Features of Apigenin, Luteolin, Hesperetin and Naringenin in Crop and Body
Reza Pashaei
Department of Biotechnology, Chemistry and Pharmacy at University of Siena, Italy.
*Corresponding Author
Reza Pashaei
Department of Biotechnology, chemistry and pharmacy at University of Siena,
Italy.
E-mail: reza.pashaei@student.unisi.it
Received: May 23, 2016; Accepted: June 13, 2016; Published: June 16, 2016
Citation: Reza Pashaei (2016) Features of Apigenin, Luteolin, Hesperetin and Naringenin in Crop and Body. Int J Food Sci Nutr Diet. 5(5), 300-304.DOI : dx.doi.org/10.19070/2326-3350-1600054
Copyright: Reza Pashaei© 2016. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
All the subgroup of flavonoids has pigment that result of these pigment are colors in fruits, vegetables, and herbs but other feature of flavonoids and subgroup of flavonoids are relevant to anti-disease property that present in all the member of flavonoids subgroups particularly in flavones and flavanones. On the other hand, subgroup of flavones and flavanones has significant advantages about anti-disease feature. This article explain role of flavonoids, flavones, flavanones, apigenin. Luteolin, hesperetin, and naringenin in nature and human body.
2.Introduction
3.Materials
3.1.Flavones
3.2.Flavanonesn
4.Conclusion
5.References
Keywords
Apigenin; Luteolin; Hesperidin; Naringenin.
Introduction
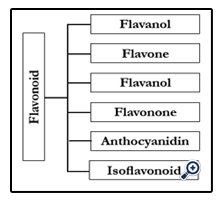
Flavonoids or bioflavonoids are a group of phytonutrients that exist in the fruits and vegetables. Flavonoids are a class of secondary plant phenolics with significant antioxidant and chelating properties [1]. The phytonutrients has more than 6000 types that the largest group of phytonutrients is flavonoids. The flavonoids divide to six subgroups: flavanol, flavone, flavonol, flavanone, anthocyanins and isoflavonoids.
Furthermore, flavonoids classified two parts:
1) isoflavonoids, derived from 3-phenylchromen-4-one (3-phenyl-1,4-benzopyrone) structure.
2) neoflavonoids, derived from 4-phenylcoumarine (4-phenyl-1,2-benzopyrone) structure.
Flavonoids are polyphenolic molecules inclusive the 15-carbon skeleton, two phenyl rings (A and B) and heterocyclic ring (C). Stress-responsive dihydroxy B-ring-substituted flavonoids have great potential to inhibit the generation of reactive oxygen species (ROS) and reduce the levels of ROS once they are formed, i.e., to perform antioxidant functions [2].
The yellow color in plants and fruit is concern to flavonoid. Banana, corn, lemon, pear, apple, pineapple, yellow peppers and yellow tomato are the most important crops that are related to flavonoids in terms of color. On the other hand, flavonoids are not only relevant to yellow fruits. The strawberry and Blueberry are the prominent instance that has the high source of flavonoids.
Other source of flavonoids in nature is related to green plants. Since flavonoids are pigments, which are ubiquitous to green plant cells and are highly diversified, as well as easily separable with modern chromatographic equipment, botanists have long used the pattern of occurrence of these compounds for taxonomical studies [18].
The significant aspect of flavonoid is related to anti disease feature because flavonoids are the strong antioxidant. Flavonoids have anti disease property against cardiovascular disease, erectile dysfunction and cancer by antioxidant. Antioxidants are such as defensive barriers versus free radicals which are small molecules in metabolic processes. The pharmacological activities of flavonoids have been attributed to their ability to alter the permeability of cell membranes, change fatty acid composition or phospholipid content, or interact with membrane protein resulting in the modification of cell membrane permeability and to their binding with specific protein targets [4]. Other property of flavonoids is concern to oxidation. Oxidation is the chemical reaction that produces the free radicals. The free radicals are negative impact on the cells that result of this damage is disease. Flavonoids found in crops particularly in coffee and tea.
Materials
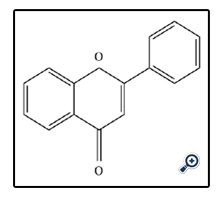
Flavones are a colorless crystalline compound of non-nitrogenous biological pigments. Flavones are usually found in plants bound to sugars as O-glycosides, beside, Flavones may also occur as C-glycosides [9]. The structure of flavones consist 2-Phenyl-1,4-benzopyrone.
Flavones organized of apigenin, luteolin, tangeritin, chrysin, baicalein, baicalin, wogonin, and nobiletin./p>
Besides, the flavones can be classified into several subgroups which are mainly indicated either by (i) hydroxylation, (ii) Omethylation, (iii) C-methylation, (iv) isoprenylation, or (v) methylenedioxy substitution [3]. It can be said that, all the flavonoids subgroup members has anti-cancer feature especially flavones that are antioxidant, anti-cancer, and anti-tumor propertes in our body. Furthermore, Flavones have been suggested by some investigators as potential anti-HIV agents [5]. For defining the source of flavones can mention to herbs particularly leaves. The herb is known to contain alkaloids and flavones [19]. Another beneficial of flavones about pharmacology property in body is relevant to COPD or Chronic Obstructive Pulmonary Disease that is related to hard breathe [20]. said that flavones has positive effect on COPD in terms of treatment this disease.
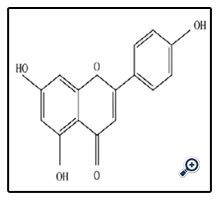
Apigenin Known as 4',5,7-Trihydroxyflavone is a natural phytochemical product of Flavones species that is present in fruits and vegetables.
One of the most important features of Apigenin is anti-cancer and anti-tumor. Nowadays, cancer is the most perilous disease but natural products such as flavonoids subgroup can treat cancer particularly skin, ovarian, breast and hematological malignancies. Apigenin, a plant flavonoid abundantly present in common fruits and vegetable has been shown to exhibit chemopreventive potential against skin cancer [10]. Recently, it was reported that apigenin inhibited the growth of human cervical carcinoma cells (HeLa) and neuroblastoma cell lines, a pediatric tumor accounting for 15% of childhood cancer deaths [3]. Apigenin is present in common crop such as apple, parsley, celery, rosemary, oregano, thyme, basil, coriander, chamomile, cloves, lemon balm, artichokes, and spinach. Apigenin can reduced risk of heart problems and neurological conditions.
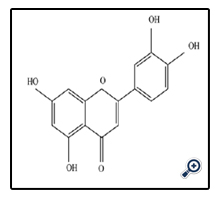
Luteolin known as 3',4',5,7-Tetrahydroxyflavone is a supper nutrient of subgroup of flavones. The flavone luteolin and the flavonol quercetin, both containing the 3,4'-dihydroxy structure in the B ring (catechol B ring), are known to possess a high antioxidant ability by scavenging free radicals [12].
Luteolin present in leaves and medicinal herbs but it can found in celery, thyme, green peppers, and tea. Besides, Oranges, grapefruit, and lemons are other great sources of luteolin. Luteolin is the natural and potent antioxidant that has anti-disease feature particularly anti-allergy because luteolin can neutralizes free radicals by antioxidant feature that is effective for reducing inflammation, and promotes nerve and muscle function. Luteolin in cancer treatment has important role because [11] said that luteolin has great promise for acting as a lead compound in the chemotherapy of leishmaniasis. Notably, luteolin is blood-brain barrier permeable, rendering it applicable to the therapy of central nerve system diseases, including brain cancer [6]. Furthermore, HL-60 or Human promyelocytic leukemia cells is kind of blood cells in human body. Luteolin inhibits the proliferation of HL-60 cells by inducing apoptosis [13].
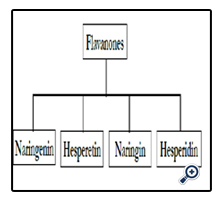
Flavanones known as 2-Phenyl-chroman-4-one are polyphenolic compounds that include the hesperidin and naringenin.
The most prominent source of flavanones is citrus such as lemon, grapefruit, and orange. Flavanones are the strong antioxidant that exists in blood. Flavanones could thus exhibit some physiological effects, and be ingested at quite a high level, but their bioavailability is still poorly known [7]. Flavanones biological activities in our body consist the significant reaction against disease, for instance can mentioned to hypotensive, antifungal, antibacterial, and antitumor features.
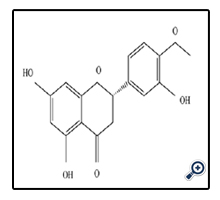
Hesperetin is the aglycone of hesperidin and its IUPAC name is ((S)-2,3-dihydro-5,7-dihydroxy-2-(3-hydroxy-4 methoxy-phenyl)-4H-1-benzopyran-4-one) [17]. Hesperetin that known as 3',5,7-Trihydroxy-4'-methoxy flavanone is subgroup of flavanones found in citrus fruits particularly in the peel. Other source of hesperetin is citrus such as orange that is opulent hesperetin.
Hesperetin has significant property in terms of biological activities that is the strong tumor inhibitor. Also possess some antioxidant activities, although this activity is poorer compared with many other polyphenols, furthermore, other possible effects of hesperetin and naringenin are on lipid metabolism [8]. One-half orange and one-half mandarin give 130 mg of hesperetin. The estrogenic features that are concern to women biological activities are another part of the pharmacological activities of hesperetin in human body. In addition, hesperetin and naringenin have also been found to exhibit estrogenic, anticarcinogenic, and antioxidative properties [16].
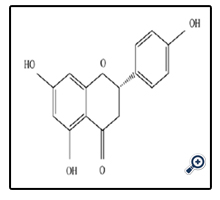
Naringenin is another member of subgroup of flavanones that play important role in body health in terms of antioxidant and anti-inflammatory. The presence of the 2, 3-double bond in conjugation with a 4-oxo group in the structure naringenin has been suggested to be important for its antioxidant activity [15]. Naringen has significant difference with other members of flavanones subgroups because naringenin can bind to DNA. Naringenin ramifications can bind to DNA in intercalative mode and they also have certain antioxidative and cytotoxic activities [21]. In subgroup of flavanones, naringenin and naringin are the strong antioxidant. Citrus fruits are the great source of naringenin with 4’, 5, 7-trihydroxy flavanone chemical formula.
Grapefruit is the famous example for naringenin between citrus fruits. One-half orange and one-half mandarin provide 30 mg of naringenin. One of the most important points about naringenin is concern to anti- infection property, particularly HCV infection that is relevant to hepatitis C virus. On the other hand, anticancer and cardiovascular protective are the other biological feature of naringenin. Naringenin has been shown to inhibit in vitro growth of in human cancer cells. Furthermore, the effects of naringin and naringenin on levels of plasma lipids, hepatic ACAT play a major role in the regulation of cholesterol metabolism, regression of atherosclerotic lesions in the aorta, and expression of endothelial VCAM-1 and MCP-1 gene [14]. Beides, narigenin can decrease the hyperlipidaemia namely can say narigenin can treat hyperlipidaemia diseases. It can be said that naringenin covering the disease for children until old persons and features of naringenin illustrates that naringenin role in terms of biological activities in our body is extensive.
Conclusion
The citrus fruits are the great source of flavonoids and subgroups of flavonoids particularly in fruits which have orange and red color but also green plant, tea and herbs are include the flavonoids and subgroups of flavonoids. The important property of flavonoids, flavones, and flavanones is anti-cancer feature but these phytonutrients has positive effect on the HIV and Hepatitis C. It can be said that, flavonoids, flavones, and flavanones play important role in treatment of most of the disease in our body.
References
- Heim KE, Tagliaferro AR, Bobilya DJ (2002) Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem 13(10): 572-584.
- Giovanni Agati, Elisa Azzarello, Susanna Pollastri, Massimiliano Tattini (2012) Flavonoids as antioxidants in plants: Location and functional significance. Plant Science 196: 67-76.
- Martens S, Mithöfer A (2005) Flavones and flavone synthases. Phytochemistry 66(20): 2399-2407.
- Morales-Flores F, Olivares-Palomares KS, Aguilar-Laurents MI, Rivero-Cruz JF, Lotina-Hennsen B, King-Díaz B (2015) Flavonoids Affect the Light Reaction of Photosynthesis in Vitro and in Vivo as Well as the Growth of Plants. J Agric Food Chem 63(37): 8106-8115.
- Li-Weber (2009) New therapeutic aspects of flavones: The anticancer properties of Scutellaria and its main active constituents Wogonin Baicalein and Baicalin. Cancer Treatment Reviews 35(1): 57-68.
- Lin Y, Shi R, Wang X, Shen HM (2009) Luteolin, a flavonoid with potentials for cancer prevention and therapy. Curr Cancer Drug Targets 8(7): 634-646.
- Manach C, Morand C, Gil-Izquierdo A, Bouteloup-Demange C, Rémésy C (2003) Bioavailability in humans of the flavanones hesperidin and narirutin after the ingestion of two doses of orange juice. Eur J Clin Nutr 57(2): 235-242.
- Iris Erlund (2004) Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutrition Research 24(10): 851-874.
- Peter C H Hollman, Ilja C W Arts (2000) Flavonols, flavones and flavanols – nature, occurrence and dietary burden. J Sci Food Agric 80(7): 1081-1093.
- Gupta S, Afaq F, Mukhtar H (2001) Selective Growth-Inhibitory, Cell-Cycle Deregulatory and Apoptotic Response of Apigenin in Normal versus Human Prostate Carcinoma Cells. Biochem Biophys Res Commun 287(4):914-920.
- Mittra B, Saha A, Chowdhury AR, Pal C, Mandal S, Mukhopadhyay S, Bandyopadhyay S, Majumder HK (2000) Luteolin, an abundant dietary component is a potent anti-leishmanial agent that acts by inducing topoisomerase II-mediated kinetoplast DNA cleavage leading to apoptosis. Mol Med 6(6): 527-541.
- Galati G, Moridani MY, Chan TS, O'Brien PJ (2001) Peroxidative metabolism of apigenin and naringenin versus luteolin and quercetin: glutathione oxidation and conjugation. Free Radic Biol Med 30(4): 370-382.
- Ko WG, Kang TH, Lee SJ, Kim YC, Lee BH (2002) Effects of Luteolin on the Inhibition of Proliferation and Induction of Apoptosis in Human Myeloid Leukaemia Cells. Phytother Res 16(3): 295-298.
- Lee CH, Jeong TS, Choi YK, Hyun BH, Oh GT, Kim EH, Kim JR, Han JI, Bok SH (2001) Anti-Atherogenic Effect of Citrus Flavonoids, Naringin and Naringenin, Associated with Hepatic ACAT and Aortic VCAM-1 and MCP-1 in High Cholesterol-Fed Rabbits. Biochem Biophys Res Commun 284(3): 681-688.
- Zbarsky V, Datla KP, Parkar S, Rai DK, Aruoma OI, Dexter DT (2005) Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson’s disease. Free Radic Res 39(10):1119-1125.
- Kim HK, Jeong TS, Lee MK, Park YB, Choi MS (2003) Lipid-lowering efficacy of hesperetin metabolites in high-cholesterol fed rats. Clin Chim Acta 327(1-2): 129-137.
- Mishra PR, Al Shaal L, Müller RH, Keck CM (2009) Production and characterization of Hesperetin nanosuspensions for dermal delivery. Int J Pharm 371(1-2): 182-189.
- Havsteen BH (2002) The biochemistry and medical significance of the flavonoids. Pharmacol Ther 96(2-3): 67-202.
- Sato Y, Suzaki S, Nishikawa T, Kihara M, Shibata H, Higuti T (2000) Phytochemical flavones isolated from Scutellaria barbata and antibacterial activity against methicillin-resistant Staphylococcus aureus. J Ethnopharmacol 72(3): 483-488.
- Tabak C, Arts IC, Smit HA, Heederik D, Kromhout D (2001) Chronic Obstructive Pulmonary Disease and Intake of Catechins, Flavonols, and Flavones. Am J Respir Crit Care Med 164(1): 61-64.
- Li TR, Yang ZY, Wang BD, Qin DD (2008) Synthesis, characterization, antioxidant activity and DNA-binding studies of two rare earth (III) complexes with naringenin-2-hydroxy benzoyl hydrazone ligand. Eur J Med Chem 43(8): 1688-1695.