Two Autopsy Cases Assessing the Association of Rare Tumors Adjacent to the Sella turcica with Cause of Death and a Review of the Literature
Morioka F1, Tani N1,2, Michiue T1,2, Oritani S1, Potente S1,3, Ishikawa T1,2*
1 Department of Legal Medicine, Osaka City University Medical School, Asahi-machi, Abeno, Osaka, Japan.
2 Forensic Autopsy Section, Medico-legal Consultation and Postmortem Investigation Support Center, c/o Department of Legal Medicine, Osaka City University Medical School, Asahi-machi, Abeno, Osaka, Japan.
3 Institute of Forensic Medicine, University Hospital, Goethe-University, Kennedyallee, Frankfurt/Main, Germany.
*Corresponding Author
Takaki Ishikawa,
Department of Legal Medicine, Osaka City University Medical School,
Asahi-machi 1-4-3, Abeno, 545-8585 Osaka, Japan.
Tel: 81-6-6645-3765
Fax: 81-6-6634-3871
Email: takaki@med.osaka-cu.ac.jp
Received: March 06, 2017; Accepted: May 05, 2017; Published: May 08, 2017
Citation: Tani N, Michiue T, Oritani S, Potente S, Ishikawa T, et al., (2017) Two Autopsy Cases Assessing the Association of Rare Tumors Adjacent to the Sella turcica with Cause of Death and a Review of the Literature. Int J Forensic Sci Pathol. 5(2), 319-325. doi: http://dx.doi.org/10.19070/2332-287X-1700071
Copyright: Ishikawa T© 2017. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Case 1 is an 80 year-old female, who consumed alcohol in a bar, fell down the stairs upon returning home, and was later found deceased. A postmortem CT did not confirm the presence of a tumor. An autopsy confirmed diffuse subarachnoid hemorrhage and a tumor, with a white surface covered by microvasculature pressing on the optic chiasma. The cause of death was traumatic subarachnoid hemorrhage due to an injury to the head while under the influence of alcohol. However, given the formation of a tumor pressing on the optic nerve, a possible causal relationship between the epidermoid cyst and the head injury cannot be excluded. Case 2 is a 60 year-old male, discovered dead in his own home. The autopsy confirmed subarachnoid hemorrhage around the base of the brain, and there was a hematoma-like tumor connected to the pituitary gland. The cause of death was determined to be pituitary bleeding due to pituitary adenoma. As seen in these cases, it is possible that a tumor at the base of the brain, which is a difficult location for identification, would impact the process of death; therefore, studying the grade of tumor, stage, and its involvement in the cause of death are diagnostically critical in forensic autopsies.
2.Introduction
3.Case Reports
3.1.Case 1
3.2.Case 2
4.Discussion
5.References
Keywords
Epidermoid Cyst; Plurihormonal Pituitary Adenoma; Sella turcica; Forensic Pathology; Endocrinology; Neurological Symptom.
Introduction
Tumors occurring adjacent to the sella turcica not only put pressure directly on the base of the cerebrum, brain stem, and cerebellum but also involve cranial nerves and blood vessels that penetrate the base of the skull [1, 2]. Therefore, in addition to the symptoms caused by pressure on adjacent cranial nerves, symptoms of intracranial hypertension are often the chief complaint [3]. As computed tomography (CT) and magnetic resonance imaging (MRI) became available, diagnosis of tumors in the sella turcica has become more accurate [4, 5]; however, understanding the properties of tumors and their relationship with surrounding tissues to examine the causal relationship with death is extremely important in forensic pathology. There have been only few reports on confirmed tumors adjacent to the sella turcica in forensic science [4, 6-8]. In this report, we examined two cases in which tumors were macroscopically confirmed near the sella turcica based on forensic pathological evidence, and reviewed past case reports.
Case 1 was an unemployed 80 year-old female, who resided with her brother, sister, and brother-in-law and had a history of hypertension. The case subject returned home after consuming alcohol at a bar. In the early morning of the following day, the subject was found dead at the landing of a staircase lying on her back. The subject was not transferred to an emergency hospital. An autopsy was performed one and a half days after the death.
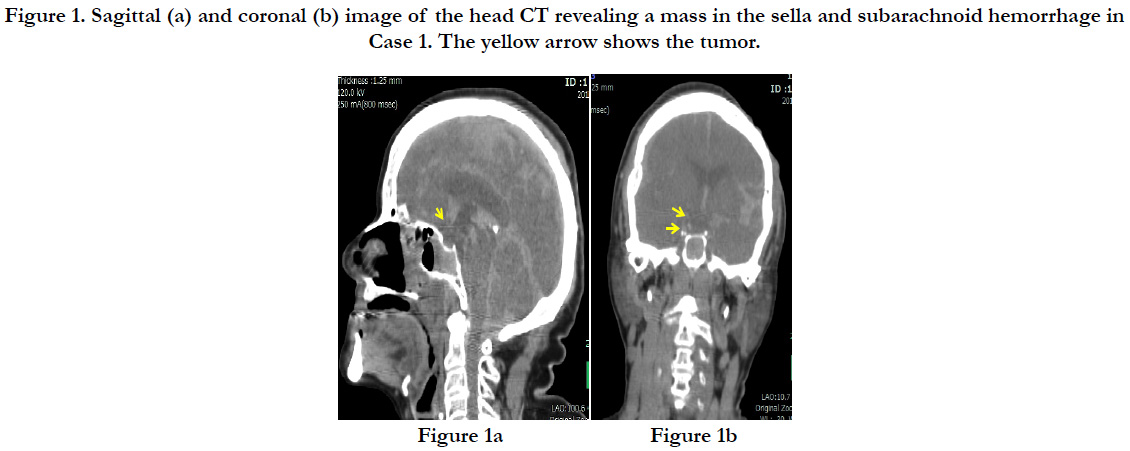
A post-mortem head CT showed subcutaneous bleeding slightly to the left of the occipital region and a linear longitudinal fracture from the top of the head to the right forehead. The brain showed mild swelling, and a subarachnoid hemorrhage was found around the base of the brain (Figures. 1a and b).
Figure 1. Sagittal (a) and coronal (b) image of the head CT revealing a mass in the sella and subarachnoid hemorrhage in Case 1. The yellow arrow shows the tumor.
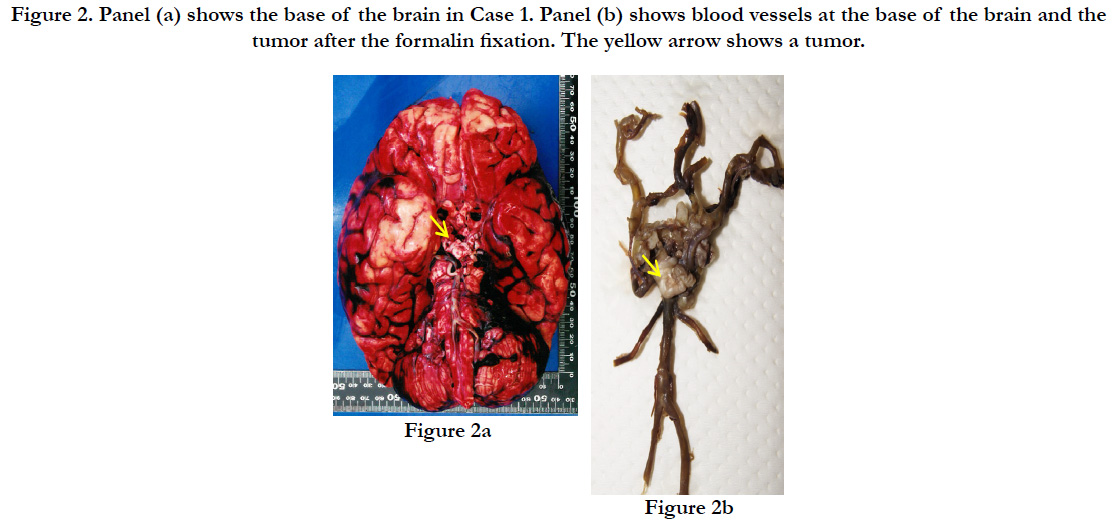
The subject’s height was 145 cm, with a weight of 53.9kg. A subcutaneous hemorrhage of 30×15×0.9 cm was found in the occipital region with a crushed intradermal area of 4×4 cm. In the skull, we found sagittal suture dehiscence around the intradermal crushed area in the occipital region and a fracture line toward the frontal bone. A diffuse subarachnoid hemorrhage was identified on the surface of the brain, and the brain was swollen (the weight of brain was 1.125g), forming uncal and tonsillar herniations (Figure. 2a). A brain contusion was present at the bilateral base of the cerebellum; however, no macroscopic contusions were found on the lower surface of the frontal and temporal lobes. Also, a slightly hardened tumor with a white surface was detected pressing on the optic chiasma at the base of the brain. The tumor was 1.5×1.5×0.5cm and covered by micro vessels extending from the surrounding main vessels with nearly-transparent mucus leaking when cut (Figure. 2b). Part of the tumor was in contact with the pituitary stalk, but no adhesion of the tumor to the pituitary gland was found. Also, no additional abnormalities were identified on major organs such as heart (370 g) and lung (left: 385 g, right: 615 g).
Figure 2. Panel (a) shows the base of the brain in Case 1. Panel (b) shows blood vessels at the base of the brain and the tumor after the formalin fixation. The yellow arrow shows a tumor.
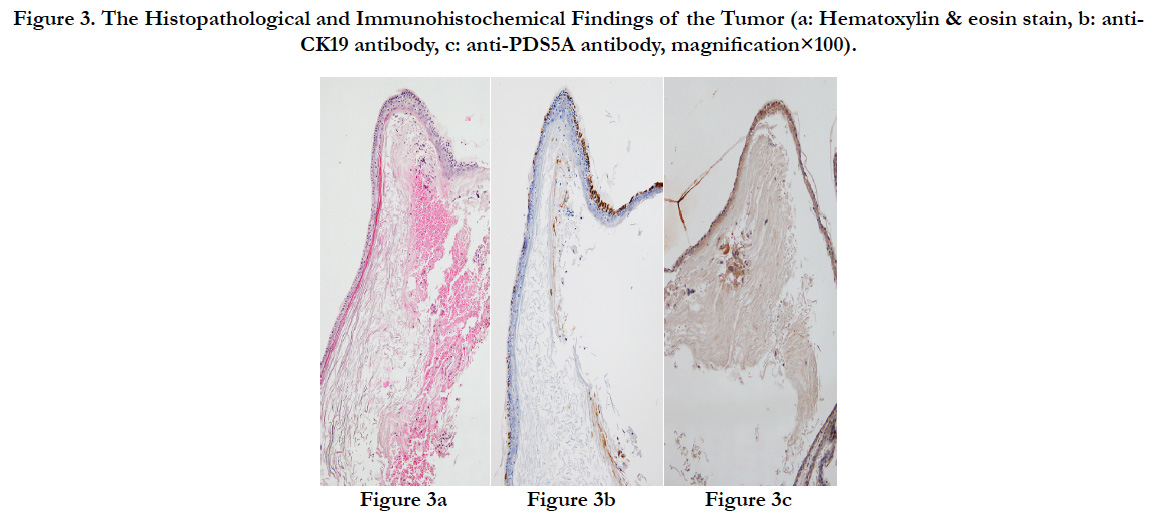
The surface of the tumor was a cyst covered by epidermis-like keratinized stratified squamous epithelium. Immunostaining revealed that the stratified squamous epithelium of the tumor was positive for CK19 that stains keratin and for PDS5A that stains stratified squamous epithelium, and the tumor was histopathologically diagnosed as an epidermoid cyst (Figures. 3a-c).
Figure 3. The Histopathological and Immunohistochemical Findings of the Tumor (a: Hematoxylin & eosin stain, b: anti-CK19 antibody, c: anti-PDS5A antibody, magnification×100).
Concentrations of 1.90 mg/mL and 2.35 mg/mL of ethyl alcohol was detected in the left intracardiac blood and urine, respectively. No drugs or toxic substances were detected in the blood.
There was no inflammation, liver dysfunction, or renal dysfunction. Pituitary gland hormones measured using right intracardiac blood were as follows: adrenocorticotropic hormone (ACTH): 2.0 pg/mL (forensic reference: median 10pg/mL, clinical reference: 7.2-63.3 pg/mL), growth hormone (GH): 0.52 ng/mL(forensic reference: median 7.0 ng/mL, clinical reference: < 2.47 pg/mL), thyroid stimulating hormone (TSH): 3.89 μIU/mL (forensic reference: median 3.5 μIU/ mL, clinical reference: 0.34-4.04 μIU/mL), prolactin (PRL): 13.23 ng/mL (forensic reference: median 20.0 ng/mL, clinical reference: 4.29-13.7 ng/mL), luteinizing hormone (LH): 0.95 mIU/ mL (forensic reference: not established, clinical reference: 1.8-7.0 mIU/mL), and follicle stimulating hormone (FSH): 4.17 mIU/ mL (forensic reference: not established, clinical reference: 5.2-14.4mIU/mL).
Case 2 was a male taxi driver in his 60s who lived alone. His medical history included hypertension and insomnia, and they had been on leave for the last several years. He was discovered lying in his living room and was estimated to have been deceased for several days.
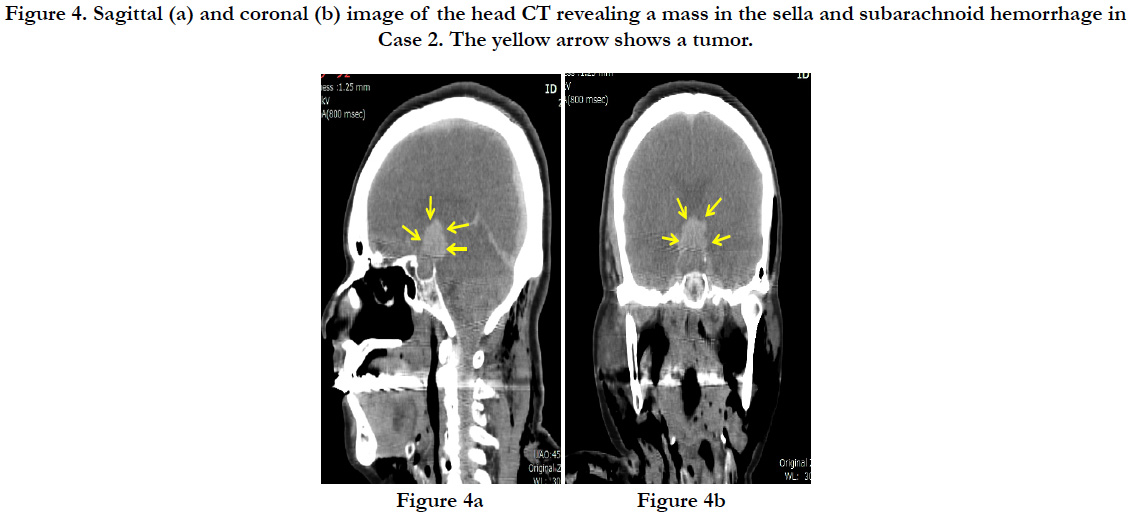
In a postmortem CT, a hematoma of 2cm in diameter was discovered in the sella turcica at the base of the brain. Diffuse subarachnoid hemorrhage was confirmed through the cerebrum, centered around the base of the brain (Figures. 4a, and b). There was no fracture of the skull. There were multiple parallel fractures on the outer parts of the right dorsal ribs, and pneumothorax was present on the right side.
Figure 4. Sagittal (a) and coronal (b) image of the head CT revealing a mass in the sella and subarachnoid hemorrhage in Case 2. The yellow arrow shows a tumor.
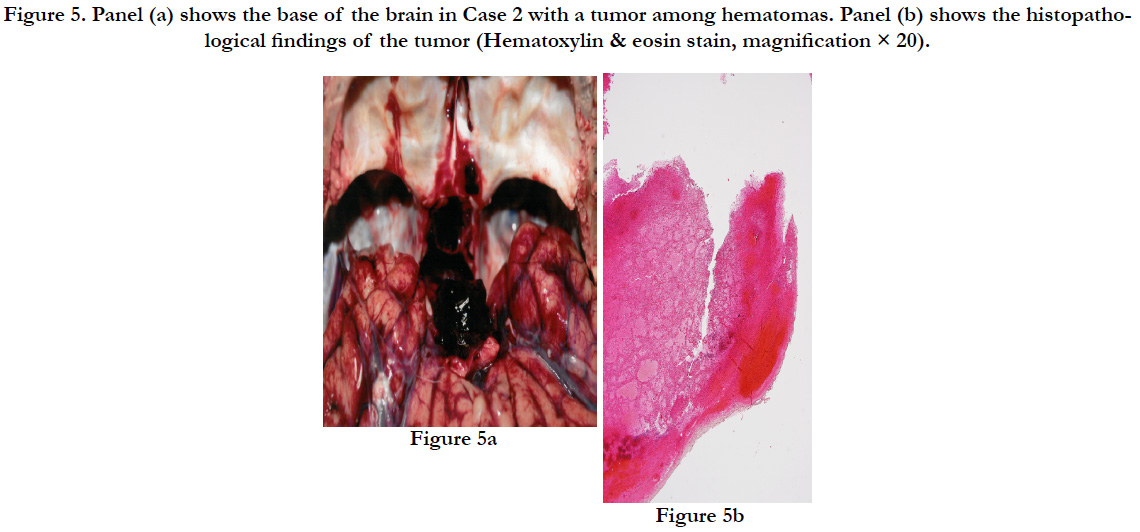
The subject’s height was 164cm with a weight of 50.6 kg. On the right side of the back, there was a subcutaneous intramuscular hemorrhage of 30×22×3 cm. In the same area, the third to tenth ribs on the right side showed parallel fractures. Some ends of the fractured ribs protruded into the thoracic cavity, forming a small contusion in the posterior surface of the right lower pulmonary lobe with bleeding in the surrounding area. The brain showed diffuse swelling (1.310 g). There was subarachnoid hemorrhage around the base of the brain, and there was a bleeding tumor of 2.5×2×2 cm (8.6 g) connected to the pituitary gland (1.7 g) (Figures. 5a).
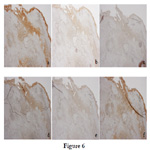
There was accumulation of acidophilic cells in the blood from the bleeding pituitary gland, presenting as pituitary adenoma (Figure 5b). The pituitary adenoma was positive for ACTH, TSH, and LH, and negative for PRL, GH, and FSH on immunostaining, suggesting a multiple hormoneproducing tumor (Figure 6a-f).
Figure 5. Panel (a) shows the base of the brain in Case 2 with a tumor among hematomas. Panel (b) shows the histopathological findings of the tumor (Hematoxylin & eosin stain, magnification × 20).
Figure 6. The immunohistochemical findings of the tumor (a: anti-ACTH antibody, b: anti-GH antibody, c: anti-PRL antibody, d: anti-TSH antibody, e: anti-FSH antibody, f: anti-LH antibody, magnification × 20).
Ethanol was not detected in the left or right intracardiac blood, and no other toxic substances were detected in the blood.
There were no findings of systemic inflammation, liver dysfunction, or renal dysfunction. Pituitary gland hormone levels, measured using right intracardiac blood, were used for reference only as several days had passed since death: ACTH: 2.0 pg/mL, GH: 0.28 ng/mL, TSH: 0.08 μg/dL, PRL: 0.1 ng/mL, LH: 0.1 mIU/mL, and FSH 0.4: mIU/mL.
Discussion
The cause of death in Case 1 was determined to be traumatic subarachnoid hemorrhage due to a contusion to the occipital region. Since the Case 1 subject was highly inebriated, there is a high likelihood that alcohol was involved in her death, but the epidermoid cyst found in this case was located at the chiasmatic groove and pressing on the optic nerve; therefore, it is possible that the subject was experiencing visual field impairment.
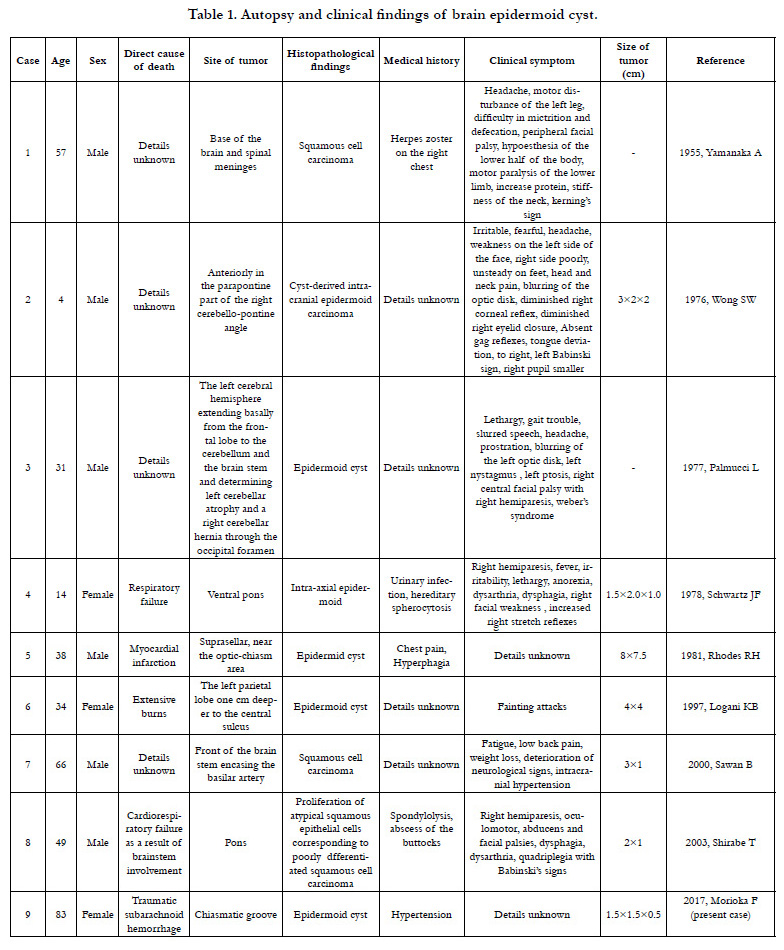
Epidermoid cysts comprise about 1% of brain tumors and have only been reported in eight autopsies published in international journals [9-16]. The ages of the subjects in these reports range from 4-66 years (median: 36 years), which is relatively young, and there were six males and two females. Only two of the eight cases had a clear and direct connection between the tumor and death [12, 16]. Among the eight reported cases, half of the patients had complications of malignant tumors, and these cases had a history of infections in their medical record [9, 16]. Though the details of a causal relationship between infection and malignant tumors are unknown, there are reports of infections spreading through the cerebrospinal fluid [17, 18]. Among the eight reported cases, the location of the tumor was often at the base of the brain, particularly in the brain stem, and the main clinical symptoms were those of the central nervous system, which were observed even in tumors measuring only about 1 cm in size.
The pituitary gland adenoma in Case 2 was an extrasellar extension that protruded from the sella turcica according to the radiological diagnostic classification [19], and diffuse bleeding had spread from the pituitary adenoma at the base of the brain. On the other hand, injuries on the back included multiple rib fractures, lung contusion, and pneumothorax, but the thoracic cavity was not open to the air, and there were no subcutaneous emphysema or injuries to large vessels. In addition to the delayed death that Case 2 presents, the lung contusion was also localized. Macroscopically, the lung contusion did not have valvular findings or extreme mediastinal displacement; therefore, there was no rationale to assert that pneumothorax was the primary condition. The direct cause of death in Case 2 was determined to be pituitary gland bleeding caused by the pituitary adenoma. The lung injury and pneumothorax caused by multiple rib fractures from contusion to the right side of the back were not the direct causes of death, but may have played a role.
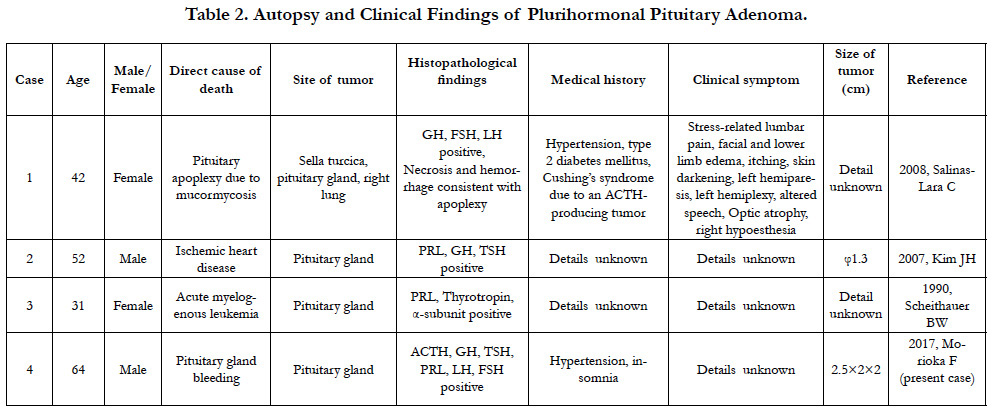
Approximately 40% of pituitary adenomas, such as the one found in Case 2, are considered nonfunctional adenomas [20]. As for functional adenomas, about 30% of all pituitary adenomas are prolactin-producting tumors, which occur approximately eight times more frequently in females than in males [21]. In addition, about 20% of pituitary adenomas are growth hormone-producing tumors, while other types of pituitary tumors are reported to be extremely rare [22]. The pituitary gland adenoma seen in Case 2 is classified as a plurihormonal adenoma, an adenoma producing multiple hormones, based on the Kovacs classification and has an incidence rate of less than 1% [23]. This is the first reported case of a plurihormonal adenoma with production of ACTH, TSH, and LH. Clinical symptoms of pituitary adenoma include headaches due to cerebral compression, visual field impairment due to optic nerve compression, and symptoms related to the hormones produced [24]. Prolactin-producing tumors result in symptoms such as lactation, amenorrhea syndrome, visual impairment, and visual field impairment [25]. However, reports of plurihormonal adenoma autopsy cases are limited to three existing reports, and its low incidence rate does not allow for a detailed understanding of factors such as symptoms and gender ratio. Since optic nerve compression is confirmed in the present case, the patient likely had symptoms of optic nerve impairment, such as visual field impairment, prior to death; however, the medical history only indicated hypertension.
There was no history of treatment for Cases 1 or 2, and only hypertension was noted in the medical history of both subjects. These two cases resulted in an autopsy due to traumatic findings. However, since the tumors were at the base of the brain, which is difficult to diagnose, they may have played a role in their deaths. Therefore, we consider that examining the malignancy and stages of the tumors, their relationship with surrounding tissues, and their involvement in the death of the subject, in addition to examining possible foul play, is diagnostically important in forensic autopsies.
References
- Stamm AC, Vellutini E, Balsalobre L (2011) Craniopharyngioma. Otolaryngol Clin North Am. 44(4): 937-952.
- Madhok R, Prevedello DM, Gardner P, Carrau RL, Snyderman CH, et al., (2010) Endoscopic endonasal resection of Rathke cleft cysts: clinical outcomes and surgical nuances. J Neurosurg. 112(6): 1333-1339.
- Arafah BM, Prunty D, Ybarra J, Hlavin ML, Selman WR (2000) The dominant role of increased intrasellar pressure in the pathogenesis of hypopituitarism, hyperprolactinemia, and headaches in patients with pituitary adenomas. J Clin Endocrinol Metab. 85(5): 1789-1793.
- Shields LB, Balko MG, Hunsaker JC (2012) Sudden and unexpected death from pituitary tumor apoplexy. J Forensic Sci. 57(1): 262-266.
- Ganaha T, Inamasu J, Oheda M, Hasegawa M, Hirose Y, et al., (2016) Subarachnoid hemorrhage caused by an undifferentiated sarcoma of the sellar region. Surg Neurol Int. 7(16): S459-462.
- Suzuki H, Hayashi K, Fukunaga T (2014) Two forensic autopsy cases of death from unexpected lesions of the pituitary gland. Leg Med. 16(1): 36- 39.
- Sun T, Liu L, Sunnassee A, Zhuo L, Zhu S (2013) Sudden death in custody due to pituitary apoplexy during long restriction in a sitting position: a case report and review of the literature. J Forensic Leg Med. 20(7): 812-815.
- Ward ME, Cowley AR (1999) Hypothermia: a natural cause of death. Am J Forensic Med Pathol. 20(4): 383-386.
- Yamanaka A, Hinohara S, Hashimoto T (1955) Primary diffuse carcinomatosis of the spinal meninges accompanied with a cancerous epidermal cyst of the base of the brain; Report of a case of autopsy. Gan. 46(2-3): 274-276.
- Wong SW, Ducker TB, Powers JM (1976) Fulminating parapontine epidermoid carcinoma in a four-year-old boy. Cancer. 37(3): 1525-1531.
- Palmucci L, Liboni W, Favero M (1977) Unusual extension of an epidermoid cyst. Zentralbl Neurochir. 38(3): 299-303.
- Schwartz JF, Balentine JD (1978) Recurrent meningitis due to an intracranial epidermoid. Neurology. 28(2): 124-129.
- Rhodes RH, Davis RL, Beamer YB, Marantz C (1981) A suprasellar epidermoid cyst with symptoms of hypothalamic involvement: case report and a review of pathogenetic mechanisms. Bull Los Angeles Neurol Soc. 46: 26-32.
- Logani KB, Agarwal K, Sharma GK (1997) Epidermoid cyst of brain--an incidental autopsy finding. J Indian Med Assoc. 95(4): 124.
- Sawan B, Vital A, Loiseau H, Dousset V, Strub D, et al., (2000) Squamous cell carcinoma developing in an intracranial prepontine epidermoid cyst. Ann Pathol. 20(3): 258-260.
- Shirabe T, Fukuoka K, Watanabe A, Imamura K, Ishii R (2003) Primary squamous cell carcinoma of the brain. A rare autopsy case. Neuropathology. 23(3): 225-229.
- Domengie F, Cottier JP, Lescanne E, Aesch B, Vinikoff-Sonier C, et al., (2004) Management of cerebrospinal fluid fistulae: physiopathology, imaging and treatment. J Neuroradiol. 31(1): 47–59.
- Robert T, Sajadi A, Uské A, Levivier M, Bloch J (2010) Fulminant meningoencephalitis as the first clinical sign of an invasive pituitary macroadenoma. Case Rep Neurol. 2(3): 133–138.
- Anthony LD, Omar NS, Bartosz TG, Pamela UF, Sharon W, et al., (2009) Simultaneous above and below approach to giant pituitary adenomas: surgical strategies and long-term follow-up. Pituitary. 12(3): 217-225.
- Chen Y, Wang CD, Su ZP, Chen YX, Cai L, et al., (2012) Natural history of postoperative nonfunctioning pituitary adenomas: a systematic review and meta-analysis. Neuroendocrinology. 96(4): 333–342.
- Antonio C, Adrian FD, Albert B (2005) The epidemiology of prolactinomas. Pituitary. 8(1): 3-6.
- Lopes MB (2010) Growth hormone-secreting adenomas: pathology and cell biology. Neurosurgical Focus. 29(4): E2.
- Wolfgang S, Dieter KL, Michael B, Rudolf F, Hans JQ, et al., (2007) Pathohistological classification of pituitary tumors: 10 years of experience with the German Pituitary Tumor Registry. Eur J Endocrinol. 156(2): 203-216.
- Richard JS, Michael PP (2005) Sellar and Parasellar Tumors. Neurosurgery: 187-204.
- Janet AS (2003) Prolactinoma. N Engl J Med. 349: 2035-2041.