Effect of Plant Growth Regulators Ivin, Methyur and Kamethur on the Organogenesis of Miniature Rose (Rosa mini L .) in Vitro
Tsygankova V.A.1*, Oliynyk O.O.2, Kvasko O.Yu.2, Pilyo S.G.1, Klyuchko S.V.1, Brovarets V.S.1
1 Department for Chemistry of Bioactive Nitrogen-Containing Heterocyclic Compounds, V.P. KukharInstitute of Bioorganic Chemistry and Petrochemistry,
National Academy of Sciences of Ukraine, 1, Murmanskaya str., 02094, Kyiv, Ukraine.
2 Department of Ecobiotechnology and Biodiversity, Faculty of plant protection, biotechnology and ecology, National University of Life and Environmental
Sciences of Ukraine, Heroiv Oborony Str.15, 03041, Kyiv, Ukraine.
*Corresponding Author
Tsygankova V.A,
Department for Chemistry of Bioactive Nitrogen-Containing Heterocyclic Compounds, V.P. Kukhar Institute of Bioorganic Chemistry and Petrochemistry, National Academy of
Sciences of Ukraine, 1, Murmanskaya str., 02094, Kyiv, Ukraine.
E-mail: vTsygankova@ukr.net
Recieved: September 28, 2022; Accepted: October 27, 2022; Published: October 31, 2022
Citation: Tsygankova V.A., Oliynyk O.O., Kvasko O.Yu., Pilyo S.G., Klyuchko S.V., Brovarets V.S.. Effect of Plant Growth Regulators Ivin, Methyur and Kamethur on the Organogenesis of Miniature Rose (Rosa mini L.) in Vitro. Int J Med Biotechnol Genetics. 2022;S1:02:001:1-8.
Copyright: Tsygankova V.A© 2022. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited..
Abstract
The effect of synthetic plant growth regulators Ivin (N-oxide-2,6-dimethylpyridine), Methyur (sodium salt of 6-methyl-2-mercapto-
4-hydroxypyrimidine) and Kamethur (potassium salt of 6-methyl-2-mercapto-4-hydroxypyrimidine) on the organogenesis
of miniature rose (Rosa mini L.) in vitro was studied. It was shown that the effect of synthetic plant growth regulators Ivin,
Methyur and Kamethur used at concentrations of 10-5M, 10-6M, 10-7M per 1 liter of MS (Murashige and Skoog) medium
on the organogenesis of shoots and roots of miniature rose (Rosa mini L.) in vitro is similar or higher than the effect of the
plant hormone auxin IAA (2-(1H-Indol-3-yl) acetic acid) used at the same concentrations. The parameters of organogenesis of
miniature rose (Rosa mini L.) in vitro (average length of shoots (cm) per explant, length of roots (cm) per explant, number of
roots (pcs) per explant, and frequency (%) of shoot rooting), measured on the 21st and 28th days of cultivation, obtained on
MS medium modified with Ivin, Methyur and Kamethur,varied depending on the concentrations of plant growth regulators.
The synthetic plant growth regulators showed the highest effect on the organogenesis of shoots and roots of miniature rose
(Rosa mini L.) in vitro when used in concentrations: Ivin at concentrations of 10-5M and 10-6M, Kamethur at concentrations of
10-5M and 10-6M, Methyur at concentrations of 10-5M and 10-7M. The plant hormone auxin IAA showed the highest effect
on the organogenesis of shoots and roots of miniature rose (Rosa mini L.) in vitro when used at concentrations of 10-6M and
10-7M. Proposed molecular mechanisms of action of the synthetic plant growth regulators Ivin, Methyur and Kamethur are
discussed. The results of the work confirm the promise of using synthetic plant growth regulators Ivin, Methyur and Kamethur
to improve the organogenesis of shoots and roots of the miniature rose (Rosa mini L.) in vitro.
2.Introduction
3.Materials and Methods
4.Results
5.Discussion
6.Conclusion
7.References
Keywords
Rosa mini L; in vitro Culture; MS Medium; Organogenesis of Shoots and Roots; IAA; Pyridine; Pyrimidine; Ivin; Methyur and Kamethur.
Introduction
The rose is rightfully considered the most elegant and incredibly
beautiful flower belonging to the genus Rosa L. and family
Rosaceae [1-3]. The genus Rosa L., with over 200 species and
thousands of cultivars grown worldwide, includes both wild rose
species and oil rose species, which are widely used as garden
ornamental plants and as a raw material for the production of
rose essential oils, water and alcohol extracts of rose used in the
perfumery, pharmaceutical and food industries [2-5]. Rose water
and alcohol extracts enriched in secondary metabolites such as
flavonoids (e.g., flavones, flavonols, anthocyanins) and rose essential
oils containing monoterpenes and sesquiterpenes have
therapeutic properties and can be used such as a respiratory antiseptic,
anti-inflammatory, mucolytic, expectorant, decongestant,
antioxidant, antiviral and anticancer agents for the prevention and
treatment of serious diseases [3-5]. The fruits of wild roses, called
rose hips, are exceptionally rich in vitamin C, 60 times the amount
of vitamin C in citrus fruit, a significant amount of iron, calcium,
and phosphorus [6].
Shrub or spray miniature roses with small flowers are in great demand among florists, designers and amateur gardeners for a
beautiful bush and abundant flowering [4, 7, 8]. The Lydia variety,
bred by the specialists of the Dutch company in 1995, is a small
plant with abundant flowering and the formation of large flower
rosettes.It is one of the most popular varieties of miniature shrub
roses belonging to the floribunda category [8]. The Lydia variety
is divided into 3 sub-varieties: Lovely Lydia, Spray White Lydia,
and Floribunda Classic Lydia. The main advantages of the Lydia
variety are frost resistance, immunity to fungal pathologies and
pests, lack of capriciousness in growing, flowering during the
growing season [8]. Growing Lydia roses is not difficult; the main
thing is to observe the regime of watering, feeding and lighting
[8]. This crop is planted in the second half of spring indoors, outdoors
in containers, or outdoors in the soil well lit by the spring
sun, but without direct sunlight and protected from drafts and
strong winds [8].
Rose propagation methods mainly include vegetative methods
such as cutting, grafting, layering, budding, and rarely seed propagation
[7, 9]. As a rule, vegetative propagation and seed propagation
methods are very time consuming and do not allow obtaining
a healthy plant. Micropropagation through in vitro culture is widely
used as an alternative biotechnological method to produce healthy
plant clones that are genetically identical to the parent plant [9].
Rose propagation in vitro involves several stages such as initiation
of aseptic cultures, proliferation and multiplication of shoots using
apical buds, nodal stem segments, shoot tip segments, or leaf
petioles through organogenesis or somatic embryogenesis, rooting
of microshoots, acclimatization and establishment in the field
[9-11].
The efficiency of rose organogenesis in vitro is influenced by
various factors, including the type of plant explant, plant species,
plant genotypes or cultivars, the composition of nutrient
media used for plant cultivation and physical factors [10]. Plant
hormones cytokinins such as BAP (6-benzyl aminopurine), 2-ip
(2-isopentyladenine) used in high concentrations, auxins such as
IBA (indole-3-butyric-acid), NAA (α-naphthalene acetic acid),
IAA (indole-3-acetic acid), and gibberellic acid (GA3) used at low
concentrations are the main factors affecting rose organogenesis
in vitro [9-15]. At the same time, the optimization of nutrient
media is carried out using plant hormones ortheir synthetic
analogues, for example, a synthetic analogue of cytokininTDS
(Thidiazuron), which make it possible to have a high stimulating
effect on the regeneration of roses in vitro [10, 12, 14, 15]. A topical
issue is the search for new synthetic analogues ofplant hormones,
auxins and cytokinins, to increase the efficiency of rose
organogenesis in vitro.
The main goal of our work is to study the effect of synthetic plant
growth regulators Ivin, Methyur and Kamethuron the organogenesis
of shoots and roots of miniature rose (Rosa mini L.) in vitro.
Materials and Methods
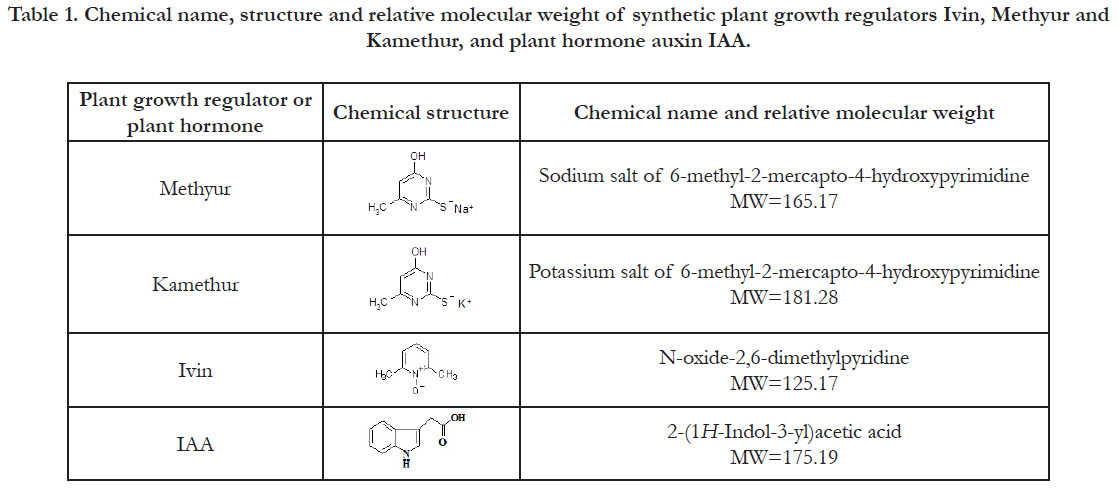
Plant growth regulators Ivin (N-oxide-2,6-dimethylpyridine),
Methyur (sodium salt of 6-methyl-2-mercapto-4-hydroxypyrimidine)
and Kamethur (potassium salt of 6-methyl-2-mercapto-
4-hydroxypyrimidine) were synthesized in the Department for
Chemistry of Bioactive Nitrogen-Containing Heterocyclic Compounds,
V.P. Kukhar Institute of Bioorganic Chemistry and Petrochemistry
of the National Academy of Sciences of Ukraine
(Table 1). The growth regulatory activity of synthetic plant
growth regulators Ivin, Methyur and Kamethur was compared
with the activity of plant hormone auxin IAA (2-(1H-Indol-3-yl)
acetic acid) (Table 1).
In experiments, explants of a miniature rose (Rosa mini L.), grown
on a hormone-free MS (Murashige and Skoog) medium [16], divided
up to 0.5 cm in size with 1-2 microbuds were used.These
explants were divided into segments with an axillary bud, one or
two leaf blades, and a stem no larger than 1,0 cm in size.
Sterilization was carried out according to the scheme: treatment
of explants in sodium hypochlorite solution - 20 min. followed
by washing in sterile distilled water; sterilization with 70% ethanol
(С2Н5ОН) - 4 sec. with washing in sterile distilled water; sterilization
in 0.1% solution of Mercury (II) chloride (HgCl2) for 7 min.
with 3 times washing with sterile distilled water.
Further, these segments were cultivated at an illumination of3000-
4000 lux, a temperature of 24 ± 2ºС, an air humidity of ~ 70%,
and a 16-hour photoperiod for 28 days on control hormone-free
MS medium, or modified MS medium containing either synthetic
plant growth regulators Ivin, Methyur and Kamethuror plant hormone
auxin IAA used at concentrations of 10-5M, 10-6M, 10-7M
per 1 liter of MS medium. MS medium for explant cultivation was
supplemented also with macro- and micro salts contained in MS
basal medium [16], sucrose (0.3%), mesoinositol (100 mg/l) and
agar (0.7%), medium pH – 5.7-5.8.
Parameters of organogenesis of miniature rose (Rosa mini L.) (average
length of shoots (cm) per explant, length of roots (cm) per
explant, average number of roots (pcs) per explant, and frequency
(%) of shoot rooting, i.e. the average number of rooted shoots
per number of explants) were measured on the 21st and 28th days of in vitro cultivation, respectively.
Statistical Analysis
All experiments were performed in three replicates. Statistical
analysis of the data was performed using dispersive Student’s-t
test with the level of significance at p≤0.05, the values are mean
± Standard Deviation (± SD) [17].
Results
The conducted studies showed that the use of synthetic plant
growth regulators Ivin, Methyur and Kamethuras components
of the MS medium had a positive effect on the formation and
growth of shoots and rootsof miniature rose (Rosa mini L.) in vitro
(Fig. 1 – Fig. 3).
Parameters of organogenesis of miniature rose (Rosa mini L.) in
vitro, measured on the 21st and 28th days of cultivation, obtained
on MS medium modified with synthetic plant growth regulators
Ivin, Methyur and Kamethur at concentration of 10-5M, 10-6M
and 10-7M, were generally similar to or higher than those obtained
on the control hormone-free medium MS, or on the medium
MS containing auxin IAA used at the same concentrations.
The effect of synthetic plant growth regulators Ivin, Methyur and
Kamethur on the organogenesis of shoots and roots of miniature
rose (Rosa mini L.) in vitro varied depending on the concentrations
of plant growth regulators (Table 2 – Table 4).
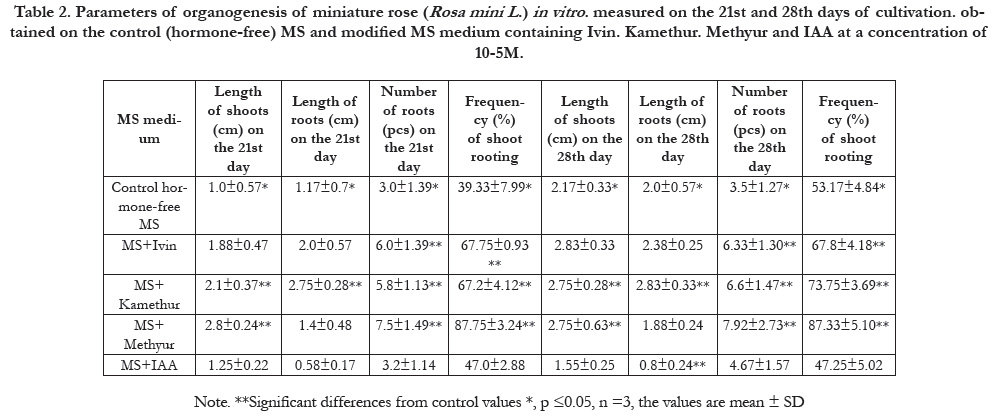
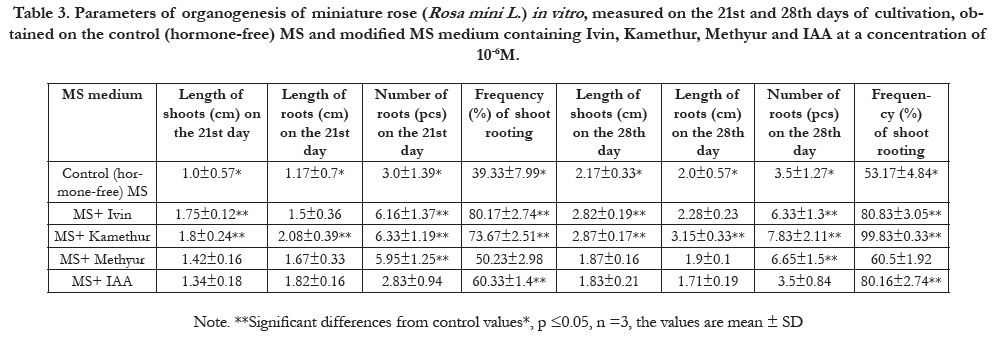
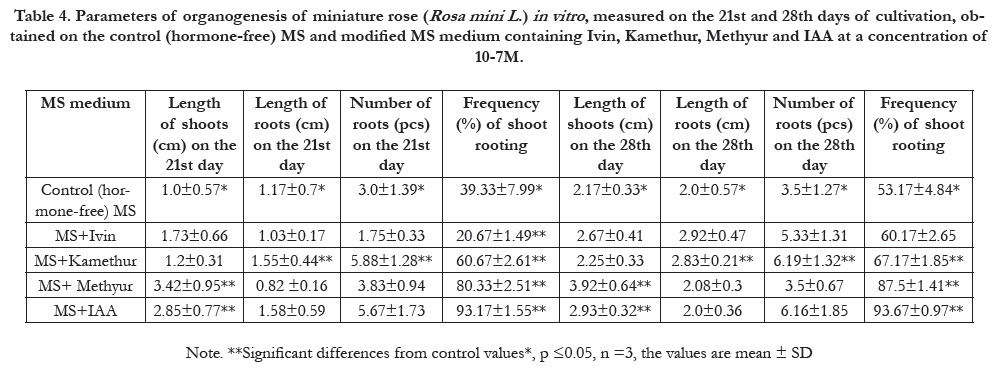
According to the parameters of length of shoots (cm) per explant,
measured on the 21st and 28th days of cultivation, synthetic
plant growth regulators showed the highest activity: Ivin at concentration
of 10-6M (1.75±0,12 cm and 2.82±0,19 cm), Kamethurat
concentrations of 10-5M (2.1±0.37 cm and 2.75±0.28 cm)
and 10-6M (1.8±0.24 cm and 2.87±0.17 cm), and Methyur at
concentrations of 10-5M (2.8±0.24 cm and 2.75±0.63 cm) and
10-7M (3.42±0.95 cm and 3.92±0.64 cm), respectively (Table 2
– Table 4).
Synthetic plant growth regulators showed the lowest activity in
relation to the parameters of length of shoots (cm) per explant,
measured on the 21st and 28th days of cultivation: Ivin at concentrations
of 10-5M (1.88±0.47 cm and 2.83±0.33 cm) and
10-7M (1.73±0.66cm and 2.67±0.41 cm), Kamethurat a concentration
of 10-7M (1.2±0.31cm and 2.25±0.33 cm) and Methyur
at concentrations of 10-6M (1.42±0.16 cm and 1.87±0.16 cm),
respectively (Table 2 – Table 4). Parameters of length of shoots
(cm)per explant indicated above did not differ statistically than
those obtained on the control hormone-free medium MS, measured
on the 21st and 28th days of cultivation (1.0±0.57 cm and
2.17±0.33cm), respectively (Table 2 – Table 4).
According to the parameters of length of roots (cm) per explant,
measured on the 21st and 28th days of cultivation, synthetic plant
growth regulator showed the highest activity: Kamethurat concentrations
of 10-5М (2.75±0.28 cm and 2.83±0.33 cm), 10-6 М
(2.08±0.39 cm and 3.15±0.33 cm) and 10-7M (1.55±0.44cm and
2.83±0.21cm), respectively (Table 2 – Table 4).
Synthetic plant growth regulators showed the lowest activity inrelation
to the parameters of length of roots (cm) per explant,
measured on the 21st and 28th days of cultivation: Ivin at concentrations
of 10-5M (2.0±0.57 cm and 2.38±0.25 cm), 10-6M
(1.5±0.36 cm and 2.28±0.23 cm) and 10-7M (1.03±0.17cm
and 2.92±0.47 cm), and Methyur at concentrations of 10-5M
(1.4±0.48 cm and 1..88±0.24 cm), 10-6M (1.67±0.33 cm and
1.9±0.1 cm) and 10-7M (0.82±0.16 cm and 2.08±0.3 cm), respectively
(Table 2 – Table 4). Parameters of length of roots (cm)
per explant indicated above did not differ statistically than those
obtained on the control hormone-free medium MS, measured on
the 21st and 28th days of cultivation (1.17±0.7cm and 2.0±0.57
cm), respectively (Table 2 – Table 4).
According to the parameters of number of roots (pcs) per
explant,measured on the 21st and 28th days of cultivation, synthetic
plant growth regulators showed the highest activity: Ivin
at concentrations of 10-5M (6.0±1.39 pcs and 6.33±1.30 pcs)
and 10-6М (6.16±1.37 pcs and 6.33±1.3 pcs), Kamethurat concentrations
of 10-5M (5.8±1.13 pcs and 6.6±1.47 pcs), 10-6М
(6.33±1.19 pcs and 7.83±2.11pcs) and 10-7М (5.88±1.28pcs and
6.19±1.32 pcs), Methyur at concentrations of 10-5M (7.5±1.49
pcs and 7.92±2.73 pcs) and 10-6М (5.95±1.25 pcs and 6.65±1.5
pcs), respectively (Table 2 – Table 4).
Synthetic plant growth regulators showed the lowest activity
in relation to the parameters of number of roots (pcs) per explant,
measured on the 21st and 28th days of cultivation: Ivin
at a concentration of 10-7M (1.75±0.33pcs and 5.33±1.31 pcs)
and Methyur at a concentration of 10-7M (3.83±0.94pcs and
3.5±0.67 pcs) (Table 2 – Table 4). Parameters of number of roots
(pcs) per explant indicated above did not differ statistically than
those obtained on the control hormone-free medium MS, measured
on the 21st and 28th days of cultivation (3.0±1.39 pcs and
3.5±1.27 pcs), respectively (Table 2 – Table 4).
According to the parameters of the frequency (%) of shoot
rooting,measured on the 21st and 28th days of cultivation, synthetic
plant growth regulators showed the highest activity: Ivinat
concentrations of 10-5M (67.75±0.93 % and 67.8±4.18 %) and
10-6M (80.17±2.74% and 80.83±3.05 %), Kamethurat concentrations
of 10-5M (67.2±4.12 % and 73.75±3.69 %), 10-6M
(73.67±2.51 % and 99.83±0.33 %) and 10-7M (60.67±2.61% and
67.17±1.85%), Methyurat concentrations of 10-5M (87.75±3.24
% and 87.33±5.10 %) and 10-7M (80.33±2.51% and 87.5±1.41%),
respectively (Table 2 – Table 4).
Synthetic plant growth regulators showed the lowest activity in
relation to the parameters of frequency (%) of shoot rooting,
measured on the 21st and 28th days of cultivation: Ivinat concentrations
of 10-7M (20.67±1.49% and 60.17±2.65%) and Methyurat
concentrations of 10-6M (50.23±2.98 % and 60.5±1.92 %),
respectively (Table 2 – Table 4). Parameters of frequency (%) of
shoot rooting indicated above did not differ statistically or were
lessthan those obtained on the control hormone-free medium MS,
measured on the 21st and 28th days of cultivation (39.33±7.99 %
and 53.17±4.84%), respectively (Table 2 – Table 4).
It has been shown that the effect of synthetic growth regulators
Ivin, Methyur and Kamethur on the organogenesis of shoots and roots of miniature rose (Rosa mini L.) in vitro is similar or higher
than the effect of the plant hormone auxin IAA used in the same
concentrations 10-5M, 10-6M, 10-7M per 1 liter of MS medium
(Table 2 – Table 4).
The plant hormone auxin IAA showed the highest activity in relation
tothe parameters of shoot and root organogenesis, measured
on the 21st and 28th days of cultivation, according to the length of
shoots (cm) per explantat concentrations of 10-7M (2.85±0.77cm
and 2.93±0.32 cm)andfor the frequency (%) of shoot rootingat
concentrations of 10-6M (60.33±1.4 % and 80.16±2.74 %) and
10-7M (93.17±1.55% and 93.17±1.55%), respectively (Table 2 –
Table 4).
The plant hormone auxin IAA showedthe lowest activity in relation
to the parameters of shoot and root organogenesis, measured
on the 21st and 28th days of cultivation, in relation to the
length of shoots (cm) per explantat concentrations of 10-5M
(1.25±0.22 cm and 1.55±0.25 cm) and 10-6M (1.34±0.18 cm
and 1.83±0.21cm), length of roots (cm) per explantat concentrations
of 10-5M (0.58±0.17 cm and 0.8±0.24 cm), 10-6M
(1.82±0.16 cm and 1.71±0.19 cm) and 10-7M (1.58±0.59cm and
2.0±0.36cm), number of roots (pcs) per explantat concentrations
of 10-5M (3.2±1.14 pcs and 4.67±1.57 pcs),10-6M (2.83±0.94
pcs and 3.5±0.84 pcs) and 10-7M (5.67±1.73pcs and 6.16±1.85
pcs), and frequency(%) of shoot rooting at concentrations of
10-5М (47.0±2.88 % and 47.25±5.02 %), respectively (Table 2
– Table 4). Parameters of organogenesis indicated above did not
differ statistically or were lessthan those obtained on the control
hormone-free medium MS, measured on the 21st and 28th days
of cultivation in relation to the length of shoots (cm) (1.0±0.57
cm and 2.17±0.33cm), length of roots (cm)(1.17±0.7cm and
2.0±0.57 cm), number of roots (pcs)(3.0±1.39pcs and 3.5±1.27
pcs), and frequency (%) of shoot rooting (39,33±7,99 % and
53.17±4.84%), respectively (Table 2 – Table 4).
Table 1. Chemical name, structure and relative molecular weight of synthetic plant growth regulators Ivin, Methyur and Kamethur, and plant hormone auxin IAA.
Table 2. Parameters of organogenesis of miniature rose (Rosa mini L .) in vitro . measured on the 21st and 28th days of cultivation. obtained on the control (hormone-free) MS and modified MS medium containing Ivin. Kamethur. Methyur and IAA at a concentration of 10-5M.
Table 3. Parameters of organogenesis of miniature rose (Rosa mini L .) in vitro , measured on the 21st and 28th days of cultivation, obtained on the control (hormone-free) MS and modified MS medium containing Ivin, Kamethur, Methyur and IAA at a concentration of 10-6M.
Table 4. Parameters of organogenesis of miniature rose (Rosa mini L .) in vitro , measured on the 21st and 28th days of cultivation, obtained on the control (hormone-free) MS and modified MS medium containing Ivin, Kamethur, Methyur and IAA at a concentration of 10-7M.
Thus, the obtained results showed both cytokinin- and auxin-like effectsof synthetic plant growth regulators Ivin, Methyur and Kamethuron theprocesses of elongation, division and differentiation of the isolated plant cells, which are the main processes of organogenesis of shoot and root meristems of miniature rose (Rosa mini L.) in vitro.
Analysis of growth regulating activity showed that synthetic plant growth regulators have a more powerful effect on the organogenesis of shoots and roots of miniature rose (Rosa mini L.) in vitro when used as components of the MS medium in concentrations: Ivin at concentrations of 10-5M and 10-6M, Kamethurat concentrations of 10-5M and 10-6M, Methyur at concentrations of 10-5M and 10-7M.
The plant hormone auxin IAA showed the highest effect on the organogenesis of shoots and roots of miniature rose (Rosa mini L.) in vitro when used as component of the MS medium at concentrations of 10-6M and 10-7M.
Figure 1. Organogenesis of shoots and roots of miniature rose (Rosa mini L. ) in vitro , measured on the 28th day of cultivation: 1-control hormone-free MS medium, 2 - MS medium containing Ivin at a concentration of 10-5M, 3 - MS mediumcontaining Kamethur at a concentration of 10-5M, 4 - MS medium containing Methyur at a concentration of 10-5M, 5 - MS medium containing plant hormone auxin IAA at a concentration of 10-5M.
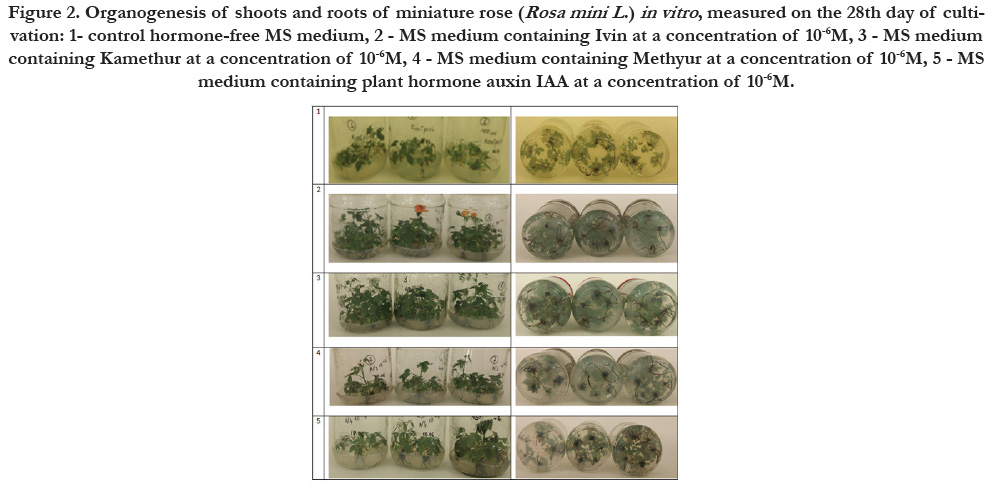
Figure 2. Organogenesis of shoots and roots of miniature rose (Rosa mini L .) in vitro , measured on the 28th day of cultivation: 1- control hormone-free MS medium, 2 - MS medium containing Ivin at a concentration of 10-6M, 3 - MS medium containing Kamethur at a concentration of 10-6M, 4 - MS medium containing Methyur at a concentration of 10-6M, 5 - MS medium containing plant hormone auxin IAA at a concentration of 10-6M.
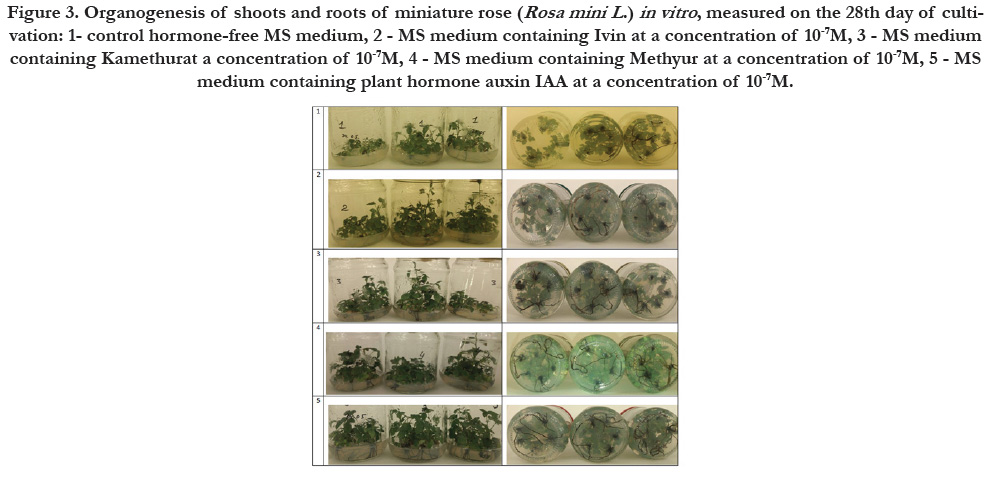
Figure 3. Organogenesis of shoots and roots of miniature rose (Rosa mini L .) in vitro , measured on the 28th day of cultivation: 1- control hormone-free MS medium, 2 - MS medium containing Ivin at a concentration of 10-7M, 3 - MS medium containing Kamethurat a concentration of 10-7M, 4 - MS medium containing Methyur at a concentration of 10-7M, 5 - MS medium containing plant hormone auxin IAA at a concentration of 10-7M.
Discussion
Rose micropropagation through in vitro culture refers to a method
of asexual propagation that produces healthy plant clones that
are genetically equal plants to the parent plant and to each other
[9]. Since the 1970s, rose micropropagation protocols have been
developed [18].
The apical buds, nodal stem segments, shoot tip segments, or
leaf petioles are used as explants for proliferation and multiplication
of shoots and roots [9-11]. To enhance the organogenesis
of stems, leaves and roots,MS basal medium [16] supplemented
with plant hormones cytokinins and auxins is used [9-15, 18]. Cytokinins
BAP, 2-ip, and TDS, and auxins IAA, NAA, and IBA are
most widely used for shoot and root organogenesis [9-15, 18].
The ratio of plant hormones auxins and cytokinins affects organogenesis
towards the proliferation of shoots or roots. Usually,
high concentrations of cytokinins and low concentrations of auxins
or gibberellic acid GA3 promote shoot proliferation through
direct organogenesis or somatic embryogenesis [9-15, 18]. To
promote root organogenesis, modified MS medium with a high
content of mineral salts, with or without the addition of auxins at
low concentrations, is used [9-15, 18].
Synthetic plant growth regulators Ivin, Methyur and Kamethurwere
previously studied by us in tissue culture of potato, tobacco
and cherry to intensify plant organogenesis in vitro [19, 20].
In our earlier published work [19], it was found that Ivin (N-oxide-
2,6-dimethylpyridine) and its derivatives, which were used as
components of culturemedia in concentration range from 0,05
mg to 10 mg per 1 liter, exhibit predominant cytokinin-like activity,
affecting the processes of increasing the growth of callus biomass,
delaying cell ageing, activating photosynthesis in plant cells
and promoting organogenesis of potato and tobacco plants in
vitro. The effect of Ivin and its derivatives on plant organogenesis
was similar or higher than that of the plant hormone cytokinin
Kinetin (6-furfurylaminopurine) used at the same concentrations.
In our work [20] it was shown that the use of Methyurand Kamethurat
concentrations of 10-5М - 10-8М as components of the
MS medium improved the development of the root system on
cherry microshoots. The plant growth regulating activity of
Kamethur and Methyur was similar or higher than that of the
auxin IBA, cytokinin BAP, and gibberellic acid.
This work is aimed at searching for new effective plant growth
regulators capable of initiating organogenesis of miniature
rose (Rosa mini L.) in vitro on MS medium without the addition
of plant hormones auxins and cytokinins. The obtained results
showed that synthetic plant growth regulators Ivin (N-oxide-2,6-
dimethylpyridine), Methyur (sodium salt of 6-methyl-2-mercapto-
4-hydroxypyrimidine) and Kamethur (potassium salt of 6-methyl-
2-mercapto-4-hydroxypyrimidine) revealed both cytokinin - and
auxin-like activity on the organogenesis of shoots and roots of
miniature rose (Rosa mini L.) in vitro. Their growth regulating effect
is similar to or exceeds that of the plant hormone auxin IAA.
The physiological and molecular mechanisms of action of the
synthetic plant growth regulators Ivin (N-oxide-2,6-dimethylpyridine),
Methyur (sodium salt of 6-methyl-2-mercapto-4-hydroxypyrimidine)
and Kamethur(potassium salt of 6-methyl-
2-mercapto-4-hydroxypyrimidine)on plant development are of
the considerable interest. The results of our previous studies with
synthetic low molecular weight heterocycles, derivatives of pyridine,
pyrimidine, pyrazole, isoflavones, oxazolopyrimidine and
oxazole showed their auxin- and cytokinin-like effects on the processes
of elongation, division and differentiation of plant cells [21-30], which are the main processes of organogenesis of plant
shoot and root meristems in vivo and in vitro, which are controlled
by plant hormones [31, 32].
Our earlier studies of the molecular mechanisms of action of
plant growth regulators Ivin and Methyur demonstrated their
stimulating effect on gene expression at the level of transcription
and translation of genetic information, due to which the time of
plant ontogenesis is almost halved [33]. In addition, we came to
the conclusion that synthetic plant growth regulators Ivin and-
Methyur affect plant growth indirectly, through the endogenous
pool of plant hormones [34].
The proposed mechanism of action of synthetic plant growth
regulators directly, through a network ofplant hormone signaling
pathway [35, 36], or indirectly, i.e. affecting the biosynthesis of
plant hormones, their stability and metabolism [36], was discussed
in the works of other authors [37-47].
Therefore, our further work will focus on study of molecular
mechanism of action of synthetic plant growth regulators Ivin,
Methyur and Kamethur.
Conclusion
Our studies showed that the use of synthetic plant growth regulators
Ivin (N-oxide-2,6-dimethylpyridine), Methyur (sodium salt
of 6-methyl-2-mercapto-4-hydroxypyrimidine) and Kamethur
(potassium salt of 6-methyl-2-mercapto-4-hydroxypyrimidine)as
components of the MS medium improves the organogenesis of
shoots and roots of miniature rose (Rosa mini L.) in vitro. The
growth regulatory activity of Ivin, Methyur and Kamethurwas
compared with the activity of plant hormone auxin IAA (2-(1HIndol-
3-yl)acetic acid). The use of synthetic growth regulators
Ivin, Methyurand Kamethurhad a positive effect on the formation
and growth of shoots and roots, measured on the 21st and
28th days of cultivation. The parameters of average length of
shoots (cm) per explant, length of roots (cm) per explant, number
of roots (pcs) per explant, and frequency (%) of shoot rooting
generally increased depending on the concentration of regulators.
The effect of Ivin, Methyur and Kamethur,used at concentrationsof
10-5M, 10-6M, 10-7M per 1 liter of MS medium,on the
organogenesis of shoots and roots of a miniature rose is similar
or higher than that of the plant hormone auxin IAA used at the
same concentrations. The application of synthetic plant growth
regulatorsIvin, Methyur and Kamethur for micropropagation of
the miniature rose (Rosa mini L.) through in vitro culture is proposed.
References
- Vosman B, Barendrecht CJ, Esselink D, Jones H, Scott E, Spellerberg B, et al. A European reference collection of rose varieties.Plant Research International; 2006.
- Datta SK. Breeding of new ornamental varieties. CurrSci. 2018 Mar 25;114(6):1194-206.
- Mileva M, Ilieva Y, Jovtchev G, Gateva S, Zaharieva MM, Georgieva A, et al. Rose Flowers-A Delicate Perfume or a Natural Healer? Biomolecules. 2021 Jan 19;11(1):127. PubMed PMID: 33478154.
- Das P, Nanda S. Medicinal Efficacy of Rose Plant: AMini Review. Pharma- Tutor. 2015 Oct 1;3(10):23-6.
- ChaharS. Rose: Ornamental As Well As Medicinal Plant. JResAgricAnim Sci. 2016;4(1):8-10.
- SternKR. Introduction to Plant Biology. McGraw-HillHigher Education. 8thEd.256 p.
- Adhikary K, Sarkar MM. Varietal evaluation of miniature rose cultivars under the plains of West Bengal, India. J PharmacognPhytochem. 2019;8(4):1618-21.
- De Hoog J. Handbook for modern greenhouse rose cultivation.Applied Plant Research.Wageningen Universityand Research.Pp 220.
- Gholami M, Khorshidi M, Agrawal V.The Effect of Benzyladenine and Indolebutyric Acid on Shooting and Rooting of Miniature Rose. COJ Biomed Sci Res. COJBSR. 2021.
- Pati PK, Rath SP, Sharma M, Sood A, Ahuja PS.In vitro propagation of rose - A Review. Biotechnology Adv. 2006 Jan 1;24(1):94-114.
- Nak-Udom N, Kanchanapoom K, Kanchanapoom K. Micropropagation from cultured nodal explants of rose (Rosa hybrida L. cv.‘Perfume Delight’). Songklanakarin J Sci Technol. 2009 Nov 1;31(6):583-6.
- liynyk OО, Kluvadenko AА, Melnychuk MD.Optimization of CultureMedia Content for Acceleration of Growth and Cultivation of Rosa DamascenaMill. in In Vitro Culture.Scientific bulletin of the National Forestry University of Ukraine.26(7):134–139.
- Stone M. Propagation of miniature roses by plant tissue culture. O’Donnell MA eds. Tested studies for laboratory teaching. 2006;27:239-63.
- Ozel CA, Arslan O. Efficient micropropagation of English shrub rose “Heritage” under in vitro conditions. IntJAgriBiol.2006;8(5):626-9.
- Chhalgri MA, Khan MT, Nizamani GS, Yasmeen S, Khan IA, Aslam MM, et al. Effect of Plant Growth Hormones on Shoot and Root Regeneration in Rose under In Vitro Conditions. Adv Life Sci. 2020 Nov 27;8(1):93-7.
- Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol plant. 1962 Jul;15(3):473-97.
- Bang H, Zhou XK, Van Epps HL, Mazumdar M, editors. Statistical methods in molecular biology. Totowa, NJ, USA:: Humana press; 2010;3(620):636.
- Canli FA, Kazaz S.Biotechnology of roses: progress and future prospects. Turk J For. 2009;10(1):167-183.
- Tsygankova VA, Blume YaB.Screening and peculiarity of the biological action of synthetic plant growth regulators. BiopolymCell. 1997;13(6):484- 492.
- Tsygankova V, Medvedieva T, Natalchuk T, Udovychenko K, Andrusevich Ya, Kopich V, et al. Study of the impact of pyrimidine derivatives on rooting microshoots of cherry (Prunus cerasus L.) under in vitro culture conditions. Materials of the 5th International scientific and practical conference “Scientific achievements of modern society”. Cognum Publishing House. Liverpool, United Kingdom.2020;1063-1076.
- Tsygankova VA, Bayer OO, Andrusevich Ya V, Galkin AP, Brovarets VS, Yemets AI, et al. Screening of five and six-membered nitrogen-containing heterocyclic compounds as new effective stimulants of Linum usitatissimum L. organogenesis in vitro. Int J Med Biotechnol Genetics S. 2016 Apr 22;S2(001):1-9.
- Tsygankova V, Ya A, Shtompel O, Romaniuk O, Yaikova M, Hurenko A, et al. Application of synthetic low molecular weight heterocyclic compounds derivatives of pyrimidine, pyrazole and oxazole in agricultural biotechnology as a new plant growth regulating substances. IntJMedBiotechnolGenetics. 2017 Mar 22;S2(002):10-32.
- Tsygankova V, Andrusevich Y, Shtompel O, Kopich V, Solomyanny R, Bondarenko O. Phytohormone-like effect of pyrimidine derivatives on regulation of vegetative growth of tomato. Int J Botany Stud.2018;3(2):91-102.
- Kornienko AM, Brovarets VS. Study of auxin-like and cytokinin-like activities of derivatives of pyrimidine, pyrazole, isoflavones, pyridine, oxazolopyrimidine and oxazole on haricot bean and pumpkin plants.IntJ ChemTechR es.2018;11(10):174-190.
- Hurenko AO, Solomyanny RM, Mrug GP, Frasinyuk MS, Pilyo SG, Kornienko AM, et al. Auxin-like effect of derivatives of Pyrimidine, Pyrazole, Isoflavones, Pyridine, Oxazolopyrimidine and Oxazole on acceleration of Vegetative growth of Flax. Int JPharmTech Res. 2018;11(3):274-86.
- Tsygankova V, Andrusevich YV, Shtompel OI, Kopich VM, Panchyshyn SY, Vydzhak RM, et al. Application of pyrazole derivatives as new substitutes of auxin IAA to regulate morphometric and biochemical parameters of wheat (Triticumaestivum L.) seedlings. JAdv Agric. 2019;10:1772-86.
- Mohilnikova IV, Tsygankova VA, Solomyannyi RM, Brovarets VS, Bilko NМ, Yemets АІ. Screening of growth-simulating activity of synthetic compounds- pyrimidine derivatives.DopovNacakadnauk Ukr. 2020;10:62-70.
- Mohilnikova IV, Tsygankova VA, Gurenko AO, Brovarets VS, Bilko NМ, Yemets АІ, et al.Influence of pyrazole derivatives on plant growth and development in vivo and in vitro.DopovNacakadnauk Ukr. 2021 Dec 23(6):108- 19.
- Tsygankova VA, Voloshchuk IV, AndrusevichYa V, Kopich VM, Pilyo SG, Klyuchko SV, et al. Pyrimidine Derivatives As Analogues Of Plant Hormones For Intensification Of Wheat Growth During The Vegetation Period. life.JAdvBiol. 8:9.
- Tsygankova VA, Andrusevich YV, Shtompel OI, Solomyanny RM, Hurenko AO, Frasinyuk MS, et al. New Auxin and Cytokinin Related Compounds Based on Synthetic Low Molecular Weight Heterocycles. InAuxins, Cytokinins and Gibberellins Signaling in Plants.Springer, Cham;2022. pp. 353- 377.
- Tsygankova VA. Genetic Control and Phytohormonal Regulation of Plant Embryogenesis. Int J MedBiotechnol. Genetics (IJMBG). 2015 Jan 23;3(1):9-20.
- Cheng ZJ, Shang BH, Zhang XS, Hu YX. Plant hormones and stem cells. Hormone Metabolism and Signaling in Plants. 2017:405-30.
- Tsygankova VA, Zayetz VN, Galkina LA, Prikazchikova LP, Blume YB. An unusual minor protein appearing in embryonic axis cells of haricot bean seeds following germination process stimulated by 6-methylthiouracil.Biopolym Cell. 1998;14(5):438-448.
- Tsygankova VA, Zayets VN, Galkina LA, Blume YB. The phytohormonemediated action of the synthetic regulators on cell extension growth in higher plants. Biopolym Cell.1999;15(5):432.
- Blázquez MA, Nelson DC, Weijers D. Evolution of Plant Hormone Response Pathways. Annu Rev Plant Biol. 2020 Apr 29;71:327-353. PubMed PMID: 32017604.
- Fàbregas N, Fernie AR. The interface of central metabolism with hormone signaling in plants.Curr Biol. 2021 Dec 6;31(23):R1535-R1548. PubMed PMID: 34875246.
- Tandon P, Arya HC. Association of auxin protectors, peroxidase, indoleacetic acid oxidase and polyphenol oxidase in Zizyphus gall and normal stem tissues grown in culture. Biochemie und Physiologie der Pflanzen. 1982 Jan 1;177(2):114-24.
- Liu ZH, Hsiao IC, Pan YW. Effect of naphthaleneacetic acid on endogenous indole-3-acetic acid, peroxidase and auxin oxidase in hypocotyl cuttings of soybean during root formation. Bot Bull Acad Sin. 1996 Oct 1;37(4):247- 253.
- Murthy BN, Murch SJ, Saxena PK. Thidiazuron‐induced somatic embryogenesis in intact seedlings of peanut (Arachis hypogaea): Endogenous growth regulator levels and significance of cotyledons. Physiol Plantarum. 1995 Jun;94(2):268-76.
- Hutchinson MJ, Saxena PK. Role of purine metabolism in thidiazuron‐induced somatic embryogenesis of geranium (Pelargonium× hortorum) hypocotyl cultures. Physiol Plantarum. 1996 Nov;98(3):517-22.
- Guo B, Abbasi BH, Zeb A, Xu LL, Wei YH. Thidiazuron: a multi-dimensional plant growth regulator. Afr JBiotechnol. 2011;10(45):8984-9000.
- Ahmad N, Faisal M, editors. Thidiazuron: From urea derivative to plant growth regulator. Singapore: Springer; 2018 Mar 23.
- Rigal A, Ma Q, Robert S. Unraveling plant hormone signaling through the use of small molecules. Front Plant Sci. 2014 Jul 30;5:373. PubMed PMID: 25126092.
- Serrano M, Kombrink E, Meesters C. Considerations for designing chemical screening strategies in plant biology. Front Plant Sci. 2015 Apr 8;6:131. PubMed PMID: 25904921.
- Dejonghe W, Russinova E. Plant Chemical Genetics: From Phenotype- Based Screens to Synthetic Biology. Plant Physiol. 2017 May;174(1):5-20. PubMed PMID: 28275150.
- Raggi S, Doyle SM, Robert S. Auxin: At the crossroads between chemistry and biology. The Chemical Biology of Plant Biostimulants.Book SeriesWiley Series in Renewable Resources. 2020 Mar 2:122-52.
- Pařízková B, Pernisová M, Novák O. What Has Been Seen Cannot Be Unseen- Detecting Auxin In Vivo. Int J Mol Sci. 2017 Dec 16;18(12):2736. PubMed PMID: 29258197.