Modified Osteo Odonto Keratoprosthesis (MOOKP) - "Tooth For An Eye"
Manikandan.G.R1*, Presanthila J2
1 Junior Resident, Department of Periodontics, Government Dental College, Trivandrum, India.
2 Professor & HOD, Principal, Department of Periodontics, Government Dental College, Trivandrum, India.
*Corresponding Author
Dr.Manikandan.G.R,
Junior Resident,
Department of Periodontics,
Government Dental College,
Trivandrum, India.
Tel: 9496815829
E-mail:bellrings4u@gmail.com
Article Type :Review Article
Received: July 16, 2015; Accepted: September 12, 2015; Published: September 21, 2015
Citation: Manikandan.G.R, Presanthila J (2015) Modified Osteo Odonto Keratoprosthesis (MOOKP)-‘Tooth For An Eye”. Int J Ophthalmol Eye Res 03(8), 133-137. doi: dx.doi.org/10.19070/2332-290X-1500028
Copyright: Manikandan.G.R© 2015. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium,provided the original author and source are credited.
Abstract
Osteo-odonto-keratoprosthesis (OOKP) (also known as "tooth in eye" surgery is a medical procedure to restore vision in the most severe cases of corneal and ocular surface patients. It includes removal of a tooth from the patient or a donor. After removal, a lamina of tissue cut from the tooth is drilled and the hole is fitted with optics. The lamina is grown in the patients' cheek for a period of months and then is implanted upon the eye. This is an excellent innovative technique blending the ophthalmology and dentistry.
2.Introduction
3.Modified OOKP
4.History
5.Indications For OOKP[12]
6.Contraindications[13]
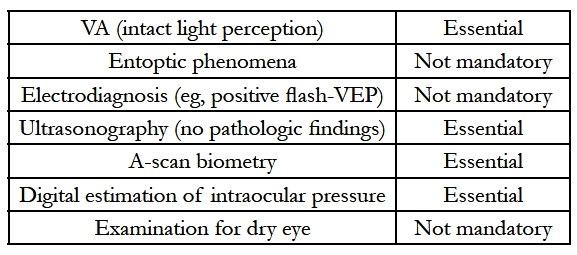
7.Preoperative Ophthalmological Assessment[16]
8.Preoperative Oral Assessment
8.1. Preparation of the Mucous Membrane Covering
9.Surgical Technique[17-19]
10.PMMA Cylinder
11.Complications[27]
12.Ocular
13.Mucous Membrane/Odal
14.Oral
15.Future Directions
16.Conclusion
17.References
Keywords
OOKP; PMMA Cylinder; Plastic Cylinder; Retroprosthetic Membrane.
Introduction
In some eyes, severe damage to the cornea and ocular surface occurs due to drug allergy, or chemical or thermal burns – resulting in a very dry, skin-like appearance of the corneal surface. In these eyes, corneal transplantation does not work, as the dry surface is unable to support the health of the transplanted biological tissue, unlike in a normal eye. To provide the patient with useful vision, an artificial cornea or keratoprosthesis is used. A keratoprosthesis is a cylinder made of a type of plastic polymer called polymethyl methacrylate (the same material used to make intraocular lenses). The cylinder is made such that the power of the cornea is built into it, so that not only does it provide a clear corneal window, but it also supplies the refractive power needed for the eye to see well. Since plastic and living tissue cannot form a firm and safe bonding, researchers have tried over the past many years to find a way to allow integration of the two tissues, so that the long-term success rate of the procedure is enhanced.
]
In some designs, researchers have implanted a plastic cylinder into a central hole in a donor cornea and retained the cylinder using a nut-and-bolt design. The donor cornea is then sutured to the patient’s eye. This is relatively fast and easy to perform, but there may be some problems in the long-term retention of the prosthesis [1].
The procedure was pioneered by the Italian ophthalmic surgeon Professor Benedetto Strampelli in the early 1960s. Strampelli was a founder-member of the International Intra-Ocular Implant Club (IIIC) in 1966.
Modified OOKP
To improve the chances of the plastic prosthesis being retained in the eye, tissues from the patient’s own body are used. The tooth is ideal because it is a hard part to which the cylinder can be fixed and also it resides in the mouth where it co-exists with soft tissues, as in the eye. Originated by Prof Strampelli in Italy – this procedure has been refined and improved by Prof Falcinelli over the last 40 years [2]. We performed the first OOKP in HK and China with his and his son Dr Johnny’s help. Since then they have traveled to HK many more times to help us with the procedure. Having performed more than 250 of these procedures, Prof Falcinelli is the most experienced surgeon for this procedure. He has also worked with Dr Rao in India 3 years ago to help start the procedure there. The procedure is at present performed only in a few centers in the world [3]. This is a fairly complex procedure, and requires to be performed in 2 stages – each of which can take up to 4 to 6 hours. The patient can see after the second stage operation. The use of a tooth requires the help of a multi-disciplinary team including a dentist – and Dr Lee has been helping with the procedure here. The term MOOKP stands for modified - osteo (bone) – odonto (tooth) – keratoprosthesis (plastic cylinder).
History
The concept of an artificial cornea is over 200 years old. The first keratoprosthesis was described in 1789 during the French Revolution by Guillaume Pellier de Quengsy. The first reported human KPro surgery with a quartz crystal implant was performed by Nussbaum in 1855, although some modern KPro experts note that while Nussbaum may have reported the first human surgery, in fact, Guillaume Pellier de Quengsy’s brother, also an ophthalmologist, may have actually been the first to perform the surgery in a human [6].
In recent decades multiple synthetic corneas have been pioneered and developed, though only three are principally used in practice: the Boston Keratoprosthesis (Massachusetts Eye & Ear Infirmary, Boston, MA), the AlphaCor (Addition Technology Inc., Des Plaines, IL) [5] and the osteo-odontokeratoprosthesis also known as the ‘OOKP’ (originally described by Strampelli, modified by Falcinelli) [7].
The Boston Keratoprosthesis has evolved from original concept to an established device over the past fifty years under the lifetime leadership of Claes Dohlman, MD, PhD. The device was approved by the FDA for marketing in 1992. An active keratoprosthesis research program continues in Boston, MA and other centers worldwide fostering continued innovation with the device. The Boston Keratoprosthesis is a collar button design keratoprosthesis. It consists of three components: a front plate with optical stem, a back plate and titanium locking c-ring The most recent design is threadless; during assembly the front and back plates are snapped together with corneal tissue sandwiched inbetween, which is then used to suture the device to the eye. It is available in type I and type II formats. (The Type II format is reserved for severe end-stage ocular surface disease desiccation, and is similar to the Type I device but requires a permanent tarsorrhaphy to be performed through which a small anterior nub of the Type II model protrudes) [8]. The Type I Boston KPro is available in either a single standard pseudophakicplano power or customized aphakic powers (based on axial length) with adult (8.5 mm diameter) and pediatric (7.0 mm diameter) sized back plates. The device is currently machined from medical grade polymethylmethacrylate (PMMA) by a small, family owned and operated precision machine shop (J.G. Machine Co., Inc.) in Woburn, Massachusetts [9].
Recent advances in design have contributed to improved outcomes. First, the addition of holes (at present 16 holes) in the back plate allows diffusion of nutritive aqueous to support donor graft stroma and keratocytes Second, in 2004, a titanium locking c-ring was added to prevent intraocular disassembly of the device. Third, in 2007, the design was changed from a threaded (screwtype) assembly to a threadless design which simplified assembly and produced less damage to the donor endothelium [10]. The most recent advance in design is the implementation of a titanium back plate which likely improves biocompatibility and retention, and may reduce complications such as retroprosthetic membranes (RPM) and stromal corneal melts [11].
Indications For OOKP[12]
Bilateral blindness in severe cases of:
1. Stevens-Johnson syndrome
2. Ocular cicatricial pemphigoid (stage 3 or 4)
3. Lyell syndrome
4. Epidermolysis bullosa acquisita
5. Trachoma (stage C0 according to WHO classification)
6. Chemical injury
7. Physical injury (fire, liquid aluminium, etc)
8. Loss of the lids (eg, Crouzon disease)
9. Vascularized corneas with complete stem cell loss and dryness following other causes (eg, other ocular surgeries, use of MMC)
10. Aniridia with severe corneal changes
11. Multiple failed penetrating keratoplasty
12. Corneal failure after vitrectomy with silicone oil filling that cannot be removed safely.
Contraindications[13]
In some patients an OOKP should not be performed because of the high risk of failure. Absolute contraindications are:
Age below 17 years. Despite the fact that children will develop bilateral amblyopia if not treated, attempts at OOKP surgery did give very unsatisfying results: allografts (with the parents as donors) have been tried in two selected cases (the Salzburg group), but the laminae were found to be completely reabsorbed within months, possibly because of the high rate of turnover of bone during growth in this age group. One patient was lost to followup; the second retained light perception following penetrating keratoplasty and surgery for endophthalmitis. It is unknown whether additional attempts might yield more favorable results [14].
In case of a phthitic eye, the risk for complications is very high, and a loss of the remaining perception of light is most often to be expected after such invasive surgery.
In eyes with no light perception there remains no hope for restoring any visual acuity. Surgery will therefore only harm the patient.
Eyes with detached retina (which cannot be reattached before OOKP) or other pathologies of the posterior segment that severely interfere with potential visual acuity should not be operated on either [15].
Because a scleral shield with painted iris and a central opening to accommodate the optic can be provided, the majority of patients are willing to accept the postoperative cosmetic appearance. Nevertheless this problem has to be discussed with the patient and the relatives extensively prior to surgery.
Preoperative Ophthalmological Assessment[16]
Mucous membrane as the ‘‘physiological’’ cover of the alveolar bone seems to be the best protection for the OOKP lamina. If sufficient buccal mucous membrane is not available, any other kind of mucous membrane, such as palatinal, lip, and vaginal mucousmembrane, can be used.
Oral hygiene should start as soon as the decision is made to go ahead with OOKP surgery.
Consider chlorhexidine and nystatin mouthwash a day or two before surgery.
Surgical Technique[17-19]
The OOKP procedure involves 2 stages performed over a period of 6-9 months.
Stage 1
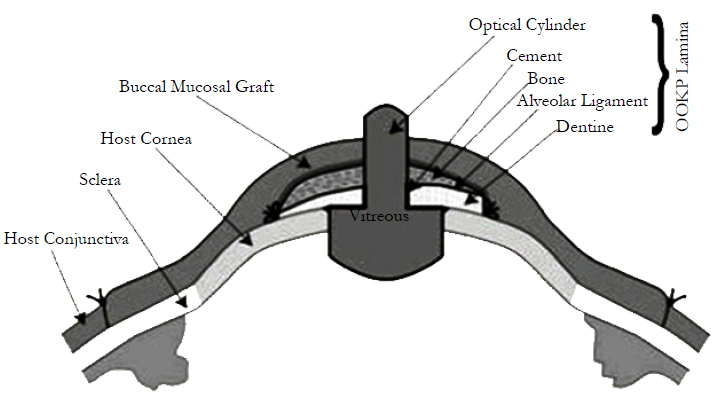
It involves ocular surface reconstruction and fashioning of an osteo-odonto lamina and its optical cylinder. A large circular piece of buccal mucosa is harvested from the cheek. The graft is trimmed of excess fat and soaked in cefuroxime solution. A lateral canthotomy is performed, followed by division of symblephara and superficial keratectomy. The buccal mucous membrane graft is sutured to the sclera bounded by the insertion of the rectus muscles to create a new ocular surface. The crown of the harvested tooth is used as a handle; whilst the attached tooth root and surrounding bone is worked into a lamina with dentine on one side and bone on the other. Periosteum is conserved and where possible glued back with fibrinogen adhesive. A hole is drilled through the dentine to accommodate a PMMA optical cylinder, which is cemented in place. The resultant osteo-odonto lamina is placed into a sub-muscular pocket under orbicularis oculi, usually in the lower lid of the fellow eye, in order to acquire a soft tissue covering [20].
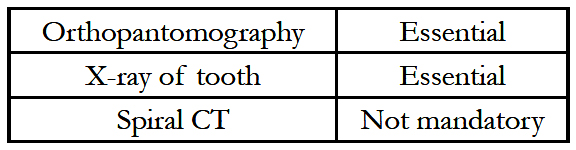
In the first stage, a canine tooth is harvested from the mouth of the patient after X-ray screening has determined that the tooth has a healthy and viable root structure. A surgical motorized saw is used to excise the canine root encased in alveolar bone from the jaw. The lamina is fashioned by sawing through the root of the tooth in a longitudinal fashion to expose the dentine and the root canal. The pulp in the root canal is scraped off and a hole is drilled in the widest part of the root – to a size of 3 to 4 mm depending on the width of the root at that point. An appropriately sized plastic cylinder of suitable power (determined from the axial length of the eye) is then glued to the hole using dental cement. A subcutaneous pocket is created in the tissues of the cheek and the lamina-cylinder complex is placed in it and the pocket is sutured closed after instilling antibiotic powder [21]. In the eye, the symblephara are released, and scar tissue is excised as described earlier. A superficial keratectomy including the Bowman’s layer is performed to expose the bare corneal stroma after which a fullthickness circular piece of cheek mucosa about 4 cm in diameter is placed over the cornea and sutured to sclera, also covering the muscle insertions.
PMMA Cylinder
A PMMA optical cylinder is cemented in the root with PMMA cement; the pulp chamber is obliterated with PMMA cement as well [22]. The completed keratoprosthesis consists of one sagittal half of the canine root with bone, ideally measuring 12 mm × 6 mm × 3 mm, carrying an optical cylinder with a margin of dentine of at least 1 mm all round. The OOKP optical cylinder comes in two different diameter sizes (3.5 and 4.0 mm) and a standard length of 8.75 mm; a wider diameter offers the benefit of a wider osteoodonto lamina. The ideal lamina should be of a size measuring 12 mm × 6 mm × 3 mm. The surgeon does not find the lamina's surface large enough to allow the insertion of an optical cylinder, two teeth can be extracted to prepare two laminae, which are then glued together with acrylic resin to prepare a larger surface. A hole of an average diameter of 3.70 mm (range, 3.3-4.0mm) is prepared leaving an edge of dentine.
The optical characteristics of the PMMA optical cylinder are, mean intraocular diameter, 4.1 mm (range, 3.6-4.6 mm); mean extraocular diameter, 3.65 mm (range, 3.3-4.0 mm); mean length, 7.75 mm (range, 7.25-8.25 mm); mean radius of the convex extraocular surface, 16 mm; mean radius of the convex intraocular surface, 6.5 mm; refractive index, 1.49; and equivalent power, 50.8 diopters [23].
Stage 2
It starts with retrieval of the osteo-odonto lamina from its submuscular pocket and excess soft tissue is removed from the bone surface. On the dentine surface, no soft tissue is allowed to remain. The lamina is reinserted into its pocket until the eye is ready to receive it. The buccal mucosal graft is reflected to allow access to the cornea. A Flieringa ring is sutured in place. The centre of the cornea is marked, and a small hole is trephined, the diameter of which corresponds to that of posterior part of the optical cylinder [24]. Relieving incisions are made and total iridodialysis, lens extraction and anterior vitrectomy are performed. The posterior part of the lamina is inserted through the central corneal hole and the lamina is sutured onto the cornea and sclera. The eye is reinflated with filtered air. The mucosal flap is replaced after cutting a hole to allow the protrusion of the anterior part of the optical cylinder Stage II surgery is performed 2 to 3 months later to allow time for a connective tissue cover to develop around the lamina implanted in the cheek. If required, the integrity of the lamina can be checked by performing a spiral computed tomographicevaluation [25]. During the second stage surgery, the lamina is retrieved from the subcutaneous location and excess connective tissue is removed from the two ends of the optic cylinder, and carefully trimmed over the rest of the lamina. The mucosal graft on the ocular surface is incised superiorly and reflected from the superior sclera and cornea, in a downward direction. The inferior attachment of the mucosal graft is left undisturbed to ensure that the blood supply is retained.
A Flieringa ring is sutured in place and a 3 mm opening is created in the center of the cornea. Three radial incisions are made in the cornea extending till the limbus. The iris is disinserted at the root and removed by gently pulling on the tissue and hypotensive anesthesia is used to control the ooze. Constant irrigation with balanced salt solution also helps wash the blood away and prevents a large clot forming in the anterior chamber. The lens is then cryo extracted and the radial corneal cuts are sutured closed [26]. A limited anterior vitrectomy is performed and the lamina is then placed over the cornea, such that the posterior part of the optic cylinder is in the anterior chamber – entering through the central corneal opening. The lamina is sutured into position using the connective tissue covering and episcleral bites. Air injection through the pars plana using a 30-gauge needle is used to maintain the intraocular pressure and avoid severe hypotony. At the conclusion of suturing, indirect ophthalmoscopy is performed to ensure that there is a good view of the disc and posterior pole, with the eye in the primary position. If this is not seen, a cylinder tilt may be responsible and the sutures are adjusted to straighten the cylinder position. Any bleeding into the vitreous cavity can also interfere with the visualization. After the cylinder and lamina are in satisfactory position, the mucosal flap is replaced and a small opening is created over the optic cylinder to allow the anterior portion of the cylinder to protrude through the mucosa. The superior edge of the mucosal flap is sutured in place and this completes the operation.
Since bleeding can occur during the ocular and oral surgical dissections, it is important that the patient is not on anti-platelet aggregators or anticoagulants. Infection is a major concern since oral tissues are used and extensive ocular surgery is performed. Intravenous antibiotics are used for the first week after surgery and oral antibiotics are continued for another week. This is more of a concern with stage I surgery. Treatment of the ocular surface mucosal graft is with antibiotic ointment and regular cleaning. Periodic follow-up is required to ensure early detection of problems with intraocular pressure and the stability of the graft. The presence of the lamina and the large mucosal graft preclude measurement of intraocular pressure using the current instrumentation. Intraocular pressure is estimated digitally and the health of the optic nerve is monitored using regular automated field measurements and visualization of the optic disc. The visual field provided by current cylinder designs is about 30 to 350 but this allows good ambulant vision to the patient. With the technique of osteo-odonto keratoprosthesis described in the previous paragraphs, prolonged retention times can be achieved with good visual function.
Complications[27]
The procedure is associated with complications. Awareness regarding these complications is necessary for early recognition and appropriate 5-7 management.
Ocular
1. Glaucoma
2. Retroprosthetic membrane
3. Vitritis
4. Expulsion of cylinder
5. Endophthalmitis
6. Retinal detachment
Mucous Membrane/Odal
1. MMG thining
2. MMG necrosis
3. Extrusion of prosthesis
Oral
1. Oroantral fistula
2. Damage to parotid duct
3. Damage to adjacent teeth
4. Mandibular fracture
During the removal of the tooth, a fracture of the tooth would render it useless as a lamina for the plastic cylinder. Sometimes, a small part of the tooth may be left behind in the bed and this would need to be extracted to prevent it from acting as a nidus for future infection. During the removal process, accidental entries into the maxillary sinus when the upper tooth is removed, and fracture of the mandible – during lower tooth removal, are possible. Both during tooth extraction and cheek mucosa excision, excessive bleeding may occur. The fashioning of the implant is also critical. It is important to avoid damage to the periosteum and the alveolar dental ligament when the tooth is cut with the dental saw. Since the saw blades can generate a significant amount of heat, a constant stream of water must be directed at the saw to ensure that there is damage to these delicate structures. The hole that is drilled in the dentine should be perpendicular to the lamina to ensure that the plastic cylinder does not tilt. The dental cement that is used to ensure proper adhesion of the cylinder and the dentine should be used to ensure complete sealing of the hole in the lamina. Once the tooth is removed, the absence of the canine(s) can pose both cosmetic and functional problems in the postoperative period. These stem from the fact that the oral cavity is no longer a closed chamber – due to the missing tooth – and can result in a lisp when speaking – and extrusion of liquids when eating.
In the eye, the scope of the surgery and the nature of the eyes operated on, both result in the potential for problems. These damaged eyes often have preoperative glaucoma. During the surgery, care must be paid when operating in the perilimbal area to avoid excessive cautery as this can damage the aqueous collecting veins and either create or worsen glaucoma. Thinned areas of the cornea and sclera must be handled with care to avoid perforations. Removal of the iris and the lens and the anterior vitrectomy can sometimes result in bleeding in the vitreous chamber. Prolonged hypotony during the implantation of the lamina should be avoided – as this can lead to expulsive hemorrhage. The use of hypotensive anesthesia during this stage is therefore, important.
After the surgery is completed, infection remains an important concern and antibiotics are essential in the postoperative period. Inflammation in the eye – due the surgical procedure – can sometimes occur and oral steroids can help. Increased intraocular pressure is not uncommon in the early postoperative period and should be carefully watched for – intravenous mannitol may be required in addition to oral acetazolamide. Later in the postoperative period, glaucoma can sometimes occur and should be watched for using regular visual field evaluations and examination of the optic disc. Retro implant membranes are rare – but can occur and may need excision – either with the YAG laser, or surgery [28]. Vitreoretinal complications can also sometimes occur, and need to be treated surgically. The mucosa covering the implant, can develop infections, or can retract and must be managed appropriately. Finally, the implant can develop periostitis – often infectious and this can compromise the integrity of the lamina. Thus, the list of complications with MOOKP is long, but withcareful attention to technique and close postoperative follow-up, many of these can be prevented or appropriately managed [29].
Future Directions
Research actively continues on the Boston Keratoprosthesis to improve design and outcomes and to expand indications for its use. KPro patients are prone to develop inflammation, RPM and cellular debris within and around the PMMA back plate, and studies are investigating alternative keratoprosthesis materials including titanium The optimal management of glaucoma and projects aimed at accurately assessing IOP in KPro eyes utilizing intraocular pressure transducers with telemetry capabilities are underway. Given the permanent need for a BCL, investigators continue to explore drug-eluting contact lenses, which could circumvent the need for patient initiated delivery of topical medications and is likely to improve outcomes given enhanced, reliable delivery of drugs. Researchers continue to challenge the dogma that KPro surgery should not be done in patients with good vision in a fellow eye, and are investigating restoring useful binocular vision in patients with corneal disease Investigators are also pursuing improving clinical outcomes with the Boston KPro in autoimmune conditions and chemical burns which have traditionally been plagued with poorer outcomes. The Boston KPro has made remarkable strides over the past decade and its continued success is promising.
Conclusion
A vast number of designs and materials of keratoprostheses with different methods of insertion have been developed and implanted in patients over the past two centuries with quite variable longterm results. Most studies report either a short follow up or a comparatively short lived visual recovery in majority of the cases. The technique with by far the best results and proven long term follow up is the osteo-odonto-keratoprosthesis (OOKP) invented by Strampelli and modified over the years by Prof. G. Falcinelli. OOKP developed some 40 years ago by Strampelli uses a biological skirt in the form of the patient's own tooth root and alveolar boneto support a polymethylmethacrylate (PMMA) optical cylinder.The osteo-odonto-keratoprosthesis serves as an excellent innovation which is an amalgamation of both ophthalmology and dentistry. More research is needed to develop further modifications.
References
- Monteiro MJ, Herold J, Liu C, Francis I (2009) Osteo-odonto-keratoprosthesis (OOKP): “A tooth for an eye” technique. Br J Oral Maxillofac Surg 47(7): e19.
- Strampelli B (1963) Osteo-odonto-Keratoprosthesis. Ann Ottalmol Clin Ocul 89: 1039-1044.
- Falcinelli G, Falsini B, Taloni M, Colliardo P, Falcinelli G (2005) Modified osteo-odonto-keratoprosthesis for treatment of corneal blindness: long term anatomical and functional outcomes in 181 cases. Arch Ophthalmol 123(10): 1319-1329.
- Falcinelli G, Missiroli A, Pettiti V, Pinna C (1987) Osteo-odonto-keratoprosthesis up-to-date. Acta Concil Ophthalmol 25: 2772-2776.
- Liu C, Paul B, Tandon R, Lee E, Fong K, et al. (2005) The Osteo-Odonto-Keratoprosthesis (OOKP). Semin Ophthalmol 20(2): 113-128.
- Cardona H (1969) Mushroom transcorneal keratoprosthesis (bolt and nut). Am J Ophthalmol 68(4): 604-612.
- Polack FM, Heinke G (1980) Ceramic Keratoprosthesis. Opthalmol 87: 693-698.
- Hollick EJ, Watson SL, Dart JK, Luthert PJ, Allan BD (2006) Legeais BioKpro III keratoprosthesis implantation: Long term results in seven patients. Br J Ophthalmol 90(9): 1146-1151.
- Casey TA (1966) Osteo-odonto-keratoprosthesis. Proc R Soc Med 59(6): 530-531.
- Liu C, Okera S, Tandon R, Herold J, Hull C, et al. (2008) Visual rehabilitation in end-stage inflammatory ocular surface disease with the osteo-odontokeratoprosthesis: Results from the UK. Br J Ophthalmol 92(9): 1211-1217.
- Chammartin M, Goldblum D, Fruh B, Wilkens L, Bosshardt D, et al. (2009) Case report of osteo-odonto-keratoprosthesis (Strampelli) and of Dacron keratoprosthesis (Pintucci). Klin Monbl Augenheilkd 226(3): 180-183.
- Viitala R, Franklin V, Green D, Liu C, Lloyd A, et al. (2009) Towards a synthetic osteo-odonto-keratoprosthesis. Acta Biomater 5(1): 438-452.
- Geerling G, Liu CS, Collin JR, Dart JK (2002) Cost and gains of complex procedures to rehabilitate end stage ocular surface disease. Br J Ophthalmol 86(11): 1220-1221.
- Lupelli L, Fletcher RJ, Palumbo P, Menghi A, Taloni M (1999) Improved optics for OOKP. Anales del Instituto Barraque 28: 159-160.
- Ricci R, Pecorella I, Ciardi A, Della Rocca C, Di Tondo U, et al. (1992) Strampelli's osteo-odonto-keratoprosthesis. Clinical and histological longterm features of three prostheses. Br J Ophthalmol 76(4): 232-234.
- Pecorella I, Maurizio T, Antonio C, Giancarlo F (2006) Progressive replacement of oral mucosa by conjunctiva in osteo-odonto-keratoprosthesis: preliminary observations. Cornea 25(2): 193-195.
- Michael R, Charoenrook V, de la Paz MF, Hitzl W, Temprano J, et al. (2008) Long-term functional and anatomical results of osteo- and osteo-odontokeratoprosthesis. Graefes Arch Clin Exp Ophthalmol 246(8): 1133-1137.
- Tan DT, Tay AB, Theng JT, Lye KW, Parthasarathy A, et al. (2008) Keratoprosthesis surgery for end-stage corneal blindness in asian eyes. Ophthalmology 115(3): 503-510.
- Tan XW, Perera PP, Tan A, Tan D, Khor KA, et al. (2011) Comparison of Candidate Materials for a synthetic osteo-odonto-keratoprosthesis device. Invest Ophthalmol Vis Sci 52(1): 21-29.
- Stoiber J, Grabner G (2005) Clinical management of severe ocular surface disease. Klin Monbl Augenheilkd 222(7): 533-551.
- Goossen C, Stempels N, Colpaert C, Tassignon MJ (1998) Strampelli osteoodonto- keratoprosthesis: Case report. Bull Soc Belge Ophtalmol 268: 129-133.
- Arora S, Bandukwala T (2010) Corneal transplantation: Dental lamina as keratoprosthesis. McMaster Univ Med J 7: 11-14.
- Skelton VA, Henderson K, Liu C (2000) Anaesthetic implications of osteoodonto-keratoprosthesis surgery. Eur J Anaesthesiol 17(6): 390-394.
- Lloyd AW, Faragher RG, Denyer SP (2001) Ocular biomaterials and implants. Biomaterials 22(8): 769-785.
- Hicks C, Crawford G, Chirila T, Wiffen S, Vijayasekaran S, et al. (2000) Development and clinical assessment of an artificial cornea. Prog Retin Eye Res 19(2): 149-170.
- De La Paz MF, De Toledo JA, Charoenrook V, Sel S, Temprano J, et al. (2011) Impact of clinical factors on the long-term functional and anatomic outcomes of osteo-odonto-keratoprosthesis and tibial bone keratoprosthesis. Am J Ophthalmol 151(5): 829-839.
- Caiazza S, Falcinelli G, Pintucci S (1990) Exceptional case of bone resorption in an osteo-odonto-keratoprosthesis. A scanning electron microscopy and X-ray microanalysis study. Cornea 9(1): 23-27.
- Hille K, Grabner G, Liu C, Colliardo P, Falcinelli G, et al. (2005) Standard for modified osteo-odonto-keratoprosthesis (OOKP) surgery according to Strampelli and Falcinelli: The Rome-Vienna protocol. Cornea 24(8): 895-908.
- Nakamura T, Kinoshita S (2011) New hopes and strategies for the treatment of severe ocular surface disease. Curr Opin Opthalmol 22(4): 274-278.