Is There Blinding Onchocerciasis in Northern Uganda?
T.L. Lakwo1*, B. Watmon2, A.W. Onapa3
1 National Onchocerciasis Control Programme, Ministry of Health, P.O. Box 1661, Kampala, Uganda.
2 Ophthalmologist, Gulu Regional Referral Hospital, Uganda.
3 Programme Manager, Envision/RTI, Neglected Tropical Disease Control, Kampala, Uganda.
*Corresponding Author
T.L. Lakwo,
National Onchocerciasis Control Programme,
Ministry of Health, Uganda,
P.O. Box, 1661, Kampala, Uganda.
Tel: 256-772-438311; Fax: 256-414-253044
E-mail: tlakwo@gmail.com
Article Type: Research Article
Received: May 08, 2014; Accepted: May 21, 2014; Published: May 22, 2014
Citation: T.L. Lakwo, B. Watmon, A.W. Onapa. (2014). Is There Blinding Onchocerciasis in Northern Uganda?, Int J Ophthalmol Eye Res, 2(2), 17-23. doi: dx.doi.org/10.19070/2332-290X-140004
Copyright: T.L. Lakwo© 2014. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Onchocerciasis is a filarial nematode Onchocerca volvulus transmitted by female black flies which breeds in fast flowing rivers.Clinical manifestations is ocular or skin lesions. The long-term armed conflict in northern Uganda deprived research on onchocerciasis
and no attempts have been made to establish the clinical manifestation of the disease in the region.
Objective: To establish whether Simulium damnosum transmitted onchocerciasis in this region was blinding.
Participants: Persons from thirteen endemic parishes in Pader district in northern Uganda were examined for clinical, parasitological and ocular manifestations of onchocerciasis.
Results: A total of 675 persons were examined and microfilaria prevalence in skin snips was 28%, while nodules and onchodermatitis were 30% and 29% respectively. The commonest skin lesion was Chronic Papular Onchodermatitis (17.5%). However, the prevalence of both microfilaria in the anterior chamber of the eye and reversible ocular lesions was 4%. The reversible ocular lesions of onchocerciasis observed were punctate keratitis stage B (0.1%), punctate keratitis stage D (0.1%) and punctate keratitis stage E (3.7%). While 16.1% of the respondents had irreversible ocular lesions attributed mainly to Optic atrophy (6.4%) and sclerosing keratitis (5.2%). There was significant association between irreversible onchocerciasis lesions and visual loss (p< 0.0001).
Conclusion: This study confirmed the occurrence of blinding onchocerciasis in Pader district, the first ever reported in indigenous
populations in Uganda.
2.Introduction
3.Materials and Methods
3.1.Study area
3.2.Study population
3.3.Study design and sample size
3.4.Data collection procedures
4.Results
4.1.Study sample and characteristics
4.2.Dermatological findings
5.Discussion
6.Conclusion
7.Acknowledgements and Disclosure
8.References
Keywords
Blinding Onchocerciasis; Onchocerca Volvulus; Pader District; Northern Uganda; Simulium Damnosum
Introduction
Onchocerciasis (river blindness) due to Onchocerca volvulus, is transmitted by female black flies of the Genus Simulium. The vector breeds in fast flowing rivers. The disease is characterized by skin manifestations such as onchodermatitis, leopard skin and hanging groin. In some cases, onchocerciasis leads to blindness, hence the name “river blindness”. The disease is known for both its ocular and dermatological effects [1]. The impact of blindness in a community is reflected in an increased mortality rate, the mortality amongst the blind people being four times higher than in the nonblind persons of the same age in a community [2].
In Uganda the disease is endemic in 37 of the 112 districts with most of the endemic areas or foci located in the western axis of the country bordering the Democratic Republic of Congo. It is estimated that over 3 million people are at risk of infection, with some 1.4 million already infected [3]. Most of the endemic foci have so far been under Mass Drug Administration (MDA) with ivermectin for over 15 years except the two districts of Kitgum and Pader where MDA could not start due to armed conflict. This conflict made research on and control of onchocerciasis in Pader district and the sub-region receive little attention. There has been information gap regarding the magnitude and clinical manifestations of onchocerciasis in this focus. Herein, we report the magnitude and ocular manifestations of onchocerciasis in Pader district.
Materials and Methods
The study was conducted in Pader district in the northern Uganda.At the time of the study, Pader had two counties (Aruu and Agago). This study was done in Aruu County (Figure 1), which has nine sub counties and 35 parishes [4]. Pader is bordered by Kitgum district in the North; Kotido and Abim to East, Gulu in the West, Apac and Lira districts in the South West and South, respectively. The total population is 326,338. The district has a network of river systems with the main one being R. Aswa and R. Agago. River Aswa is one of the tributaries of River Nile.
Figure 1. Map showing the sub-counties where the surveys were conducted in Pader district, northern Uganda
The study population included persons aged five years and above who lived in the sampled parishes for more than five years before displacement. Following the resettlement, the population is now generally stable with very limited migration. Their main occupation is subsistence farming through which they earn their livelihood. Cultivation is usually done along the river banks that provide fertile land for cultivation and grazing. Besides there is small scale fishing in the rivers and hunting which generally exposed the population to the risk of Simulium bites.
This study followed a population-based descriptive cross sectional design. The sample size was calculated using the Kish and Frankel [5]. The calculated sample size for the thirteen parishes was 701.
Multistage cluster sampling method [6] was used based on the REMO survey report of 2008 from the National onchocerciasis Control Programme Ministry of Health. The sub counties with nodule prevalence of above 25% were selected. Three to four parishes were randomly selected in each of the four sub counties. Then individuals were consecutively recruited until the sample size of sixty individuals for the parish was reached.
Multistage cluster sampling method [6] was used based on the REMO survey report of 2008 from the National onchocerciasis Control Programme Ministry of Health. The sub counties with nodule prevalence of above 25% were selected. Three to four parishes were randomly selected in each of the four sub counties. Then individuals were consecutively recruited until the sample size of sixty individuals for the parish was reached.
The variables included age, sex, duration of stay in the community,and source of ivermectin in the community. Other variables were the presence of onchocercal skin lesions, presence of nodules, skin snip for microfilaria and ocular manifestations [7]. The method of assessing the reversible and non reversible onchocerciasis lesions was used. The data on ivermectin treatment were collected using pre-tested open and closed ended questionnaires.
The district authorities were informed about the purpose and schedule of the study. Communities were mobilized through their leaders, local FM radio announcements and talk shows. Prior to enrolment and examination of participants, health education focusing on onchocerciasis and other Neglected tropical diseases endemic in the area was given for one hour. The study procedures were explained to the community and their consent sought.
The subjects underwent thorough clinical examinations of the skin for onchocercal lesions adopting methods used by others [8,9]. Examinations were by programme staff with over 15 years experience in onchocerciasis research and control. These examinations included clinical assessments for Acute Papular onchodermatitis (APOD), Chronic Papular onchodermatitis (CPOD), Lichenified onchodermatitis (LOD), depigmentation (DPM) and subcutaneous nodules as described by Murdoch, et.al. [10].
Visual acuity was assessed in all the subjects using the Snellen chart [2]. Visual impairment or blindness was assessed using the ability to count fingers or perceive light. In children, the assessment of visual acuity was based on WHO procedures [7]. Torch examination followed by hand held portable slit lamp examinations at x16 magnification were done. All the subjects were then made to bend down and lower their heads in between the knees for at least five minutes prior to slit lamp examination in order to view any microfilariae in the anterior chamber. The eyes were assessed for reversible and irreversible lesions in the cornea, presence of microfilariae in the anterior chamber and evidence of inflammation. The posterior segment of the eye was examined for any choroidal, retinal and optic nerve lesions using a direct ophthalmoscope. Ocular manifestations among the study participants were assessed to determine the distribution of reversible anterior segment lesions in the cornea.
Two skin snips or biopsies were taken from the right and left iliac crests using Walser Punch [11]. The snips were put in 0.1 ml. of saline in a microtitre plate and left for 24 hours to allow the microfilaria to come out of the snip. The snips were later examined for microfilaria using microscope [2]. Standard techniques of sterilization and infection control measures strictly adhered to [12].
The questionnaire was pre tested and data was collected by trained and qualified research staff. At the end of the day’s work the questionnaires were cleaned to ensure correct entry.
Data entry templates were designed by a biostatistician. Data entry clerks conducted “double data entry” to minimize clerical errors. Entry was initially in Excel and was later exported to SPSS for analysis. The significance p-value used was 0.05.
Ethical clearance was obtained from the Uganda National Council of Science and Technology. The district authority and community leaders gave consent to conduct the study. Informed consent was obtained from the community members and individuals. The parents and guardians consented for the children below 18 years. Confidentiality was observed.
Results
A total of 675 participants were enrolled in the study. Females were slightly more (52%) than males (48%) and the male: female ratio was 1:1. Their ages ranged from 5 to 65 years. The study participants were mainly the Acholi tribe from northern Uganda.
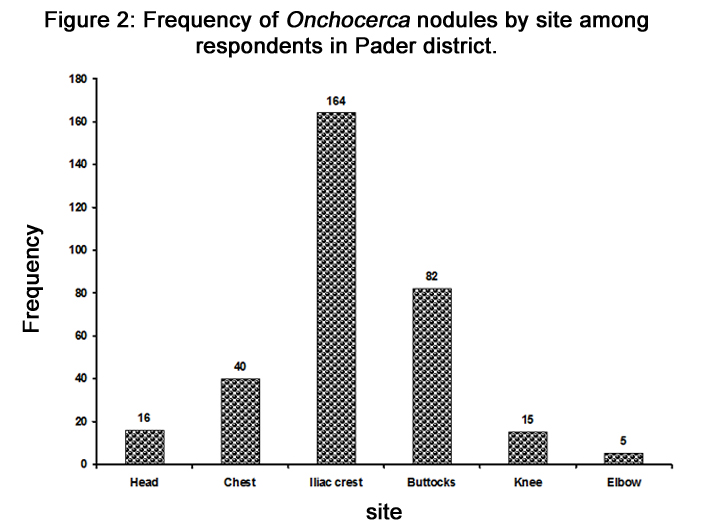
The prevalence of the nodules was 30% among the participants clinically examined. The most common sites for nodules were the iliac region (50.1%) followed by the buttocks/coccyx (25.5%; Figure 2). Nodule prevalence was lowest amongst those 5-14 years. The youngest participants with nodules were two nine year old males. The relationships between nodules and visual loss were assessed for all the nodule sites. In all cases, there was significant association between presence of nodules at different sites and visual loss (Table 1).
Table 1: Statistical relationship between nodules location/site and visual loss among study participants in Pader district (n=675)
All the four types of the reactive skin lesions were recorded among those examined. CPOD was most common lesion (17.5%), followed by APOD (17.1%). LOD affected 11.3%, while 11% had DPM. CPOD occurred in 57.8% of the females compared to 42.2% males. The majority (66%) of the 118 individuals with CPOD was aged 45 years or more; 57.8% were females while males constituted 42.2%.the respondents had multiple types of lesions.
Skin snip were taken from 318 people from five parishes. Of these 318, 94 (29.6%) were positive for O. volvulus microfilariae with males having 54 (57.4%) and females 40 (35.6%), giving an overall prevalence of 29.6%. The youngest participants with positive snips were aged five years (male and female). There were more males affected among age group 5-14 years and >65 years of age (Figure 3). Positive skin snip was significantly associated with presence of nodules (χ2= 72.665; df = 41; p<0.001).
Figure 3.Distribution of skin snip positive persons by age group and sex in Pader district, northern Uganda.
A total of 675 respondents were subjected to ophthalmic examinations.The majority (95.7%) of those examined had clear and normal cornea. However, reversible lesions (PKB, PKD andPKE) were seen in about 4.0% of the respondents (Table 2).
Table 2: Reversible ocular lesions in individuals examined in 13 parishes in Pader district, northern Uganda
NB: No cases of Punctate keratitis stage A (PKA) and Punctate keratitis stage C (PKC) were seen.
Of the 675 respondents, 28 (4%) had microfilaria in the anterior chamber of the eye (Figure 4). In eight people examined (1%), the anterior chamber was neither accessible nor visible for evaluation. The microfilaria in the anterior chamber was most common among age group 15-24 years; and least in 55-64 years (Figure 4). In all five parishes investigated the presence of microfilaria in the anterior chamber of the eye was significantly associated with presence of nodules (χ2 = 207.006; df = 82; p< 0.0001).
Irreversible lesion were seen in 119 out of 675 people examined,giving a prevalence of 16.1%. The commonest form of irreversible lesions were optic atrophy (39.4%) followed by sclerosing keratitis (32.1%). There were significant associations between irreversible ocular lesions with visual loss (χ2 = 188.454, df=15, p<0.0001) and the presence of nodule and visual loss (χ2= 91.416,p<0.0001). In terms of age groups, optic atrophy was observed to be more or less uniformly distributed while sclerosing keratitis was most common among age group 45-54 years (Table 3).
Of the 675 respondents, 197 (29.2%) had visual impairment in one or both eyes and the remaining 478 (70.8%) had normal vision. The leading causes of visual loss were cataract and optic atrophy (Figure 5). Majority of the participants with cataracts were those above 55 years old. Of the 51 people with optic atrophy, 24 had positive skin snips. And of the 94 people with positive snips 24 (25.5%) had optic atrophy. Other causes of visual impairment not related to onchocerciasis include uveitis, refractive errors, phthsis bulbi and glaucoma. The significant retinal diseases were chorioretinitis and retinitis pigmentation.
Discussion
The results of clinical, parasitological and ocular examinations conducted in Pader district revealed the presence of onchocerciasis presenting both skin and ocular manifestations. The high nodule prevalence reported (30%) among the respondents in Pader district agrees with what was observed in Kabarole district in western Uganda before treatment started in 1991 [11]. In Pader district, the nodules were mainly cited in the iliac crest, trochanter and coccyx. In similar studies conducted elsewhere, nodules were mostly seen over bony prominences like the iliac crest, coccyx, trochanter, chest wall and limbs [13].
It was observed that the commonest type of onchodermatitis in the communities was CPOD, and this agrees with earlier studies conducted in Nebbi District in Northwestern Uganda [8,9]. Similar findings were also documented by Hagan [14] in Cameroon, Ghana, Tanzania and Nigeria. The prevalence of CPOD was higher among the women, an observation that was also reported by Brieger, et al. [15] in Oyo state in Nigeria. It is known that differences in prevalence of onchocerciasis skin disease (OSD) relate to exposure to the vector.
The prevalence of positive skin snips (29.6%) reported here confirmed the high endemicity of the disease in the area. This is probably associated with the occupational activities of the population in relation to main vector biting behaviour [2]. This also explains the high prevalence of microfilaria observed among age groups 4-15 years and those >65 years. In Pader district, children of this age group (4-15 years) are involved in fishing and swimming in rivers breeding Simulium vectors. On the other hand, the older age group might have been involved in these activities during their youthful age, all exposing them to the bites of the vector. Due to lack of intervention in the district due to armed conflict, microfilarial densities have been building up and onchocerciasis clinical manifestations are known to arise from infections cumulating over several years of transmission [16].
The results revealed that reversible eye lesions (PKB, PKD and PKE) were seen in 4.0% of the respondents after one round of ivermectin treatment. This finding agrees with Abiose [17] who reported that a single treatment with ivermectin reduced the microfilaria in the cornea from 9% to 2% four months after treatment. In the surveyed communities two cases (0.2%) had dead microfilaria in the cornea (each for PKB and PKD). The most common eye lesion was PKE (4.0%) which indicates evidence of inflammatory reactions left in the cornea.
In the present study the prevalence of microfilaria in the anterior chamber of the eye was (4%). Dadzie et al [18] reported a reduction of 20%, four months after a single treatment with ivermectin. The low level of microfilaria in the anterior chamber of the eyes among the surveyed communities could have been because the communities had received ivermectin in the previous six months.
In this study Iritis or Uveitis (both active and inactive) was observed in 8% of the respondents. Fischer et al [11] reported a prevalence of 5.8% in Kabarole district of Western Uganda. Abiose [17] reported that with the death of microfilaria, a torpid iritis or uveitis develops. A more severe anterior uveitis may develop with the formation of inferior, posterior and peripheral anterior synaechiae which may be complicated by secondary cataract and glaucoma.
The commonest forms of irreversible lesions were optic atrophy (39.4%) and sclerosing keratitis (32.1%). The prevalence of optic atrophy observed was much higher than that reported in savanna communities (6%-9%) of northern Nigeria [17]. Atrophy of choriocapillaries, choroido-retinal scaring and sub-retinal fibrosis is advanced lesions which are sometimes seen [17]. Berghout, [19] on the other hand reported that in patients with palpable nodules, serious pathology of posterior segment of the eye was found twice as frequently as in non-onchocerciasis patients. These are consistent with our findings where there was significant relationship between the presence of nodules and irreversible ocular lesions (χ2= 91.416, p<0.0001), and the occurrence of irreversible lesions with positive skin snip (χ2 = 400.982; df = 205, p<0.0001). The development of ocular lesions correlates with the degree and duration of infection [20,21].
A survey in Adjumani and Moyo districts, Northwestern Uganda revealed that 2.8% of those examined had irreversible eye lesions comprising sclerosing keratitis, chorioretinitis and optic atrophy. All cases with blinding lesions were either Sudanese refugees or Ugandans who had lived in southern Sudan between 1979 and 1990 [22]. This study demonstrated a link between visual loss and irreversible lesions in the eye, the occurrence of optic atrophy, nodules and positive skin snip. The main rivers in this focus are R. Pader and R. Aswa, and they form the major breeding sites for the Simulium flies. The inflow of R. Aswa into R. Nile that joins in Sudan could possibility attribute to an overlap of this focus into Sudan, a situation to be further investigated.
The majority of the respondents with optic atrophy were below 45 years and 24 of them had positive skin snips, thus there was a significant association between positive skin snip and visual loss. Globally cataract is the leading cause of blindness and accounts for 50% total blindness while the other diseases each account for the following: Glaucoma’s 12.3%, Maculopathy age related macular degeneration 8.7%, corneal opacities due to trachoma 8.7% and Onchocerciasis 0.8% [23]. This prevalence of cataract (29%) observed in Pader district is relatively less than the reported global figure. This is probably attributed to the established ophthalmic surgical outreach program in the region. However, the observed high prevalence of optic atrophy, which is distributed in all age groups, could be due to onchocerciasis, a situation also reported in hyperendemic areas of West Africa [17,24].
Conclusion
This study has confirmed the presence of blinding onchocerciasis in Pader district the first ever reported in indigenous populations in Uganda. The association between visual loss with presence of microfilaria in the anterior chamber of the eye was noted.
Acknowledgements and Disclosure
Our sincere appreciations are extended to the Neglected Tropical Disease Control Programme and ENVISION/RTI for the financial support for the field investigations. The African Programme for Onchocerciasis Control and WHO country office (Uganda) for supporting the REMO survey. We are also indebted to all the team members (E.Tukesiga, B.V. Abwang, W. Oyet, J. Luciamoi, P. Odonga), Local Councils and communities of the surveyed parishes ,the District Health Office Pader, for the various support rendered to us that enabled the survey to be completed smoothly. Appreciations to the District Health Officers of Kabarole, Mbarara and Lamwo districts; and the Medical Superintendent of Gulu Regional Referral Hospital for accepting to release their staff to participate in the survey. All investigators had access to the study data at all times and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- Kim A, Benton B (1995) Cost-Benefit analysis of onchocerciasis Control Programme (OCP), Technical Paper No.2, 282, World Bank, Washington.
- WHO (1995). Onchocerciasis and its Control. Report of a WHO Expert Committee on Onchocerciasis, Technical Report Series 852:1-104.
- Ndyomugyenyi R (1998) The burden of onchocerciasis in Uganda. Ann. Trop. Med. Parasit 92:S133-S137.
- Uganda Bureau of Statistics (UBOS). Population and social statistics. Uganda Bureau of statistics; 2002.
- Kish L, Frankel M.R (1974) Inference from complex samples. Journal of the Royal Statistical Society, Series B 36: 1 – 37.
- Bennet S, Words T, Liyange W.M, Smith D (1991) Simplified general methods for cluster sample surveys in developing countries. World Health Stat Q 44: 98-106
- WHO (2001). Certification of Elimination of Human Onchocerciasis: Criteria and Procedures. Geneva, Switzerland: World Health Organization
- Okello D, Ovuga E, Ogwal-Okeng J (1995) Dermatological problems of onchocerciasis in Nebbi district, Uganda. East African Medical Journal 72: 295-298.
- Ovuga E, Ogwal-Okeng, Okello D (1995) Social anthropological aspects of onchocercal skin disease in Nebbi district, Uganda. East African Medical Journal, 72: 649-653.
- Murdoch M, Hay R, Mackenzie C, Williams J, Ghalib H, et al (1993). A clinical classification and grading system of the cutaneous changes in onchocerciasis. Br. J. Dermatol. 129: 260-269.
- Fischer P, Kipp W, Bamuhiga J, Binta-Kahwa J, Kiefer A, et al. (1993) Parasitological and clinical characterization of S. neavei transmitted onchocerciasis in western Uganda. Trop.Med. Parasit. 44: 311-321
- WHO (1989). Guidelines on sterilization and disinfection methods effective against human immunodeficiency virus (HIV) (2nd Edition). WHO AIDs series 2:11.
- Krupp M.A, Chatton M.J (1978) Current medical diagnosis and treatment. Large medical publications 94022: 892-893.
- Hagan M (1998) Onchocercal dermatitis: clinical aspects. Annal. Trop. Med. Parasit 92: S 85-S96.
- Brieger W.R, Osasanya OO, Kale OO, Oshiname F.O, Oke G.A (1997) Gender and ethnic differences in onchocerciasis skin disease in Oyo state, Nigeria. Tropical Medicine and International Health 2:529-534.
- Thylefors B, Philippon B, Prost A (1978) Transmission potentials of Onchocerca volvulus and associated intensity of onchocerciasis in Sudan savanna area. Tropenmed. Parasit 29: 346-354.
- Abiose A (1998) Onchocercal eye disease and impact of mectizan treatment. Annals of Tropical Medicine & Parasitology 92: S11-S22.
- Dadzie K.Y, Remme J, Alley E.S, De Sole G (1990a.) Changes in ocular onchocerciasis four and twelve months after community-based treatment with ivermectin in a holoendemic onchocerciasis focus. Trans. Roy. Soc. Trop. Med.Hyg 84: 103-108.
- Berghout E (1987) Onchocerciasis and optic atrophy in the savannah area of Ghana. Trop Geog. Med. 39:323-329
- Fuglsang H, Anderson J (1977) The concentration of microfilariae in the skin near the eye as a simple measurement of the severity of onchocerciasis in community and as indicator of danger to the eye. Tropenmed Parasitol 28:63-67
- WHO (1987) Expert Committee on Onchocerciasis. Wld Hlth. Org. Technical Report series 752: 66.
- Ukety, Nyathirombo A, Watmon B, Habomugisha (2007) Evaluation of Onchocerciasis treatment in eight sentinel villages in Adjumani and Moyo districts, Uganda.
- Kocur Ivo (2006) What is new at the back of the eye? Community Eye Health 19(57):1-3.
- Newland HS, White AT, Greene BM, Murphy RP, Taylor HR (1991) Ocular manifestations of onchocerciasis in a rain forest area of West Africa. Br J Ophthalmol 75(3): 163–169.