ABO Typing In Forensic Analysis: to be or not to be in the epoch of genotyping
Sandip Ghosh*
Assistant Director, Biology Division, Forensic Science Laboratory, Government of West Bengal, West Bengal, India.
*Corresponding Author
Sandip Ghosh,
Assistant Director, Biology Division, Forensic Science Laboratory, Government of West Bengal, West Bengal, India.
Tel: 91-033-25565430/91- 9434706988
Fax: 91- 033-25565430
E-mail: sandip@rocketmail.com
Received: February 25, 2022; Accepted: March 28, 2022; Published: March 30, 2022
Citation: Sandip Ghosh. ABO Typing In Forensic Analysis: to be or not to be in the epoch of genotyping. Int J Forensic Sci Pathol. 2022;9(3):487-494. doi: dx.doi.org/10.19070/2332-287X-22000101
Copyright: Sandip Ghosh©2022. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Increasing crime rate, in the modern era, has prompted the scientists to develop fast and effective forensic strategies to distinguish between guilty and innocent. Since ages, investigators have relied on ABO typing which was the only method available mostly to exclude the innocent from the suspect list, though most of the ABO-typing methods are low throughput and time consuming. Moreover, as the aim of every investigation is to establish the identity of the offender, the ABO typing methods, being used, even if done properly, were proven to be non-beneficial to single out the perpetrator and thus futile in the court of law. Due to lack of accuracy, specificity, conclusiveness and availability of sample, ABO typing is fast being replaced by genotyping, which is a more suitable method to convict the offender. The pros and cons of different ABO-typing methods and the usefulness of genotyping techniques in this context have been discussed here. In spite of being overshadowed by genotyping, ABO typing is still being used as a tool in forensic science in developing countries because of its cost-effectiveness. However, it is concluded that the potential challenge for forensic investigators to identify suspects possibly poses a future, where genotyping will be the ultimate tool and ABO typing may become obsolete.
2.Keywords
3.Introduction
4.Methodology
5.Case Report
6.Discussion
7.Conclusion
8.References
Keywords
ABO-Typing; Absorption-Inhibition; Absorption-Elution; STR-PCR; Touch DNA Analysis; Epigenomics.
Introduction
Forensic science is an interdisciplinary field that uses scientific
knowledge from varied fields like biotechnology, toxicology,
chemistry, physics and others to analyze and characterize physical
evidence found at the scene of crime. In criminal matters,
particularly those involving violence, specimens of blood, semen,
and other body fluids or tissues are used as evidence as they provide
information that may solve the case. Blood is considered one
of the most important biological samples that are frequently obtained
from the crime scene. It is regarded as a very important
forensic tool since analysis of different aspects of bloodstains
can provide valuable information which helps the investigators
to have a clear understanding of the circumstances under which a
crime has been committed. The use of blood in forensic analysis
is a method for identifying individuals suspected of committing a
crime, solving disputes in paternity etc [1].
The term Forensic serology has generally been used to refer to the
identification and individualization of biological evidence, including
all the activities and tests associated with the evaluation and
typing of biological evidence in criminal matters. The word serology
was derived from serum, the fraction of blood containing
antibodies. Blood grouping was long the only means of individualizing
biological evidence, and serology classically encompassed
blood groups and blood typing [2].
Origin and ABO typing
The importance of blood grouping in medico-legal issues,lies in
the fact that blood groups are considered genetic markers since
they are strongly inherited following the Mendelian laws, and are
unaffected by environmental factors such as nutrition,diet, age,
occupation, diseases. It can withstand stringent conditions, such as
high heating or drying [3, 4] and thus remain unchanged throughout
life [5] Blood grouping is the classification of erythrocytes or
red blood cells (RBCs) based on surface markers or antigens like
A, B, D, H, etc., present on their cell membrane [6]. There are
more than 300 antigens present on the RBC membrane and based
on these antigens, 38 blood group systems have been identified by International Society of Blood Transfusion which includes ABO,
Rh, P, Kell, MNS, Lewis, Kidd, Diego, etc [7]. Due to complexity,
and expense of testing for possible reactions among all known
antigens, the simpler ABO and Rh blood typing system remains
the primary, cost-effective and conspicuous method in practice
for clinical and forensic use [8].
The ABO blood group system, first described in 1901 by Karl
Landsteiner is the most basic system which divides blood into
four groups, or types: A, B, AB and O [9] Blood group specificity
depends upon the inheritance of the ABO and H genes and the
subsequent expression of these antigens on the red blood cells.
For e.g., an individual having blood group A will expressantigen
"A" on the surface of the RBCs and will contain "anti-B" antibody
in the plasma.The Rhesus-system is the second most imperative
blood group system after ABO[10]. This system is based
on the expression of the "D" antigen or Rh factor on the RBC
membrane and accordingly, the status of a person is indicated
as either Rh-positive (D-antigen present) or Rh-negative (D-antigen
absent). Landsteiner's description of blood types gave a new
opening to forensic science and based on this, forensic scientists
could definitively compare blood evidence left at a crime scene to
the blood of a suspect. The Rh factor enabled forensic scientists
to better study the blood of suspects and to potentially exclude
individuals as the source of blood at crime scenes and narrow
down the list of suspects [11]. Therefore blood-typing could be
used to help prove innocence, but not toidentify a suspect beyond
a reasonable doubt, the standard necessary for a criminal conviction
in many criminal courts.
However, accuracy of ABO blood typing is a major concern
for the forensic serologist since inception. Small sample quantity,
degraded and contaminated blood, hemolysis, putrefaction,
mummification and skeletonization of the body during the post
mortem [12] often makes the typing method mostly inconclusive
either due to confusing observations or due to lack of sufficient
stained sample present.
Species identification
Species identification along with ABO- typing of different human
samples in Forensic serology has long been one of the well
accepted test in criminal investigation [13]. Beside blood, several
other evidences, hair, semen and saliva may present in the crime
scene, which can be used to link the suspect in accordance with
the Locard’s principal.
The amount of biological evidence collected at a crime scene is
often extremely small, and thus it becomes particularly important
for the forensic scientists to preserve as much of the evidence as
possible and select the accurate tests for analysis that require trace
amounts of sample.
After detection, the sample requires the test for species identification.
Species identification is important prior to ABO-typing because
the forensic materials may be contaminated with the animal
body fluids. For most of the body fluids the primary analysis for
species determination is by ‘precipitin’ test [14] which used the
principle of interaction between antigens and the anti-sera raised
against the blood cells of the same species. Precipitin tests can be
performed in various ways. In every instance, however, the goal is
to bring the antigens and antibodies, both in solution, into contact
with one another.
The earliest precipitin test method was the "ring test," where an
aqueous solution of antigen is layered over the more dense antiserum
solution in a tube. The formation of precipitate formation
at the interface between the layers indicates a positive test. This
method was largely abandoned when gel-based methods, such
as Double Diffusion’ [15] was developed, which was also based
on the same principle. Though used globally these methods have
some limitations. First, due to unavailability of enough quantity
of sample it is always difficult to titrate the required quantity of
antigen-antibody to get a visible result and the second, is to eliminate
the cross-reactivity of antisera [16] against one species with
bloods of evolutionary closed species ( e. g., human &chimpanzee).
Both the limitations are some-way related to the particular
batch of antisera being used. Therefore, it is always necessary to
test the new batch of antisera every time against the homologous
blood. The precipitin test can also be done using the antisera,
raised against the hemoglobin of the species, which detects the
origin of species as well as confirms the presence of blood. Unfortunately,
the blood stain often found in the crime scene is dried
and is not a true mixture of blood cell and serum. Depending on
the environment and clotting time (before drying or after drying)
the ratio of cell/serum often varies.The antisera raised against
serum or hemoglobin will therefore, produce different results for
different cell/serum mixture for the same species.
Nevertheless, the "anti-human" sera raised against human serum
proteinsreact with some human physiological fluids, e. g. human
semen, saliva, and semen-free vaginal swab extracts although to a
lesser extent than they do with serum or blood. Therefore, the antihuman
sera can be used to test for human proteins in biological
fluids other than serum. It must also sometimes be considered in
the interpretation of positive test results when the specimens are
or may be mixtures of blood and/or various physiological fluids.
There are a number of more involved technical modifications of
precipitin tests that have been proposed or used, particularly to
help in differentiating closely related species [17].
ABO - Typing
Agglutination is the main criteria to detect antigen-antibody interaction,
using the principle of immunochemistry.
The main avenues for ABO typing are (1) Direct method and (2)
Indirect technique .The direct method could be classified into two
types ; (i) Forward method, depending on the presence/absence of
antigen [18] on the cell surface,& (ii) Reverse method depending
presence/absence of antibody in the serum part [19]. The indirect
method was mainly the antigen-antibody combined technique, for
which two basic methods have been reported namely, absorption inhibition
[20, 21] and absorption-elution [22, 23]. Both these
methods are used to detect the blood group antigens remaining on
the ruptured cell membrane. Some other high throughput methods,
such as Enzyme linked immune-sorbent (ELISA) assay [24,
25] and Micro-plate method [26] have also been tried. The latter
two methods, when used in combination with absorption-elution,
helped to increase the efficiency of the result.
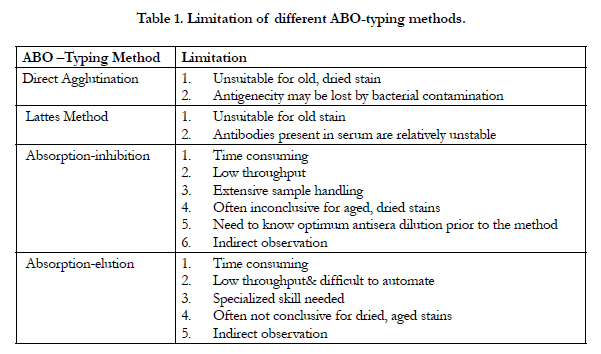
Forward method
It is the simplest ABO-typing method, which is mainly used for the fresh blood. The antigen on the cell surface and anti-sera raised
against the blood antigen reacts together and a cell-cell attachment
occurs through antigen-antibody bridge. This cell-cell attachment
(agglutination) can be visualized in the microscope. The
efficiency of agglutination depends on the proper cell structure,
antibody binding capacity, osmotic fragility etc [27, 28]. In dried,
aged stains the antigens are partially or completely destroyed.
Sunlight and humidity are also known to be destructive for the
antibody stability [29]. Moreover, hemolysis or partial hemolysis
has been a common observation during suspension of cells in
saline, although, later it was shown that the hemolysis could be
partially avoided by using 40% saccharose solution of pH 6 [30].
Lattes method
An alternative reverse approach has been reported by Lattes 19
based on serum antibody. It used the unique property of the
ABO system, that an individual’s serum contained antibodies corresponding
to the antigen that he or she lacked. This process has
its own limitations for the analysing forensic samples as the serum
antibodies are far less stable in dried stains. Though some laboratories
reported the presence of antigen in the dried stain, they
could not opine the antigen type conclusively which may be due
to less sensitivity of the method. Mistyping can also occur in the
stain having some other unrelated antibodies in the serum, which
causes the agglutination of the test cells. The possibility of misinterpretation
of result has been a common observation in report
using older cells.
Absorption-inhibition
Absorption-inhibitionwas the first combinedmethod reported by
Schutze, 1921, where he used the dried blood sample for ABO
typing. The method was less sensitive, whichneeded the predeterminedtiter
value of the anti-sera to work with. A two-dimensional
absorption –inhibition method [21] was proposed, which took
best features of inhibition-titration and titration-inhibition at the
same time and was shown to be more sensitive than either of
them.
Absorption-elution
The absorption-inhibition method was subsequently replaced by
Absorption-Elution method [22] and was consequently refined by
the method developed by Kind (1960), which was predominately
used by most of the laboratoriesfor its high sensitivity. Though
ABH antigen detection was reported even for 42 weeks aged
samples by absorption –elution method [4], some discrepancies
were also observed [12]. In this context, a comparative study between
direct agglutination and absorption-elution methods was performed, which showed some cross reactivity in absorptionelution
method for the blood stored at room temperature for 60
days [28]. That result could not comprehensively eliminate the
possibility of mistyping for the aged, dried stains [31] even using
absorption-elution method. Haemolysis might also produce
negative results [32]. Though, ABH antigens were shown to be
stable [3] at high heat, activity changes was reported for the dried
blood stains on standing [4]. Surface type (cotton, synthetic fabrics,
wooden surface) was also shown to affect the efficiency of
this method with age [33]. High sensitivity of this method can
also be a disadvantage as some other body secretions like, semen,
saliva which are also the source of ABH antigen might interfere
with the result.
However, all ABO typing methods for dried, aged blood
stains, were complex, tedious, time-consuming and low throughput,
which were difficult to automate. As it involved, extensive sample
handling, and complex techniques, which were coupled with human
skill and experience, a lot of discrepancies and false positive/
negative results were also found [12]. The method also became
vulnerable while determining the blood group of wet stains and/
or decayed bodies [12] and possibilities of mistyping occurred in
aged blood sample [4]. The failure might be attributed to the loss
of antigenicity or to the acquired antigenicity by bacterial contamination
[34] and/or partial digestion of erythrocyte membrane by
proteolytic enzymes [35]. All these limitation made most of the
methods unreliable to work with.
ABO typing in other body fluids
In the 1930s, it was found that the blood group antigens, were not
confined to erythrocytes but were also present in various other
body secretions and tissue fluids, such as saliva, semen, sweat,
urine, vaginal secretions, etc., from which blood groups could be
determined [36], provided the subject had the secretor status [37,
38]. The status of an individual as secretor or non-secretor is determined
by the presence of Lewis antigens, Lea and Leb, which
are not intrinsic to the RBC membrane but are synthesized by
intestinal epithelial cells and circulate in plasma [39, 40]. The ABO
group specific substances are typically present in high concentrations
in body fluids of secretors and the secreted substances can
withstand drying and retain their antigenic activity over a prolonged
period. The forensic serologists took advantage of these
characteristics to group stains of body fluids such as semen [41]
and saliva [42] that assisted in the investigation of crimes where
there was lack of blood sample. Studies on the secretor status of
blood group antigens had extensively been studied for saliva [37,
38, 43] vaginal sample [44], sweat stains [45] and semen [46]. ABH
antigens were also expressed in some indoor pets also [46], so the
secretion from the saliva of rabbit, cat, dog interacted with the antibodies
raised against ABH antigen [47]. It is therefore important
to exclude false positive reaction and mis-judgement of species
identification in the blood group determination.
Due to unavailability of adequate techniques, analysis of anti-A
and anti-B haem-agglutinins in saliva or other body fluids were
not utilized as evidence in the medico legal cases initially [48].
Later, a lot of modifications were done to develop highly precise
techniques to determine blood group antigens in body fluids.
At present, two methods are used to type blood and body
fluids for ABO grouping, the absorption-inhibition (AI) and the
absorption-elution (AE) methods. Absorption-inhibition showed
a 100% positive correlation for ABO blood grouping in dried salivary
samples and was found to be a more effective technique for
recognizable proof and secretor status determination in different
populations [49]. A different study carried out the estimation of
blood group antigens on cigarette butt [50] also showed the Absorption-
inhibition method to be more fruitful. But in a comparative
study [42] absorption-elution technique was demonstrated
to be more sensitive, specific, and effective in determination of
ABO blood grouping in dried saliva stain.
ABO-typing from other tissue
ABH antigen is expressed in human cells including dermal epithelium,
endothelial cells of blood vessel Hassall’s corpuscle [51],
renal tubules, secretary cell of respiratory tract [52], and male reproductive
organs [53]. Indirect typing method were mostly tried
for hair [54, 55] tooth [56-59] and bone tissue [60, 61]. Some
histochemical techniques have also been used for hair [52, 62] and
other tissue [53]. However, the histochemical method is very time
consuming which requires a lot of skill dependent steps to reach
to a conclusive result.
Advent of DNA technology
Unfortunately, the limitations of ABO-typing methods (Table 1)
for forensic samples along with variable reports by different laboratory,
made the results inconclusive in many cases. The effect of
autolysis, dehydration, loss of antigens by temperature, humidity,
and aging, or contamination by microbes or animals may haveled
to variations in the study. Moreover, even though, properly done,
it did not always have the potential to produce concrete evidence
to individualize the offender and therefore may be proven futile
in the court of law.
DNA Fingerprinting
Forensic science is nothing but the art of piecing together a crime
scene in order to determine how the crime was committed and
who was responsible. DNA evidence is one of the most prominent
pieces of evidence, which is lacking in ABO typing, method
to individualize the perpetrator.
Restriction Fragment Length Polymorphism ( RFLP)
Restriction fragment length polymorphisms, or RFLPs as they
are commonly known, were the first type of DNA fingerprinting
which came onto the scene in the mid-1980’s [63]. that focus
on the size differences of certain genetic locations. Some other
methods, utilizing the RFLP principle were used, such as, amplified
fragment length polymorphism [64], and terminal restriction
fragment length polymorphism [65].
Variable Number Tandem repeats VNTR
Variable number tandem repeats, or VNTRs represent specific
locations on a chromosome in which tandem repeats of 9-80 or
more bases repeat a different number of times between individuals.
These regions of DNA are readily analyzed using the RFLP approach
and a probe specific to a VNTR locus. The fragments
are a little shorter than RFLPs (about 1-2 kilo base pairs), but are
created through the exact same process.
STR-PCR analysis
After the invention of Polymerase Chain Reaction by Mullis [66],
the DNA fingerprinting is largely being done by STR marker
analysis [67-69]. Unlike VNTRs which analyze mini-satellites that
have repeat of 9-80 base pairs STRs use microsatellites which
have repeat sequences of only 2-5 base pairs [70], tandemly repeated
at a specific locus, mostly in the non-coding regions , introducing
the “less is more” philosophy to the world of DNA fingerprinting.
This was a big step forward in forensic science since
the length of DNA fragment being analyzed is short enough to be
amplified by polymerase chain reaction (PCR), which enabled the
analysis of a very small sample of DNA. This method is quicker
and easier than any previously known method and match it to
a person’s identity. The STR typing method is nowadays mostly
performed by the robotics starting from, DNA extraction, amplification
(PCR) and all through DNA profiling. That is why, a
skilled or trained personnel may not be needed to perform the
analysis, though the interpretation part would still require some
knowledgeable person in this field.
Next Generation Sequencing (NGS)
Next generation sequencing (NGS) was another landmark in
DNA fingerprint analysis, which is capable of sequencing thousands
of genomic regions of a species simultaneously to get idea
about the phenotype, age of the unknown source retrieved from
the crime scene [71]. A promising NGS approach was reported
with simultaneous analysis of 10 STRs, 386 ancestory-specific
SNP s, and the complete mtDNA [72]. Other two studies [73, 74]
has been reported, where they analysed 27 autosomal, 7-X chromosomal,
24-Y chromosomal, 94 identity specific SNPs and 56
ancestory informative SNPs in a single sequencing. The method
has been shown to be applicable in degraded and low template
samples [75] as well.
Rapid-Hit technology
This is also a high-throughput method which does not require any
human intervention in whole process. It takes help of the simple
“sample in-profile out’ principle and analysis is done in a single
step, including extraction, amplification and sequencing within 2
hrs [76]. The minimum time taken for genotyping makes it very
useful for the criminal justice system and has a tremendous potential
to use it at the crime-spot, to include/exclude the suspect.
This method is currently under validation for law enforcement
use.
Merits & demerits of DNA- typing
The crime rate around the globe is increasing alarmingly and the
need for fast and effective methods of crime and criminal detection
is also increasing. As the criminal justice system comes of age,
it really requires individualization of a suspect from a crime scene.
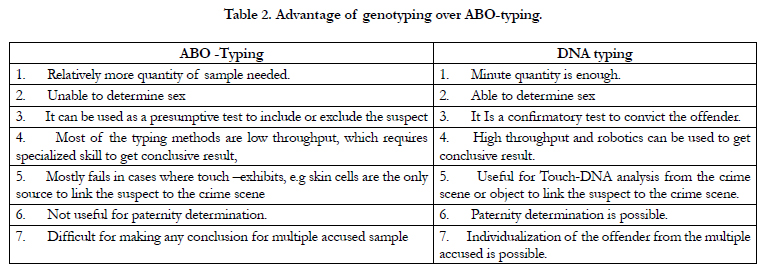
ABO-typing could be considered as the evidence which was able
to place an individual in a general class but failed to identify the
offender conclusively. For example, blood typing can be used to
establish whether someone has type A, B, AB, or O blood, which
can be useful in helping to investigate a crime by either including
or excluding an individual from the list of suspects but cannot
pin down the actual culprit. On the contrary, individual evidence,
such as fingerprints and DNA, can be used to unravel the identity
of an individual. DNA analysis has made possible the accurate
typing of very small traces of body fluid evidence to a very high
level of individuality in many cases. A laboratory worker might be
virtually certain, therefore, that a biological trace has originated
from a particular person.
Apart from the advantages over mere ABO typing (Table. 2) the
DNA typing has a vast potential in the criminal justice system in
future. Touch DNA is one of them. It was a break-through technology
developed [77-79], which did not require any biological
fluids to type DNA, instead it could isolate the DNA from the
touch site of the object having the skin epithelial cells of the accused
remain at the crime scene. When a crime is committed, the
offender often may touch items, like weapon, clothing, victims
body, etc [80], which were used as a DNA source of the perpetrator.
It helped massively in investigating “cold cases” which were
closed due to lack of evidence.
However, STR profile data was found to be ineffective, where the
suspect is unknown and any genetic data was unavailable to compare
with. In those cases, DNA profile was compared with those
in data banks, kept in many state and nationally for the offender,
which could link the perpetrator to the crime scene. For nonmatching
DNA profiles, a statistical approach [81] was proposed.
The ancestry-informative SNP marker PCR multiplexing was also
an effective method to infer the geographic background of the
donor by using five major sub-continental population groups [82].
New investigative lead was also provided using uni-parentally inherited
markers (mitochondrial DNA and non-recombinant Ychromosome)
to infer the ancestries of the maternal/paternal
background of a DNA sample and thus, helped to narrow down
the suspect list.
Another, limitation what the forensic scientists often encounter is
the inability to determine the age from the skeletal remain, since
a lot depends on the age of person in question. Though the morphological
analysis of the skeleton was the only way [83] using
mostly hard tissues, such as,tooth, bone etc, the method have been
shown to produce ambiguous result [84]. A promising method for
more accurate age prediction arose from the field of epigenomics,
where epigenetics and the DNA-methylation markers have been
proposed to estimate age, tissue type and even differentiate between
monozygotic twins [85]. A significant change of age-related
DNA methylation level at CpG dinucleotide islands was shown
to be associated with increasing age in epigenome-wide association
study [86-88]. Gene expression had also been reported to
correlate with human age [89]. But both these method required
relatively higher amount of DNA to work with, which is normally
absent in the typical forensic specimen [90].
Though omnipotent, DNA typing has some inherent limitation
in forensic cases. As it is unable to establish the nature of the
biological material which may or may not be important in a particular
case. The importance of identification versus typing tests
with an evidence item has to be considered in the context of the
case. A successful DNA typing on a licked envelope flap or on a
swabbing from a bite mark, was likely to be informative regardless
of whether saliva could be identified or not. On the other hand,
the identification and species-determination aspects of a forensic
examination can sometimes be more important for a suspected
hit-and-run case. The suspect may be absolved of suspicion by a
finding that bloodstains on his vehicle were of nonhuman origin.
In addition, a virtual match between evidence and a person might
have little meaning if there is an innocent explanation for the
finding. This would be true, for example, when a victim's genetic
profile is matched to bloodstains on a suspect's clothing in a case
in which the victim and suspect lived in the same household or
the suspect is able to offer a plausible explanation for the stains.
Not withstanding the DNA typing revolution, some activities long
associated with "forensic serology" remain important and continue
to be a vital part of forensic biological evidence analysis.
Blood and physiological fluid stains and traces still require identification.
More importantly, those aspects of "forensic serology"
most characteristic of its association with criminal sciences remain
critical if biological evidence analysis is going to help to
unravel a case. Recognizing the crucial physical evidence in a given
case, using experience and judgment to select the most important
and informative specimens for typing in terms of the case, and interpreting
stain patterns are essential skills that cannot be replaced
by any DNA typing technique.
Conclusions
Degraded samples, loss of antigenecity due to effect of environmental
factors, lack of ability to individualize the perpetrator,
made the ABO-typing methods unfruitful to the judiciary
and rapidly being replaced by high-throughput genotyping. Sex
determination and age estimation is often required for the human
or skeletal remains found in the crime scene to get a preliminary
idea about the crime. Limitation to discriminate between male and
female is another feature that makes the traditional. ABO- typing
methods inapplicable in various cases. On the contrary, while the
STR-PCR can determine the sex, new approaches like, epigenomics
can infer about the approximate age of the skeletal remains.
Paternity dispute is another area often encountered in both criminal
and civil cases, such as, guardianship, inheritance, adultery and
fornication, where, ABO–typing is of no use as a conclusive evidence
as the A, B, AB and O blood groups are never unique. Genotyping
is the only option toconfirm paternity of the disputed
off-spring in such cases.Non-secretory status of human subject
can also make the other body fluids, other than blood, unsuitable
for ABO typing. The problem can be addressed by genotyping
where saliva, semen, or other body fluids may be used to individualize
a person. In addition, in absence of any detectable biological
fluid also, touch-DNA can be handy to get results in presence of
skin cell remains in the crime scene. Thus, genotyping, in its present
form, mostly fulfils the requirements for the forensic experts,
to unravel a case conclusively to court of law. However, in spite
of being eclipsed by genotyping, ABO typing is still being used
and may be continued to be in use as a routine-tool for forensic
analysis in developing countriesbecause of its cost-effectiveness.It
is only a matter of time when forensic experts will rely on genotyping
methods and the ABO-typing will either be discarded or
confined only to a primary screening.
References
- Okroi M, Voswinckel P. “Obviously impossible”—the application of the inheritance of blood groups as a forensic method. The beginning of paternity tests in Germany, Europe and the USA. InInternational Congress Series. 2003 Jan 1;1239:711-714. Elsevier.
- Gaensslen RE. Forensic analysis of biological evidence. Forensic Sciences. 2000;1.
- Nishi K, Tanaka N, Okazaki S, Maeda H, Tsuji T, Nagano T. Effect of heat on blood group A-and B-active glycolipid extracted from AB human erythrocytes. Jpn J Legal Med. 1979;33:86-90.
- Maeda H, Nishi K, Okazaki S, Tsuji T, Tanaka N, Nagano T. Activity changes of blood group antigens in dried blood stains on standing. J Wakayama Med Soc. 1979;30:211-8.
- Shetty M, Premlata K. ABO blood grouping from tooth material. J Indian Acad of Forensic Med. 1972;32:336-338.
- Pourazar A. Red cell antigens: Structure and function. Asian J Transfus Sci. 2007 Jan;1(1):24-32. PubMed PMID: 21938229.
- ISBT (2019). "Table of Blood Group Systems v 6.0 (August 2019)" (PDF). International Society of Blood Transfusion. Archived (PDF) from the original on 27 September 2019. Retrieved 19 January 2020.
- Harbison C. ABO Blood Type Identification and Forensic Science (1900- 1960). Embryo Project Encyclopedia. 2016 Jun 2.
- Owen R. Karl Landsteiner and the first human marker locus. Genetics. 2000 Jul;155(3):995-8. PubMed PMID: 10880463.
- Westhoff CM. The Rh blood group system in review: a new face for the next decade. Transfusion. 2004 Nov;44(11):1663-73. PubMed PMID: 15504174.
- Muehlberger CW, Inbau FE. Scientific and Legal Application of Blood Grouping Tests. Am. Inst. Crim. L. & Criminology. 1936;27:578.
- Nishi K, Rand S, Nakagawa T, Yamamoto A, Yamasaki S, Yamamoto Y, et al. ABO Blood Typing from Forensic Materials-Merits and demerits of detection methods utilized in our laboratories, and biological significance of the antigens. Anil Aggrawal's Internet Journal of Forensic Medicine and Toxicology. 2005;6(2).
- Ladd C, Bourke MT, Scherczinger CA, Pagliaro EM, Gaensslen RE, Lee HC. A PCR-based strategy for ABO genotype determination. J Forensic Sci. 1996 Jan;41(1):134-7. PubMed PMID: 8934712.
- Uhlenhuth PT. EineMethodezurUnterscheidung der verschiedenenBlutarten, imbesonderenzum differential diagnostischenNachweise des Menschenblutes. 1901.
- Ouchterlony O. Antigen-Antibody Reactions in Gels. 25B Ark Kemi Mineral Geol. 1948;14.
- Nuttall GH. On the Formation of Specific Anti-Bodies in the Blood following upon Treatment with the Sera of different Animals, together with their Use in Legal Medicine. J Hyg (Lond). 1901 Jul;1(3):367-87. PubMed PMID: 20474125.
- Sensabaugh GF. Molecular Evolution and the Immunological Determination of Species. Int. Microform J. Leg. Med. 1976;11:art.219.
- Landsteiner K. Ueber Agglutinationserscheinungen normal enmenschlichenBlutes. 1901 [Agglutination phenomena of normal human blood]. Wien KlinWochenschr. 2001 Oct 30;113(20-21):768-9. German. PubMed PMID: 11732110.
- Lattes L, Bertie LWH. L'Individualità Del Sangue Nella Biologia, Nella Clinica E Nella MedicinaLegale. Individuality of the Blood in Biology and in Clinical and Forensic Medicine... Translated by LW Howard Bertie... from the French Edition of 1929, Thoroughly Revised and Brought Up to Date by the Author. With a Bibliography. Oxford University Press; 1932.
- Schütze H. Hæmagglutination and its Medico-Legal Bearing, with Observations upon the Theory of Isoagglutinins. British journal of experimental pathology. 1921 Feb;2(1):26.
- . Lee HC, Gaensslen RE, Pagliaro EM, Novitch B. Two-dimensional absorption- inhibition. J Forensic Sci. 1988 Sep;33(5):1127-38. PubMed PMID: 3193074.
- Siracusa V. La sostanzaisoagglutinabiledelsangue e la suadimostrazione per la diagnosiindividualedellemacchie. Arch AntropolCriminPsichiat Med Leg. 1923;43:362-5.
- Kind S. Absorption-elution grouping of dried blood smears. Nature. 1960 Feb 6;185:397-8. PubMed PMID: 14409131.
- Parigian MJ. An ELISA procedure for the detection of soluble ABH blood group substance in semen, saliva, and vaginal samples. J Forensic Sci. 1995 Jan;40(1):122-5. PubMed PMID: 7876793.
- Lapenkov MI, Gurtovaia SV, AleksandrovaVIu, Kapinos TA. Combination of absorption-elution and gel-filtration methods for group identification of blood spot specimens. Sud Med Ekspert. 2009 May-Jun;52(3):13-5. Russian. PubMed PMID: 19569533.
- Mudd JL. A microplate method for reverse ABO typing of bloodstains. J Forensic Sci. 1986 Apr;31(2):418-25. PubMed PMID: 3711823.
- Tsuji T, Kimura A, Nishi K, Ito N, Mizumoto J, Wada K. Effects of red cell shape changes on hemagglutination. Nihon HoigakuZasshi. 1985 Apr;39(2):138-42. PubMed PMID: 4087512.
- Nishi K, Ito N, Mizumoto J, Wada K, Tsuji T, Kimura A. Reliability of blood grouping of aged blood by two direct hemagglutination methods and the absorption elution method. Nihon HoigakuZasshi. 1985 Apr;39(2):131-7. PubMed PMID: 4087511.
- Wang J, Yiu B, Obermeyer J, Filipe CD, Brennan JD, Pelton R. Effects of temperature and relative humidity on the stability of paper-immobilized antibodies. Biomacromolecules. 2012 Feb 13;13(2):559-64. PubMed PMID: 22257068.
- Michailow R. A method for treatment of partially haemolysed blood with lost agglutination properties, allowing its blood group determination (author's transl). Z Rechtsmed. 1977 Jul 5;80(1):35-8. German. PubMed PMID: 883425.
- Açikgöz H, Kendi I, Bilge Y. Environmental Factors Affecting Blood Stains. Ankara University Faculty of Law Journal. 2007; 56(3):1-10.
- Özer A. Practical Hematology Ege University Faculty of Medicine Publications. Izmir: Ege University Printing House Serology. 1980;65-105.
- Mishra SK, Yadav AS. Variations in ABH Antigenic Stability of Dried Bloodstains from Different Surfaces with Age. Indian Journal of Forensic Medicine & Toxicology. 2012 Jul 1;6(2):202.
- Shanmugam P, Roy R, Sethi J. Impact of age and microbial growth on the accuracy of preliminary tests and blood grouping of dried blood stains in forensic investigations. Indian Congress of Forensic Medicine & Toxicology. 2015.
- Bosmann HB. Red cell hydrolases. 3. Neuraminidase activity in isolated human erythrocyte plasma membranes. Vox Sang. 1974;26(6):497-512. Pub- Med PMID: 4137161.
- Schiff F, Sasaki H. Der Ausscheidungstypus, ein auf serologischemWegenachweisbaresmendelndes Merkmal. KlinischeWochenschrift. 1932 Aug;11(34):1426-9.
- Motghare P, Kale L, Bedia AS, Charde S. Efficacy and accuracy of ABO blood group determination from saliva. Journal of Indian Academy of Oral Medicine and Radiology. 2011 Jul 1;23(3):163.
- Saboor M, Ullah A, Qamar K, Mir A, Moinuddin. Frequency of ABH secretors and non secretors: A cross sectional study in Karachi. Pak J Med Sci. 2014 Jan;30(1):189-93. PubMed PMID: 24639859.
- Grubb R. Correlation between Lewis blood group and secretor character in man. Nature. 1948 Dec 11;162(4128):933. PubMed PMID: 18104581.
- Henry S, Oriol R, Samuelsson B. Lewis histo-blood group system and associated secretory phenotypes. Vox Sang. 1995;69(3):166-82. PubMed PMID: 8578728.
- Verma RP, Arya E. Determination of serological markers (blood group markers) of biological fluid (semen) obtained from crime scene for individualization of the donor (s). Int J SciEng Res. 2014;5:2307-1.
- Sen MP, Vanishree M, Hunasgi S, Surekha R, Koneru A, Manvikar V. A comparison of absorption inhibition and absorption elution methods for estimation of ABO blood groups in saliva. J Med RadiolPathol Surg. 2015 Jan;1(1):1-4.
- Thaler R, Froum S, Chuba JV, Scopp IW. A quantitative study on the relationship of salivary blood group substances to periodontal disease. J Periodontal Res. 1976 Apr;11(2):116-20. PubMed PMID: 132517.
- Graves MH, White JM, Fitzpatrick FA, Kuo MC. A comparison of absorption- inhibition and absorption-elution methods in the detection of ABO(H) antigens found in vaginal samples submitted in sexual offense cases. J Forensic Sci. 1978 Apr;23(2):345-52. PubMed PMID: 122745.
- Kaur G, Sharma VK. Comparison of absorption-inhibition and absorptionelution methods in the detection of ABO (H) antigens in sweat stains. Current science. 1988 Nov 20:1221-3.
- Nishimura A, Yamamoto Y, Nishi K. Expression of ABH and ABH-related antigen in secretory cells of indoor pets. Species analysis should be necessary prior to ABO blood grouping in stain analysis. Anil Aggrawal’s Internet J. Forens Med. Toxcology. 2001 Jul;2(2).
- Nishi K, Tanegashima A, Yamada M, IkeharaY, Yamamoto Y, et al. Lectinand Immuno-Histochemistry on Mucous Substances of the Taste Buds and Lingual Glands in Some Mammals. In Advances in Forensic Haemogenetics 1994;(pp. 638-640). Springer, Berlin, Heidelberg.
- Harrington JJ, Martin R, Kobilinsky L. Detection of hemagglutinins in dried saliva stains and their potential use in blood typing. J Forensic Sci. 1988 May;33(3):628-37. PubMed PMID: 3385376.
- Thrumiaya T, Gayathri R, Priya VV. Efficacy and accuracy of ABO blood group determination from saliva. Journal of Advanced Pharmacy Education& Research| Apr-Jun. 2017;7(2).
- Ruth MS, Purnadianti M, Marini MI. Blood group analysis from cigarette butts by absorption inhibition method: An experimental study. Journal of International Oral Health. 2020 May 1;12(3):275.
- Nishi K, Rand S, Fechner G, Brinkmann B. Immunohistochemical localization of A, B, H, Le a and Le b antigens in human thymus using avidin–biotin complex technique. ActaCrimJpn. 1998;54:217-23.
- Nishi K, Ito N, Hirota T, Fechner G, Rand S, Brinkmann B. Different expression of blood group A antigen in the mucous cells of salivary glands from Japanese and German nonsecretor individuals. Jpn J legal Med.1989; suppl. 220.
- Nishi K, Fukunaga T, Yamamoto Y, Yamada M, Kane M, Tanegashima A, et al. ABH-related antigens in human male genital tract. A histochemical examination. Int J Legal Med. 1992;105(2):75-80. PubMed PMID: 1520640.
- Yada S, Ishimoto G, Okane M. Blood grouping an 88-years-old hair rope. Acta Crime Apon. 1968;34:90-2.
- Miyasaka S, Yoshino M, Sato H, Miyake B, Seta S. The ABO blood grouping of a minute hair sample by the immunohistochemical technique. Forensic Sci Int. 1987 May-Jun;34(1-2):85-98. PubMed PMID: 3297953.
- Smeets B, van de Voorde H, Hooft P. ABO bloodgrouping on tooth material. Forensic Sci Int. 1991 Sep;50(2):277-84. PubMed PMID: 1748363.
- Neiders ME, Standish SM. Blood group determinations in forensic dentistry. Dent Clin North Am. 1977 Jan;21(1):99-111. PubMed PMID: 264468.
- Suzuki K. Blood group determination in the tissues of human teeth. Jap J Legal Med. 1957 Mar;11:168-77.
- Ramnarayan B, Manjunath M, Joshi AA. ABO blood grouping from hard and soft tissues of teeth by modified absorption-elution technique. J Forensic Dent Sci. 2013 Jan;5(1):28-34. PubMed PMID: 23960412.
- Borgognini SM. New trends in blood group determination in human bones. Proc VIIth Intern CongrAnthropEthnolSci, Tokyo-Kyto. 1968 Sep;114.
- Lee HC, Gaensslen RE, Pagliaro EM, Carroll-Reho J. ABH Typing of Human Bone. In 39th Annual Meeting, Am Acad Forensic Sci. San Diego, CA; 1987 Feb.
- Saito Y, Kimura A, Sagawa K, Inoue H, Yasuda S, Nosaka M, et al. Analysis of blood group active glycolipids in hairs and nails, and development of a novel blood grouping method. J Wakayama Med Soc. 2003;54:21-9.
- Jeffreys AJ, Wilson V, Thein SL. Hypervariable 'minisatellite' regions in human DNA. Nature. 1985 Mar 7-13;314(6006):67-73. PubMed PMID: 3856104.
- Vos P, Hogers R, Bleeker M, Reijans M, van de Lee T, et al. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res. 1995 Nov 11;23(21):4407-14. PubMed PMID: 7501463.
- Liu WT, Marsh TL, Cheng H, Forney LJ. Characterization of microbial diversity by determining terminal restriction fragment length polymorphisms of genes encoding 16S rRNA. Appl Environ Microbiol. 1997 Nov;63(11):4516-22. PubMed PMID: 9361437.
- Mullis K, Faloona F, Scharf S, Saiki R, Horn G, Erlich H. Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring HarbSymp Quant Biol. 1986;51 Pt 1:263-73. PubMed PMID: 3472723.
- McCord BR, Gauthier Q, Cho S, Roig MN, Gibson-Daw GC, Young B, et al. Forensic DNA Analysis. Anal Chem. 2019 Jan 2;91(1):673-688. PubMed PMID: 30485738.
- Goodwin W, Linacre A, Hadi S. An introduction to forensic genetics. John Wiley & Sons. 2nd ed. 2011 Jun 28;53-62.
- Oostdik K, Lenz K, Nye J, Schelling K, Yet D, Bruski S, et al. Developmental validation of the PowerPlex(®) Fusion System for analysis of casework and reference samples: A 24-locus multiplex for new database standards. Forensic SciInt Genet. 2014 Sep;12:69-76. PubMed PMID: 24905335.
- Tautz D, Renz M. Simple sequences are ubiquitous repetitive components of eukaryotic genomes. Nucleic Acids Res. 1984 May 25;12(10):4127-38. PubMed PMID: 6328411.
- Gettings KB, Kiesler KM, Faith SA, Montano E, Baker CH, Young BA, et al. Sequence variation of 22 autosomal STR loci detected by next generation sequencing. Forensic SciInt Genet. 2016 Mar;21:15-21. PubMed PMID: 26701720.
- Allen M, Nilsson M, Havsjö M, Edwinsson L, Granemo J, Bjerke MH, et al. for simultaneous analysis of 10 STRs, 386 SNPs and the complete mtDNA genome. In Presentation at the 25th Congress of the International Society for Forensic Genetics 2013; 2-7.
- Guo F, Yu J, Zhang L, Li J. Massively parallel sequencing of forensic STRs and SNPs using the Illumina® ForenSeq™ DNA Signature Prep Kit on the MiSeqFGx™ Forensic Genomics System. Forensic SciInt Genet. 2017 Nov;31:135-148. PubMed PMID: 28938154.
- Moreno LI, Mills DK, Entry J, Sautter RT, Mathee K. Microbial metagenome profiling using amplicon length heterogeneity-polymerase chain reaction proves more effective than elemental analysis in discriminating soil specimens. J Forensic Sci. 2006 Nov;51(6):1315-22. PubMed PMID: 17199616.
- Børsting C, Morling N. Next generation sequencing and its applications in forensic genetics. Forensic SciInt Genet. 2015 Sep;18:78-89. PubMed PMID: 25704953.
- Tan E, Turingan RS, Hogan C, Vasantgadkar S, Palombo L, Schumm JW, et al. Fully integrated, fully automated generation of short tandem repeat profiles. Investig Genet. 2013 Aug 6;4(1):16. PubMed PMID: 23915594.
- Williamson AL. Touch DNA: forensic collection and application to investigations. J Assoc Crime Scene Reconstr. 2012;18(1):1-5.
- Minor J. Touch DNA: from the crime scene to the crime laboratory. Forensic Magazine. 2013 Apr;4.
- Sowmyya T. Touch DNA: an investigative tool in forensic science. Int J Curr Res. 2016;8(02):26093-7.
- Wickenheiser RA. Trace DNA: a review, discussion of theory, and application of the transfer of trace quantities of DNA through skin contact. J Forensic Sci. 2002 May;47(3):442-50. PubMed PMID: 12051321.
- Pereira L, Alshamali F, Andreassen R, Ballard R, Chantratita W, Cho NS, et al. PopAffiliator: online calculator for individual affiliation to a major population group based on 17 autosomal short tandem repeat genotype profile. Int J Legal Med. 2011 Sep;125(5):629-36. PubMed PMID: 20552217.
- Eduardoff M, Gross TE, Santos C, de la Puente M, Ballard D, Strobl C, et al. Inter-laboratory evaluation of the EUROFORGEN Global ancestry-informative SNP panel by massively parallel sequencing using the Ion PGM™. Forensic SciInt Genet. 2016 Jul;23:178-189. PubMed PMID: 27208666.
- Meissner C, Ritz-Timme S. Molecular pathology and age estimation. Forensic Sci Int. 2010 Dec 15;203(1-3):34-43. PubMed PMID: 20702051.
- Bauer CM, Niederstätter H, McGlynn G, Stadler H, Parson W. Comparison of morphological and molecular genetic sex-typing on mediaeval human skeletal remains. Forensic SciInt Genet. 2013 Dec;7(6):581-586. PubMed PMID: 23941903.
- Vidaki A, Kayser M. Recent progress, methods and perspectives in forensic epigenetics. Forensic SciInt Genet. 2018 Nov;37:180-195. PubMed PMID: 30176440.
- Horvath S, Zhang Y, Langfelder P, Kahn RS, Boks MP, van Eijk K, et al. Aging effects on DNA methylation modules in human brain and blood tissue. Genome Biol. 2012 Oct 3;13(10):R97. PubMed PMID: 23034122.
- Weidner CI, Lin Q, Koch CM, Eisele L, Beier F, Ziegler P, et al. Aging of blood can be tracked by DNA methylation changes at just three CpG sites. Genome Biol. 2014 Feb 3;15(2):R24. PubMed PMID: 24490752.
- Garagnani P, Bacalini MG, Pirazzini C, Gori D, Giuliani C, Mari D, et al. Methylation of ELOVL2 gene as a new epigenetic marker of age. Aging Cell. 2012 Dec;11(6):1132-4. PubMed PMID: 23061750.
- Pan F, Chiu CH, Pulapura S, Mehan MR, Nunez-Iglesias J, Zhang K, et al. Gene Aging Nexus: a web database and data mining platform for microarray data on aging. Nucleic Acids Res. 2007 Jan;35(Database issue):D756-9. PubMed PMID: 17090592.
- Freire-Aradas A, Phillips C, Lareu MV. Forensic individual age estimation with DNA: From initial approaches to methylation tests. Forensic Sci Rev. 2017 Jul;29(2):121-144. PubMed PMID: 28691915.