hemorrhage. Takase et al., [6] detected the area of the foveal avascular zone (FAZ) by en-face OCTA (AngioVue, Avanti OCT; Optovue), and then evaluated the diferences between healthy and diabetic eyes. They found that the FAZ area in the deep plexus layer was significantly larger in diabetic eyes than in healthy eyes. Therefore they concluded that en-face OCTA is a useful non-invasive screening tool for detecting early microcirculatory disturbance in diabetic patients. Samara et al., [7] measured the vascular density and FAZ area in deep and superficial retinal vascular networks using OCTA in 17 patients with branch retinal vein occlusion (BRVO). The BRVO eyes were compared with their fellow eyes, and the study determined that the superficial and deep vascular density in BRVO eyes measured by quantitative OCTA was decreased. The mean FAZ area in BRVO eyes was significantly lower in the deep vascular network when compared to the fellow eyes. Moreover, vascular density and FAZ area appear to correlate with visual function. Adeleh et al., [8] evaluated the association between vessel density and severity of visual field loss in primary open-angle glaucoma (POAG). They imaged 153 eyes, which contain 31 normal eyes, 48 glaucoma suspects, and 74 glaucoma eyes, by OCTA (Angiovue; Optovue, Fremont, CA). The study concluded that decreased vessel density was significantly associated with the severity of visual field damage independent of the structural loss. So OCTA is a promising technology in glaucoma management.
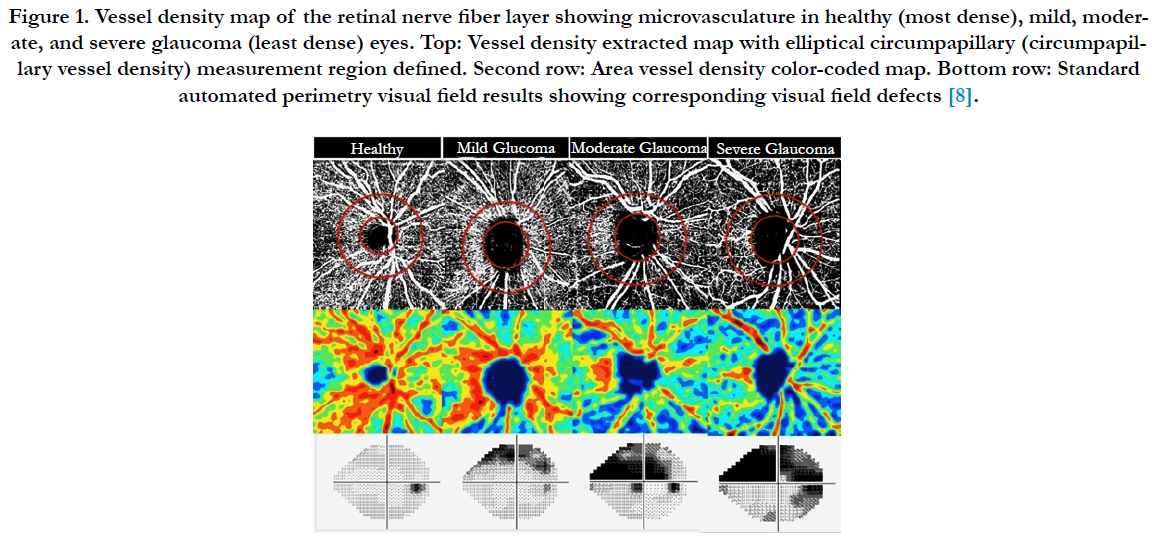
The above application of OCTA has shown promise in the evaluation of common ophthalmologic diseases such as AMD, diabetic retinopathy, retinal vascular occlusions (RVO) and glaucoma. OCTA provide an available and noninvasive device to monitoring CNV in AMD [9, 10] and other related disorders. It is helpful to image the FAZ in eyes with unexplained visual loss and can provide information of the deep retinal capillary (Figure 1).
Figure 1. Vessel density map of the retinal nerve fiber layer showing microvasculature in healthy (most dense), mild, moderate, and severe glaucoma (least dense) eyes. Top: Vessel density extracted map with elliptical circumpapillary (circumpapillary vessel density) measurement region defined. Second row: Area vessel density color-coded map. Bottom row: Standard automated perimetry visual field results showing corresponding visual field defects [8].
Despite potential benefits and broad application of OCTA in various retinal diseases [11], OCTA exist some other unavoidable limitations. In the past, the major and most reported limitation is several type of artifacts that require careful interpretation. Although different methods, such as eye tracking software and projection-resolved OCTA, have been employed to help remove these artifacts, correction of artifacts entirely is also challenging. Another limitation includes the inability to show leakage [12] and a limited view of microaneurysms, both of which give us very important information in disease analysis. In addition, imaging a larger field of view [13] and monitoring slow flow (e.g. microaneurysms) more sensitively are important areas for future development. OCTA is a novel and evolving field. It will be advantageous in improving patient care and reducing the morbidity of retinal diseases through earlier detection and intervention [10, 11].
Photoacoustic Imaging (PAI)
Photoacoustic imaging is a promising imaging technique that is particularly useful for imaging deep retinal and choroidal structures without compromising the spatial resolution
[14]. It has been demonstrated to image hemoglobin, melanin, and exogenous contrast agents [15]. OCT and ultrasound (US) techniques are widely used methods for diagnostic imaging of the eye. They detect discontinuities in optical refractive index and acoustic impedance, respectively. PAI depicts optical absorption, which is independent of tissue characteristics imaged by OCT and US.
PAI is based on optical excitation and ultrasonic detection. A short pulse laser (nanosecond pulse duration) illuminates and excites the eye. The retina of the eye absorbs some of the delivered laser energy, generates heat, undergoes transient thermoelastic expansion, and produces ultrasonic signal. An ultrasound transducer is focused on the retinal surface and detects ultrasonic signals, which are then imaged for both anatomic PAI and functional analysis [16-18].
Since 2010, several groups have built ocular PA imaging systems and reported imaging for the eye. Our group performed PAI of an enucleated pig eye and the eye of living rabbits. We acquired 3D images of the posterior pole (retina, choroid, optic nerve) of the rabbit eye by ultrasound and photoacoustic imaging [18-20]. Silverman et al., [21] imaged fresh ex vivo pig eyes by PAI and US. They demonstrated choroidal/scleral images, and the PAI image shows sharp depiction of the choroidal surface but with limited penetration due to the strong absorption of the green laser by melanin. This article demonstrated high-resolution PAI of ex vivo eyes. Zhang et al., [22] showed a system simultaneously acquired photoacoustic ophthalmoscopy (PAOM) and OCT B-scan images of eyes. They demonstrate contrast mechanism of PAOM is that the stronger the optical absorption, the stronger the generated ultrasonic signals. They demonstrate that the hemoglobin in the blood vessels and the melanin in the RPE cells are strong absorbers of the illuminating light, in contrast other retinal tissues have very low optical absorption. Due to the difference of optical absorption, we can get the functional and anatomical imaging of the blood vessels and the RPE.
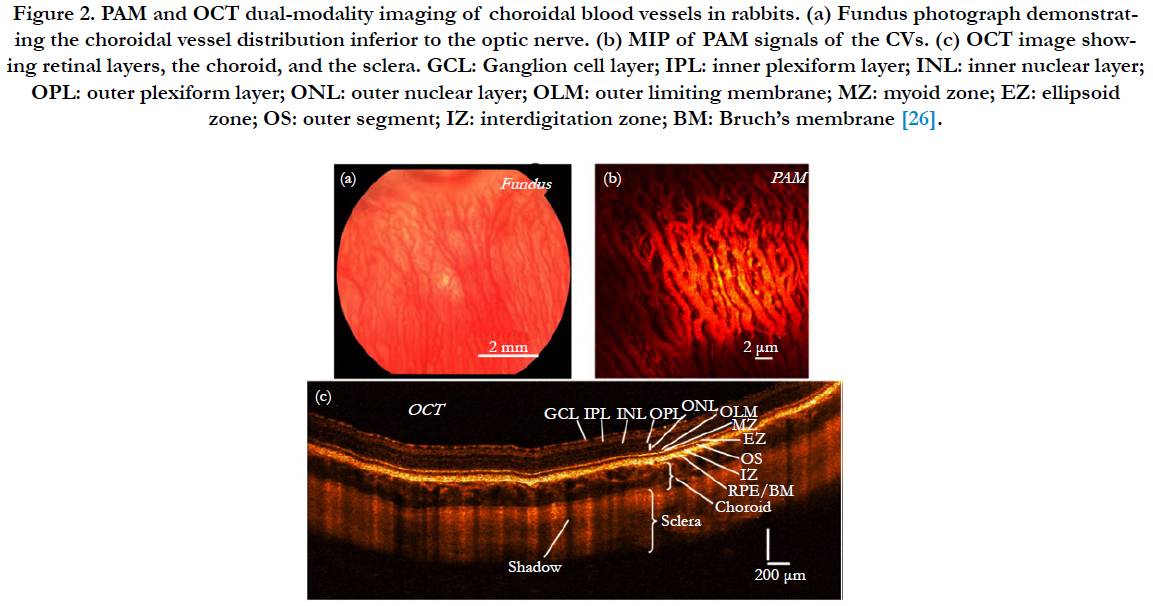
Moreover, PAI as an ophthalmic imaging modality is based on optical absorption, it can be integrated with other well-established clinical ophthalmic imaging techniques in order to acquire more detailed anatomic and functional evaluation of the eye by multiple optical contrasts [17, 23]. Song et al., [24] developed a retinal imaging platform integrating PAOM with scanning laser ophthalmoscopy (SLO), SD-OCT, and FA. In this system, all the imaging modalities shared the same optical scanning and delivery mechanisms, which enable registered retinal imaging from all the modalities. They use this system to achieve high-quality in vivo images in rat eyes. Liu et al., [25] successfully measured retinal oxygen saturation using an integrated PAOM and OCT system. The proposed imaging modality can be a powerful tool for the investigation of the pathophysiology of retinal diseases and retinal disease detection in clinical practice. We have also recently developed a multimodal system which contains PAI, OCT, and fluorescence microscopy which we are using to image the vasculature in vivo of rabbit and mice eye [26-30] (Figure 2).
Figure 2. PAM and OCT dual-modality imaging of choroidal blood vessels in rabbits. (a) Fundus photograph demonstrating the choroidal vessel distribution inferior to the optic nerve. (b) MIP of PAM signals of the CVs. (c) OCT image showing retinal layers, the choroid, and the sclera. GCL: Ganglion cell layer; IPL: inner plexiform layer; INL: inner nuclear layer; OPL: outer plexiform layer; ONL: outer nuclear layer; OLM: outer limiting membrane; MZ: myoid zone; EZ: ellipsoid zone; OS: outer segment; IZ: interdigitation zone; BM: Bruch’s membrane [26].
PAI is expected to find broad applications in retinal diseases. It has provided us with abundant experience and knowledge in preclinical studies [31]. PAI has been able to map the hemoglobin oxygen saturation in retinal vessels, which is critical in studying the physiology and pathology of various blinding diseases such as diabetic retinopathy (DR) and neovascular AMD. Multimodal imaging systems will likely play an important role in the development of PAI in future, especially to promote the evaluation of PAI compared with other modalities in both clinical use and fundamental investigation in detecting and management blinding diseases [23, 32, 33]. Future improvements in laser sources and detectors, image processing software, and eye tracking to reduce the influence of eye movement will open up opportunities for PAI in retinal diseases detection and treatment methods.
Molecular Imaging
Molecular imaging of the eye plays an increasingly powerful role in ocular therapeutic discovery in preclinical processes. The images of the retina will be at the cell and molecular level. Molecular imaging can be used both in basic research and in clinical ophthalmology. The developments have important implications in early disease detection, treatment surveillance, and targeted therapy in a range of pathologies.
Molecular imaging can take place using multiple imaging systems and some special biomarkers [34]. The modalities for MI may include PAI, optical imaging, ultrasound, OCT, and fluorescence to obtain fundamental images at the cellular and molecular level. Biomarkers are important as imaging tools and targets, such as intrinsic imaging contrast in tissue, targeted injectable contrast agents, reporter gene imaging and cell labelling, antibody labelling, protein expression, enzyme activity, nanoparticles, and apoptosis [35].
MI can precede retinal structural changes in a number of diseases lead to vision loss, including AMD, glaucoma, diabetic retinopathy, and retinopathy of prematurity [34, 36, 37]. King et al., [38] suggests that ROS a good early indicator of the changes of disease- related in the RPE. Takeda et al., [39] indicates that the C-C chemokine receptor 3 (CCR3) is a possible target for AMD diagnosis and therapy. CCR3 can play a role as a potentially important biomarker of CNV in AMD. Tsuda et al., [37] used a cell-impermeable, fluorescent nucleic acid dye compound, SYTOX orange (SO) as the biomarker. Confocal scanning laser ophthalmoscope (cSLO) is used as the imaging modality. In a murine modal with optic nerve crushed (ONC), SO was injected into the vitreous cavity. After ten minutes, retinal ganglion cells (RGC) death was visualized with cSLO in vivo. Real time imaging with SO was able to quickly quantify RGC death. Evans et al., [40] discovered HYPOX- 1 and HYPOX-2 are a promising step toward translation of hypoxia-sensitive molecular imaging agents in preclinical animal models and patients. Dearling et al., [41] reviewed the use of molecular imaging to measure in vivo distribution of nanoparticles, and summerized that nanoparticles are an important and promising target for developing the application of molecular imaging. Thus molecular imaging of the retina is poised to play an important role in disease diagnosis and monitoring, as well as assessment of therapeutic efficacy.
Molecular imaging act as a great powerful method to translate basic science into clinical application. It provides a way to evaluate and quantify pathways in both animal modals and patients. In addition, molecular imaging can be used in targeted, individualized therapies and pharmacodynamic measurements for optimal dosing [23]. Molecular imaging holds significant promise as a novel tool as further biomarkers are developed.
References
- Malihi M, Jia Y, Gao SS, Flaxel C, Wilson DJ, et al., (2017) Optical coherence tomographic angiography of choroidal neovascularization ill-defined with fluorescein angiography. Br j ophthalmol. 101(1): 45-50, doi:10.1136/bjophthalmol-2016-309094.
- Chalam KV, Sambhav K (2016) Optical Coherence Tomography Angiography in Retinal Diseases. J ophthalmic vis res. 11(1): 84-92. doi:10.4103/2008-322X.180709.
- Spaide RF, Klancnik JM, Cooney MJ (2015) Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA ophthalmology. 133(1): 45-50. doi:10.1001/jamaophthalmol. 2014.3616.
- Jia Y, Bailey ST, Tan O, Liu JJ, Lu CD, et al., (2014) Quantitative optical coherence tomography angiography of choroidal neovascularization in age-related macular degeneration. Ophthalmol. 121(7): 1435-1444. doi:10.1016/j.ophtha.2014.01.034.
- Novais EA, Moult EM, Lee B, Dang S, Witikin AJ, et al., (2016) Choroidal Neovascularization Analyzed on Ultrahigh-Speed Swept-Source Optical Coherence Tomography Angiography Compared to Spectral-Domain Optical Coherence Tomography Angiography. Am j ophthalmol. 164: 80-88. doi:10.1016/j.ajo.2016.01.011).
- Takase N, Nozaki M, Kato A, Oqura Y, Ozeki H, et al., (2015) Enlargement of Foveal Avascular Zone in Diabetic Eyes Evaluated by En Face Optical Coherence Tomography Angiography. Retina. 35(11): 2377-2383. doi:10.1097/IAE.0000000000000849.
- Samara WA, Sridhar J, Ho AC, Hsu J, Khan MA, et al., (2016) Quantitative Optical Coherence Tomography Angiography Features and Visual Function in Eyes With Branch Retinal Vein Occlusion. Am j ophthalmol. 166: 76-83. doi:10.1016/j.ajo.2016.03.033.
- Yarmohammadi A, Zanqwil LM, Diniz-Filho A, Saunders LJ, Medeiros FA, et al., (2016) Relationship between Optical Coherence Tomography Angiography Vessel Density and Severity of Visual Field Loss in Glaucoma. Ophthalmol. 123(12): 2498-2508. doi:10.1016/j.ophtha.2016.08.041.
- Sambhav K, Grover S, Chalam KV (2017) The Application of Optical Coherence Tomography Angiography in Retinal Diseases. Surv ophthalmol. doi:10.1016/j.survophthal.2017.05.006.
- Ma J, Desai R, Nesper P, Gil M, Skondra D, et al., (2017) Optical Coherence Tomographic Angiography Imaging in Age-Related Macular Degeneration. Ophthalmol eye dis. 9: 1179172116686075, doi:10.1177/1179172116686075.
- Falavarjani KG, Sarraf D (2017) Optical coherence tomography angiography of the retina and choroid; current applications and future directions. J curr ophthalmol. 29(1): 1-4. doi:10.1016/j.joco.2017.02.005.
- Spaide RF, Fujimoto JG, Waheed NK (2015) Optical Coherence Tomography Angiography. Retina. 35(11): 2161-2162. doi:10.1097/IAE.0000000000000881.
- . Mo S, Garq R, Carroll J, Rosen B, Chui TY, et al., (2017) Visualization of Radial Peripapillary Capillaries Using Optical Coherence Tomography Angiography: The Effect of Image Averaging. PloS one. 12(1): e0169385. doi:10.1371/journal.pone.0169385.
- Hu Z, Wang X, Liu Q, Paulus YM (2015) Photoacoustic Imaging in Opthalmaology. Int J Ophthalmol Eye Res. 3(8): 126-132.
- Wang LV, Hu S (2012) Photoacoustic tomography: in vivo imaging from organelles to organs. Science. 335(6075): 1458-1462. doi:10.1126/science. 1216210.
- Hu Z, Liu Q, Paulus YM (2016) New Frontiers in Retinal Imaging. Int J Ophthalmic Res. 2(3): 148-158. doi:10.17554/j.issn.2409-5680.2016.02.48.
- Liu W, Zhang HF (2016) Photoacoustic imaging of the eye: A mini review. Photoacoustics. 4(3): 112-123. doi:10.1016/j.pacs.2016.05.001.
- de la Zerda A, Paulus YM, Robert Teed, Bodapati S, Dollberg Y, Khuri-Yakub, BT, Blumenkranz, MS, Moshfeqhi M, Gambhir SS (2010) Photoacoustic ocular imaging. Opt lett. 35(3): 270-272. doi:10.1364/OL.35.000270.
- Tian C, Qian W, Liu S, Liu B, Wang X, et al., (2016) Plasmonic Nanoparticles with Quantitatively Controlled Bioconjugation for Photoacoustic Imaging of Live Cancer Cells. Adv Sci. 3(12): 1600237. doi:10.1002/advs.201600237.
- Tian C, Ting Feng, Cheng Wang, Shengchun Liu, Qian Cheng, et al., (2016) Non-Contact Photoacoustic Imaging Using a Commercial Heterodyne Interferometer. Ieee sensors j. 16(23): 8381-8388.
- Silverman RH, Kong F, Kim HH, Chen YC, shungg HH, et al., (2010) High-resolution photoacoustic imaging of ocular tissues. Ultrasound med biol. 36(5): 733-742. doi:10.1016/j.ultrasmedbio.2010.02.006.
- Jiao S, Jiang M, Fawzi A, Shung KK, Carmen A, et al., (2010) Photoacoustic ophthalmoscopy for in vivo retinal imaging. Optics express 18, 3967-3972, doi:10.1364/OE.18.003967.
- Zackrisson S, van de Ven SM, Gambhir SS (2014) Light in and sound out: emerging translational strategies for photoacoustic imaging. Cancer res. 74(4): 979-1004, doi:10.1158/0008-5472.CAN-13-2387.
- Song W, Wei Q, Kuai D, Jiao S, Liu T, et al., (2012) Integrating photoacoustic ophthalmoscopy with scanning laser ophthalmoscopy, optical coherence tomography, and fluorescein angiography for a multimodal retinal imaging platform. J biomedical optics. 17(6): 061206. doi:10.1117/1. JBO.17.6.061206.
- Liu W, Zhang HF (2014) Noninvasive in vivo imaging of oxygen metabolic rate in the retina. Conf proc IEEE Eng Med Biol Soc. 3865-3868. doi:10.1109/EMBC.2014.6944467.
- Tian C, Zhang W, Mordovanakis A, Wang X, Paulus YM (2017) Noninvasive chorioretinal imaging in living rabbits using integrated photoacoustic microscopy and optical coherence tomography. Optics express. 25(14): 15947-15955.
- Kim KH, Luo W, Zhang C, Tian C, Fan X, et al., (2017) Air-coupled ultrasound detection using capillary-based optical ring resonators. Sci rep. 7(1): 109. doi:10.1038/s41598-017-00134-7.
- Keswani RK, Tian CC, Peryea T, Wang X, Girish G, et al., (2016) Repositioning Clofazimine as a Macrophage-Targeting Photoacoustic Contrast Agent. Sci rep. 6: 23528. doi:10.1038/srep23528.
- Tian C, Wang W, Cheng Q, Wang X, Liu S, et al., (2015) Dual-pulse nonlinear photoacoustic technique: a practical investigation. Biomed opt express. 6(8): 2923-2933. doi:10.1364/BOE.6.002923.
- Tian C, Xie Z, Fabiilli ML, Wang X (2015) Imaging and sensing based on dual-pulse nonlinear photoacoustic contrast: a preliminary study on fatty liver. Opt lett. 40(10): 2253-2256. doi:10.1364/OL.40.002253.
- Liu T, Li H, Song W, Jiao S, Zhang HF (2013) Fundus camera guided photoacoustic ophthalmoscopy. Curr eye research. 38(12): 1229-1234. doi:10.3 109/02713683.2013.815219.
- Song W, Wei Q, Feng L, Liu X, Zhang HF, et al., (2013) Multimodal photoacoustic ophthalmoscopy in mouse. J biophotonics. 6(6-7): 505-512. doi:10.1002/jbio.201200061.
- Kim J, Lee D, Jung U, Kim C (2015) Photoacoustic imaging platforms for multimodal imaging. Ultrasonography. 34(2): 88-97.doi:10.14366/ usg.14062.
- Capozzi ME, Gordon AY, Penn JS, Jayagopal A (2013) Molecular imaging of retinal disease. J ocul pharmacol ther. 29(2): 275-286. doi:10.1089/ jop.2012.0279.
- Eter N (2010) Molecular imaging in the eye. Br j ophthalmol. 94(11): 1420- 1426, doi:10.1136/bjo.2009.158105.
- Frimmel S, Zandi S, Sun D, Zhang Z, Melhorn MI, et al., (2017) Molecular Imaging of Retinal Endothelial Injury in Diabetic Animals. J ophthalmic vis res. 12(2): 175-182. doi:10.4103/jovr.jovr_243_16.
- Tsuda S, Tanaka Y, Ito A, Yasuda M, Nakazawa T, et al., (2016) Real-time imaging of RGC death with a cell-impermeable nucleic acid dyeing compound after optic nerve crush in a murine model. Exp eye res. 146: 179-188. doi:10.1016/j.exer.2016.03.017.
- King A, Gottlieb E, Brooks DG, Murphy MP, Dunaief JL (2004) Mitochondria- derived reactive oxygen species mediate blue light-induced death of retinal pigment epithelial cells. Photochem photobiol. 79(5): 470-475.
- Takeda A, Baffi JZ, Cho WG, Nozaki M, Pan Y, et al., (2009) CCR3 is a target for age-related macular degeneration diagnosis and therapy. Nature. 460(7252): 225-230. doi:10.1038/nature08151.
- Evans SM, Kim M, Craft JR, Uddin MI, Moore CE, et al., (2014) Molecular probes for imaging of hypoxia in the retina. Bioconjug chem. 25(11): 2030- 2037. doi:10.1021/bc500400z.
- Dearling JLJ, Packard AB (2017) Molecular imaging in nanomedicine - A developmental tool and a clinical necessity. J Control Release. 261: 23-30. doi:10.1016/j.jconrel.2017.06.011.