Clinical Perspective: Diabetes and Calcific Aortic Stenosis
Lin C1, Hasoon M2, Chilton R3*
1 Interventional Cardiology, UT Health Science Center at San Antonio, San Antonio Texas, USA.
2 Cardiology Service, UT Health Science Center at San Antonio, San Antonio, Texas, USA.
3 Interventional Cardiology, Audie L. Murphy Memorial VA Hospital, San Antonio, Texas, USA.
*Corresponding Author
Robert Chilton,
Interventional Cardiology, Audie L. Murphy Memorial VA Hospital, San Antonio, Texas, USA.
E-mail: Chilton@uthscsa.edu
Received: December 28, 2017; Accepted: Janaury 27, 2018; Published: January 30, 2018
Citation: Lin C, Hasoon M, Chilton R. Clinical Perspective: Diabetes and Calcific Aortic Stenosis. Int J Diabetol Vasc Dis Res,. 2018;6(1):214-216. doi: dx.doi.org/10.19070/2328-353X-1800043
Copyright: Chilton R© 2018. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
2.Basic Science Clinical Perspective: Diabetes and Calcific Aortic Stenosis
3.Human Research and Calcific Aortic Stenosis
4.References
Background
Calcific aortic valve stenosis (CAVD) has similar classical coshared risk factors that are associated with endothelial cell dysfunction and cardiovascular disease. For example, lifestyle habits similar to those predisposing to coronary artery disease, inflammation, hypertension, advanced age, malegender, hyperlipidemia, diabetes mellitus, cigarette smoking, metabolic syndrome, and end-stage kidney disease have all been linked with the development of CAVD [1-6]. Diabetes mellitus (DM) is a powerful known risk factor for CV disease and aortic stenosis. However, mechanisms causing CAVD still remain unknown to a large extent [5]. Pro-atherogenic factors cause amplification of vascular wall inflammation and increased endothelial oxidative stress, thereby activating aortic valve interstitial cells (cells that lie between other cells) toward anosteogenic phenotype favoring the development of CAVD in DM [7].
Over the past 5 years, research in the basic sciences has reached a clinical translational level. From the initial recognition of mineralization and hyperlipidemia by Lobstein and Virchow’s calcification of arteriosclerotic vessels, an increased prevalence of CAVD has become a new treatment target [8]. These calcific vascular changes have major CV implications due to reduced arterial compliance (increased vascular stiffness and impaired Windkessel physiology), leading to vascular wall stress that increases myocardial oxygen consumption and can ultimately cumulatein heart failure (Figure 1).
Basic Science Clinical Perspective: Diabetes and Calcific Aortic Stenosis
Key drivers of aortic valve mineralization are primarily metabolic, inflammatory, and osteogenic. The most important changes in this area are from two key papers, and including a possible benefit in the use of DPPIV inhibitors in treatment of diabetes in animals with CAVDand vascular calcification [9-12]. If this is replicated in human studies, it would be a major advance and potential use for both diabetic and non-diabetic patients with CAVD and CV disease.
Prior human trials involving statins have not yielded beneficial clinical results in patients with CAVD. Earlier animal models with statin therapy in hypercholesterolemic mice had promising findings, demonstrating reduced aortic valve thickening and slowed fibrocalcific progression through suspected endothelial nitric oxide signaling pathways. However, the use of statin therapy has not been associated with slowed calcific aortic stenosis progression in randomized human studies. Finding the perfect animal model for these studies has been problematic at best [13-15].
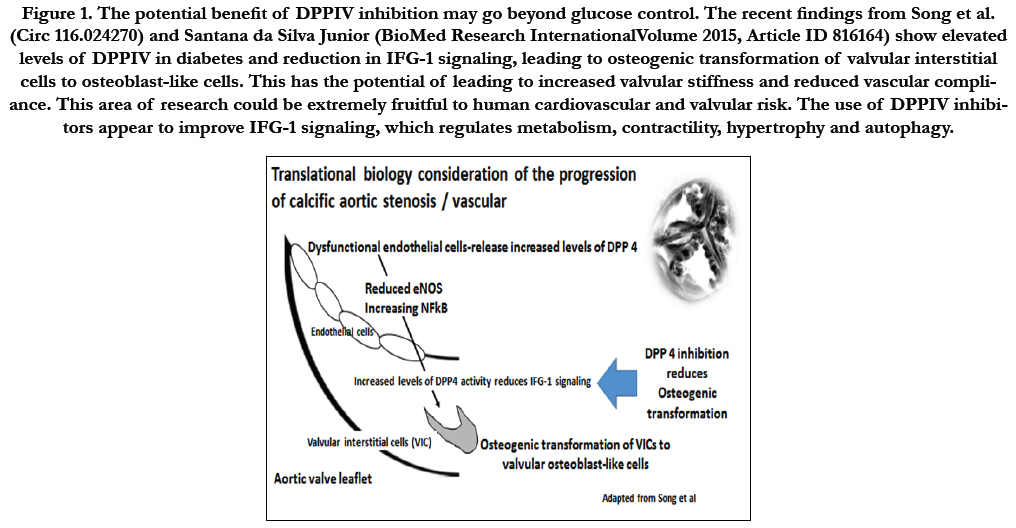
Figure 1. The potential benefit of DPPIV inhibition may go beyond glucose control. The recent findings from Song et al. (Circ 116.024270) and Santana da Silva Junior (BioMed Research InternationalVolume 2015, Article ID 816164) show elevated levels of DPPIV in diabetes and reduction in IFG-1 signaling, leading to osteogenic transformation of valvular interstitial cells to osteoblast-like cells. This has the potential of leading to increased valvular stiffness and reduced vascular compliance. This area of research could be extremely fruitful to human cardiovascular and valvular risk. The use of DPPIV inhibitorsappear to improve IFG-1 signaling, which regulates metabolism, contractility, hypertrophy and autophagy.
New basic research is of critical importance in vascular calcification and atherosclerosis. First, basic scientific evidence has proposed diabetes mellitus-prone LDLr−/−/ApoB100/100/ IGF-II mice as a new model of calcified aortic valve disease. Of special interest is the discovery of insulin growth factor II amplifying the valvular obstruction. In this study, mice lacking low-density lipoprotein receptors and expressing only apolipoprotein B100 (LRKOB100) can develop aortic stenosis at a very old age (≈20 months). However, the addition of IGF (LRKOB100/IGF) increased the features of metabolic syndrome, with susceptibility to type 2 DM, and significantly amplified the severity of CAVD. Thus, in an animal model, abnormal lipids and a diabetic metabolic state were noted to increase calcific aortic valve gradients by echocardiography [13]. Second, studies from Ikushima and colleagues reported important findings suggesting that sCD26 has major influence on T-cell migration by interacting with M6P/IGFIIR (mannose 6-phosphate/insulin-like growth factor II receptor). An insulin-like growth factor receptor was involved with trans-endothelial migration, with the endothelial cell surface acting as a receptor for sCD26 [16]. Lastly, research by Choi et al., and associates from Seoul, South Korea, found a reduction inaortic valve areas and pressure gradients with the use of a DPPIV inhibitor in New Zealand rabbits and with cell-based aortic valve tissue studies from humans [17].
Human Research and Calcific Aortic Stenosis
Two human studies evaluating CAVD patients with DM are important to this topic. First, the plasma from diabetes and hyperlipidemic patients changed human aortic valve interstitial cells to an osteoblast-like phenotype. Cattan-Levy et al., reported that plasma from both types of patients showed an inflammatory phenotype (increased IL6, IL1b) in 24 hours and an osteogenic phenotype (osteocalcin, BSP, CBFA1, phosphatase alkaline) in 4 days using RT-PCR, when compared to healthy control subjects. In addition, primary valve interstitial cells isolated from non-calcified human aortic valves were cultured in 3D collagen scaffolds in the presence of plasma from patients with calcified aortic disease and from healthy subjects. They also reported that the plasma from patients with dyslipidemia and diabetes induced an osteogenic phenotype, which was positively correlated with the levels of Lp (a) [18].
The second study in humans was by Mosch and colleagues, in which 45 patients with CAVD were evaluated histologically. Results showed increased calcification in diabetic patients on gross examination (p<0.01), with reduced expression of the proinflammatory protein S100A9 (p<0.01) in diabetic individuals [19].
In summary, it is possible these findings will help initiate a new era in treatment of CAVD in diabetes patients, if further clinical research supports these findings. In addition, accelerated atherosclerosis in humans with increased calcification may represent a new target for treatment via possible stabilization of further osteogenic phenotypes, thus reducing the early need for valve replacement in young adults with and without diabetes.
References
- Manning WJ. Asymptomatic aortic stenosis in the elderly: a clinical review. JAMA. 2013 Oct 9;310(14):1490-7. doi: 10.1001/jama.2013.279194. Pub- Med PMID: 24104373.
- Mohty D, Pibarot P, Després JP, Côté C, Arsenault B, Cartier A, et al., Association between plasma LDL particle size, valvular accumulation of oxidized LDL, and inflammation in patients with aortic stenosis. Arteriosclerosis, thrombosis, and vascular biology. Arterioscler Thromb Vasc Biol. 2008 Jan;28(1):187-93. Epub 2007 Nov 1. PubMed PMID: 17975118.
- Pate GE. Association between aortic stenosis and hypertension. The Journal of heart valve disease. 2002 Sep;11(5):612-4.
- Ngo MV1, Gottdiener JS, Fletcher RD, Fernicola DJ, Gersh BJ. Smoking and obesity are associated with progression of aortic stenosis. Am J Geriatr Cardiol. 2001 Mar-Apr;10(2):86-90. PubMed PMID: 11253465.
- Katz R, Wong ND, Kronmal R, Takasu J, Shavelle DM, Probstfield JL, et al., Features of the metabolic syndrome and diabetes mellitus as predictors of aortic valve calcification in the Multi-Ethnic Study of Atherosclerosis. Circulation. 2006 May 2;113(17):2113-9. Epub 2006 Apr 24. PubMed PMID:16636166.
- Milosz D, Czupryniak L, Saryusz-Wolska M, Zasadzinska G, Borkowska A, Cieplucha E, et al., Adiponectinemia, inflammatory process activity, and endothelial dysfunction in patients with type 2 diabetes and acute coronary syndrome with ST elevation in relation to the severity of lesions in the coronary arteries. Polskie Archiwum Medycyny Wewnetrznej. 2007 Aug;117(8):343.
- Kang S, Tsai LT, Rosen ED. Nuclear Mechanisms of Insulin Resistance. Trends Cell Biol. 2016 May;26(5):341-351. doi: 10.1016/j. tcb.2016.01.002. Epub 2016 Jan 25. Pub Med PMID:26822036 PubMed Central PMCID:PMC4844850.
- Thompson B, Towler DA. Arterial calcification and bone physiology: role of the bone-vascular axis. Nat Rev Endocrinol. 2012 Sep;8(9):529-43. doi: 10.1038/nrendo.2012.36. Epub 2012 Apr 3. Pub Med PMID:22473330 PubMed Central PMCID:PMC3423589.
- Coté N, Mahmut A, Bosse Y, Couture C, Pagé S, Trahan S, et al., Inflammation is associated with the remodeling of calcific aortic valve disease. Inflammation. 2013 Jun;36(3):573-81. doi:10.1007/s10753-012-9579-6. PubMed PMID:23225202.
- El Husseini D, Boulanger MC, Fournier D, Mahmut A, Bossé Y, Pibarot P, et al., High expression of the Pi-transporter SLC20A1/Pit1 in calcific aortic valve disease promotes mineralization through regulation of Akt-1. PLoS One. 2013;8(1):e53393. doi: 10.1371/journal.pone.0053393. Epub 2013 Jan 4. Pub Med PMID:23308213 PubMed Central PMCID:PMC3537628.
- Capoulade R, Clavel MA, Dumesnil JG, Chan KL, Teo KK, Tam JW, et al., Insulin resistance and LVH progression in patients with calcific aortic stenosis: a substudy of the ASTRONOMER trial. JACC Cardiovasc Imaging. 2013 Feb;6(2):165-74. doi: 10.1016/j.jcmg.2012.11.004. Pub Med PMID:23489530.
- Katz R, Wong ND, Kronmal R, Takasu J, Shavelle DM, Probstfield JL, et al., Features of the metabolic syndrome and diabetes mellitus as predictors of aortic valve calcification in the Multi-Ethnic Study of Atherosclerosis. Circulation. 2006 May 2;113(17):2113-9. Epub 2006 Apr 24. Pub Med PMID:16636166.
- Le Quang K, Bouchareb R, Lachance D, Laplante MA, El Husseini D, Boulanger MC, et al., Early development of calcific aortic valve disease and left ventricular hypertrophy in a mouse model of combined dyslipidemia and type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol. 2014 Oct;34(10):2283-91. doi: 10.1161/ATVBAHA.114.304205. Epub 2014 .ug 14. Pub Med PMID: 25231636.
- Rajamannan NM, Subramaniam M, Springett M, Sebo TC, Niekrasz M, McConnell JP, et al., Atorvastatin inhibits hypercholesterolemia-induced cellular proliferation and bone matrix production in the rabbit aortic valve. Circulation. 2002 Jun 4;105(22):2660-5. Pub Med PMID:12045173 PubMed Central PMCID:PMC3951862.
- Rajamannan NM, Subramaniam M, Stock SR, Stone NJ, Springett M, Ignatiev KI, et al., Atorvastatin inhibits calcification and enhances nitric oxide synthase production in the hypercholesterolaemic aortic valve. Heart. 2005 Jun;91(6):806-10. Pub Med PMID:15894785 PubMed Central PMCID: PMC1768932.
- Ikushima H, Munakata Y, Iwata S, Ohnuma K, Kobayashi S, Dang NH, et al., Soluble CD26/dipeptidyl peptidase IV enhances transendothelial migration via its interaction with mannose 6-phosphate/insulin-like growth factor II receptor. Cell Immunol. 2002 Jan;215(1):106-10. Pub Med PMID:12142042.
- Choi B, Lee S, Kim SM, Lee EJ, Lee SR, Kim DH, et al., Dipeptidyl Peptidase- 4 Induces Aortic Valve Calcification by Inhibiting Insulin-like Growth Factor-1 Signaling in Valvular Interstitial Cells. Circulation. 2017 May 16;135(20):1935-1950. doi: 10.1161/CIRCULATIONAHA.116.024270. Epub 2017 Feb 8. Circulation. 2017 May 16;135(20):1935-1950. doi:10.1161/CIRCULATIONAHA.116.024270. Epub 2017 Feb 8. PubMed PMID:28179397.
- Cattan-Lévy L, Jacob-Lenet MP, Dehoux M, Deschildre C, Codogno I, Morvan M, Gaston AT, Even G, Michel JB, Messika-Zeitoun D, Nicoletti A. Plasma from patients with calcified aortic disease triggers an osteoblastlike phenotype switch in human aortic valve interstitial cells. Atherosclerosis. 2016 Sep 1;252:e234.
- Mosch J, Gleissner CA, Body S, Aikawa E Histopathological assessment of calcification and inflammation of calcific aortic valves from patients with and without diabetes mellitus. Histol Histopathol. 2017 Mar;32(3):293-306. doi: 10.14670/HH-11-797. Epub 2016 Jun 29. Pub Med PMID: 27353274 PubMed Central PMCID: PMC5199639.