Growth Differentiation Factor-15 (GDF-15) a Precursory Biomarker for Diabetic Patients with Coronary Artery and Chronic Kidney Disease
Anitha Rani A1, Ezhilarasi K2, Viswanathan V3*
1 Post Doctoral Research Fellow, Prof. M. Viswanathan Diabetes Research Centre, Royapuram, Chennai, Tamil Nadu, India.
2 Research Coordinator, Prof. M. Viswanathan Diabetes Research Centre, Royapuram, Chennai, Tamil Nadu, India.
3 Head & Chief Diabetologist, M.V. Hospital for Diabetes and President, Prof. M.Viswanathan Diabetes Research Centre, West Madha Church Street, Royapuram, Chennai, Tamil Nadu, India.
*Corresponding Author
Vijay Viswanathan,
Head & Chief Diabetologist, M.V. Hospital for Diabetes and President, Prof. M.Viswanathan Diabetes Research Centre,
No: 4, West Madha Church Street, Royapuram, Chennai 600013, Tamil Nadu, India.
E-mail: drvijay@mvdiabetes.com/researchcommunication@mvdiabetes.com
Received: July 27, 2017; Accepted: August 31, 2017; Published: September 14, 2017
Citation: Anitha Rani A, Ezhilarasi K, Viswanathan V. Growth Differentiation Factor-15 (GDF-15) a Precursory Biomarker for Diabetic Patients with Coronary Artery and Chronic Kidney Disease. Int J Diabetol Vasc Dis Res,. 2017;5(4):202-207. doi: dx.doi.org/10.19070/2328-353X-1700041
Copyright: Viswanathan V© 2017. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Growth-differentiation factor-15 (GDF-15) is emerging as an important biomarker, thus the current study aimed to determine the levels of GDF-15 among, T2DM subjects with chronic kidney disease (CKD) and coronary artery disease (CAD).
Methodology: The present cross sectional study comprised of 80 T2DM subjects with mean age of 60.15 ± 1.8 years. The study subjects were divided into four groups Group-I: T2DM subjects (T2DM), Group-II: T2DM subjects with chronic kidney disease (CKD), Group-III: T2DM subjects with established coronary artery disease (CAD) and Group-IV: T2DM subjects with CKD and established CAD. Anthropometric, demographic and biochemical parameters were measured in all study subjects. The levels of GDF-15 were measured in plasma using ELISA.
Results: The level of GDF-15 was significantly higher in diabetic subjects having both CAD and CKD when compared to other study groups and significant difference was observed between the study groups (p<0.0001). Comparisons of GDF-15 levels between group’s shows statistical significance among the group I vs. other groups (p <0.0001), similar scenario were observed in group II vs. III (p< 0.05), IV (p <0.0001) and group III vs. IV (p< 0.05). There is a positive correlation with the level GDF-15 with duration of diabetes, systolic blood pressure, HbA1c, Urea, Creatinine and Triglyceride. Negative correlation were observed in eGFR (r = - 0.35, p < 0.001) and HDL (r = - 0.25 p = 0.03).
Conclusion: The levels of GDF-15 are markedly increased in diabetic subjects having both CAD and CKD. The study finding highlights the major role of GDF-15 as a promising biomarker of CAD and CKD in T2DM subjects.
2.Introduction
3.Materials and Methods
3.1 Subjects
3.2 Assessment of Plasma GDF-15
4.Statistical Analysis
5.Results
6.Discussion
7.Limitations
8.Conclusion
9.Acknowledgement
10.References
Keywords
GDF-15; Type 2 Diabetes; Diabetic Coronary Artery Disease; Diabetic Chronic Kidney Disease; Bio Marker.
Introduction
Global burden of diabetes is huge and in India, approximately 65.1 million people are with diabetes [1]. Diabetes is associated with an increased risk of cardiovascular disease, which is the important cause of morbidity and mortality among patients with diabetic nephropathy [2]. Myocardial damage is associated with the development of proteinuria and focal glomerulosclerosis [3]. Clinical studies suggest that impaired renal function is a risk factor for the development of cardiovascular disease conversely; experimental evidence, proof that cardiac injury may in turn deteriorates kidney function [3, 4]. Recent studies have demonstrated an association between elevated GDF-15 levels and progression of kidney and cardiovascular disease in patients with diabetes [5-8].
Currently, the diagnosis of diabetic nephropathy (DN) is based on the elevated urinary albumin excretion level. However the emerging evidences suggested that the risk of DN starts developing well before the elevation of urinary albumin level. The potential treatment can be offer to the patients as a preventive measure, if the onset of DN is diagnosed earlier. The development of focal glomerulosclerosis and Proteinuria is associated with myocardial damage. The elevated level of circulating troponins is associated with both Chronic Kidney Disease (CKD) and cardiac injury. The expression of serum GDF-15 is also increases with BMI (body Mass Index), age and insulin resistance. Increased level of GDF- 15 is associated with CVDs and also with incidence of CKD which leads to rapid decline in renal function. Thus the higher levels were also predictive of deterioration of kidney function.
Growth differentiation factor-15 (GDF-15) is a stress-responsive member of transforming growth factor-β (TGF-β) super family. GDF-15 is produced as α ≈ 40 kDa propeptide forms. The N terminus is cleaved and released as α ≈ 30 kDa disulphide linked dimeric active protein [9-10] GDF-15 expression is markedly increased in response to tissue injury, including heart and kidney [8, 11]. Previous studies have suggested that higher GDF-15 levels were associated with deterioration of kidney function and in the development and progression of coronary artery disease [7, 12, 13]. GDF-15 is expressed in heart under normal physiological conditions, but increases rapidly in response to cardiovascular injury [14]. Increased expression of GDF- 15 was observed in the animal and human heart after myocardial infarction and remains elevated in the infracted myocardium [15].
Despite a growing body of evidence substantiating the role of GDF-15 in tissue injury little is known about the association of this biomarker in coronary artery disease and kidney disease. Deeper understanding of the role of GDF-15 may provide insights into potential process of renal injury, and the complex interaction of coronary artery and renal disease. Hence the present work was designed to determine the levels of GDF-15 in T2DM subjects with chronic kidney disease (CKD) and coronary artery disease (CAD).
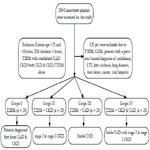
The cross section study has been conducted among T2DM patients, attending outpatient department in a tertiary care hospital in India. Consecutive patients were recruited based on the inclusion and exclusion criteria. The study protocol was approved by the institutional ethics committee. T2DM subjects with age above 25 years and less than 65 years, diabetic duration more than 5 years, T2DM with established CAD, CKD and both CKD & CKD were included in the study. Patients with Type-1 diabetes and patients with a previous/current diagnosis of urolithiasis, UTI, gestational diabetes, liver cirrhosis, lung diseases, foot ulcers, cancer, viral hepatitis and patients who are not willing to give consent were excluded. All the subjects included in the study were on treatment with oral hypoglycemic agents and or with Insulin and known hypertensive’s were on antihypertensive medication. Well established stable CAD patients were recruited for CAD group, for CKD group, stage 2 and stage 3 CKD patients with three or more results of urine protein creatinine ratios (>30 mg/ dl) and confirmed clinical diagnosis of diabetic nephropathy were included in the study. For CKD and T2DM groups, patients who underwent regular diabetic examination, and diagnosed free of CAD by their medical history of CAD or angiography, normal electrocardiography, and without chest pain symptoms, were recruited as controls (Figure 1).
Demographic and anthropometric details like age, height, weight, BMI, blood pressure, duration of diabetes, family history of diabetes and duration of hypertension were obtained from patients’ proforma and medical records. Biochemical parameters like fasting and postprandial glucose level, HbA1c, lipid profile, urea, creatinine, urinary albumin and urinary protein values were also recorded. All biochemical parameters were estimated using BS400 biochemistry autoanalyzer, HbA1c was measured using HPLC method using variant turbo equipment (Bio-Rad). Creatinine was estimated by Jaffe’s kinetic method, urinary albumin was estimated by immunoturbidimetric procedure. Urinary protein was determined using pyrogallol method.
Fasting blood samples were collected and centrifuged at 2000 rpm/min for 10 min. After centrifugation plasma samples were gently transferred into sterile vials for the estimation of plasma GDF-15 levels and stored at 20°C until tested. Plasma GDF-15 levels were measured with a solid phase enzyme linked immunosorbent assay following the manufacturer's instruction (Raybio human GDF-15 kit, Norcross, GA). The minimum detectable dose of GDF-15 levels was determined to be 2 pg/ml. The coefficient of mean variations in the samples was <5%.
Statistical Analysis
All statistical analyses were performed using IBM SPSS Version 20. ANOVA, Multiple comparison-Bonferroni were used to compared dependent variables, Pearson's correlation coefficient test was used to observe correlation of plasma GDF-15 with biochemical and clinical parameters and the p value of <0.05 was considered statistically significant.
Results
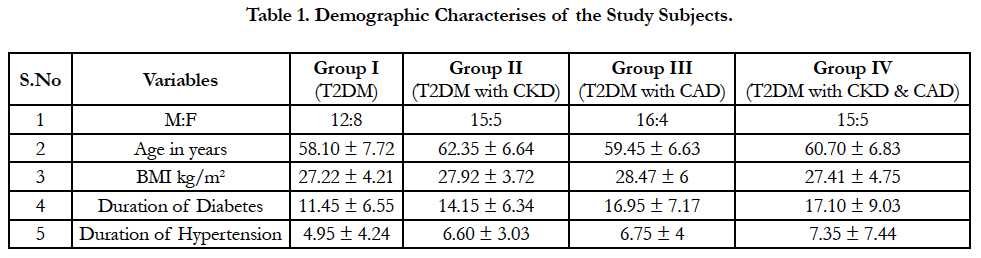
A total of 80 consecutive patients (M: F = 58:22) were included in the study with the mean age of 60.15 ± 1.8 years and mean Diabetic duration was 14.91 years. The study subjects were divided into four groups Group-I: T2DM subjects (n=20; M: F= 12:8), Group-II: T2DM subjects with CKD (n=20; M:F= 15:5), Group-III: T2DM subjects with established CAD (n=20; M:F= 16:4) and Group-IV: T2DM subjects with CKD and established CAD (n=20; M:F= 15:5).
The demographic detail of the study group was presented in Table 1. The duration of Hypertension was higher in Group IV when compared to other groups. Our study population showed 46.25% of the subjects had family history of diabetes. Stastical significance was observed in the Systolic blood Pressure (p=0.01), HbA1c (p=0.01), Postprandial blood sugar levels (p=0.02), Urea (p<0.001), Creatinine (p<0.001), eGFR (p<0.001), Triglyceride (p=0.01), High Density Lipoprotein (p=0.02) and VLDL (p= 0.04) between the study groups (Table 2).
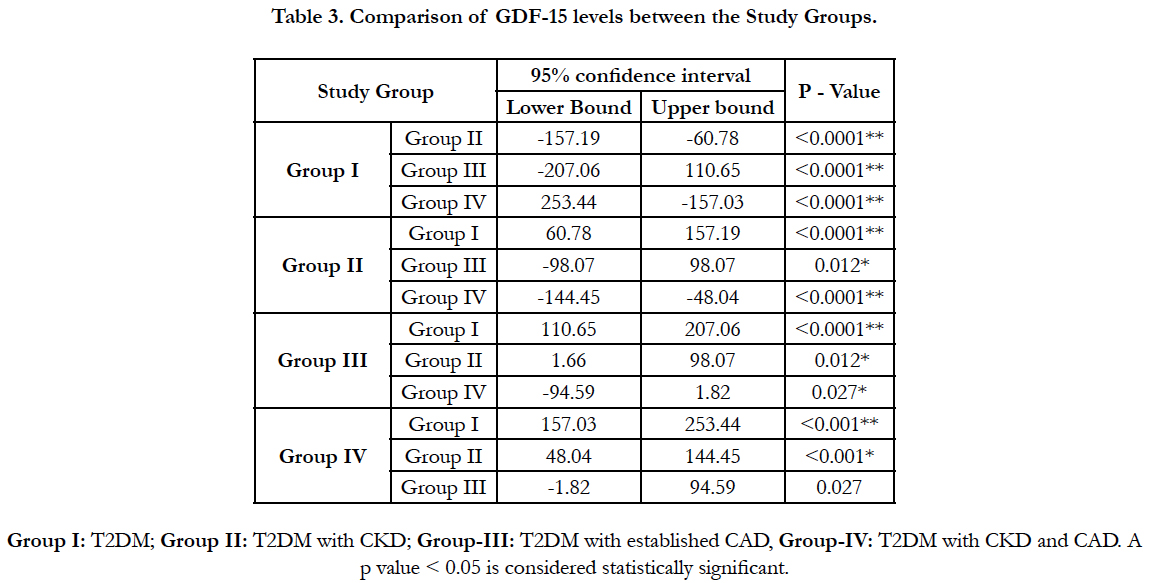
The level of GDF-15 was markedly increased in diabetic subjects with both CKD and CAD (Group IV: 520.52 ± 62.18) compared to other groups. It is also observed that the mean levels of GDF-15 was higher in T2DM subjects with CAD (group III: 474.14 ± 65.14) compared to T2DM subjects with CKD (424.28 ± 53.79) and subjects with T2DM (Group I: 315.29 ± 40.77). Significant difference in the plasma levels of GDF-15 (p<0.001) was observed between the groups. Comparisons of GDF-15 levels between the groups shows statistical differences between group I vs. group II (p<0.0001), group III (p<0.0001), group IV (p< 0.0001), group II vs. group III (p=0.012), group IV (p<0.0001) and group III vs. IV (p=0.027) (Table 3).
Among the study groups GDF-15 levels are positively correlated with duration of diabetes (r=0.32; p=0.001), systolic blood pressure (r=0.28; p=0.01), HbA1c (r=0.35; p=0.001), urea (r= 0.42; p=0.001), creatinine (r=0.32; p=0.001) and triglyceride (r=0.31; p=0.01). A negative correlation were noticed in eGFR (r= - 0.35; p=0.001) and HDL (r = -0.25; P=0.03). Other variable doesn’t show any correlation with GDF -15.
When focusing on each groups separately, group II (CKD) showed negative correlation with eGFR (r = -0.48; p=0.03), group III showed negative correlation with Post prandial blood sugars (r=-0.46; p=0.04) and group IV showed positive correlation with Urea (r=0.47; P= 0.04) and Creatinine (r=0.46; P=0.04) and negative correlation with eGFR (r= -0.25; p=0.03).
Discussion
The association of GDF-15 levels in diabetes, cardiovascular and kidney disease has been studied previously, perhaps for the first time we have examined the levels of GDF-15 in all the four groups (T2DM, CKD, CAD, and CKD & CAD). In healthy condition GDF-15 is rarely much expressed [16]. The plasma concentration of GDF-15, increased and up-regulated under pathological conditions such as hypoxia, inflammation or oxidative stress [17]. GDF-15 plays a role as an endocrine factor if present in circulation [18]. GDF-15 is released from macrophages, vascular cardiomyocytes, adipocytes, smooth muscle cells and endothelial cells after tissue injury, anoxia, and proinflammatory cytokine responses [10, 19, 20].
GDF-15 highly expressed in response to biomechanical stress, ischemia, anoxia and different kinds of cytokines and growth factors like interleukin-1 (IL-1, IL-2, IL-4, IL-6), TNF-, angiotensin II, macrophage colony stimulating factor (M-CSF), and TGF-β. GDF-15 plays a major role in the regulation growth, cell differentiation and inflammatory response [21].
Cardiomyocytes in the infarct border zone have been identified as the main source of GDF-15 [22]. The controversy exists in the production sites of GDF-15 during heart failure conditions. Even though GDF-15 is strongly released from the infracted condition of the human heart, it might also be released from the macrophages [23]. Earlier study also reported that there is no evidence for the myocardial expression of GDF-15 in patients having advanced non-ischemic heart failure. Perhaps, the circulating GDF-15 levels were increased the same as cardiac troponin and natriuretic peptides levels in serum [24].
It has been established that levels of GDF-15 correlated strongly with age [25], specifically higher in elderly adults [26], these changes would reflect both in cardiovascular and renal changes, inflammations and pathophysiological process. The elevated levels of GDF-15 in subjects with T2DM with both CAD and CKD could reflect both cardiovascular and renal perturbations, inflammation and other independent pathophysiological processes [14, 27]. Several animal and human studies highlighted the association between GDF-15 with CAD and CKD; perhaps there is a paucity of data in diabetic subjects with both cardio and renal complications. The elevated level of GDF-15 was already reported in diabetic conditions, hypertension, and deterioration of kidney function [15, 26]. Further, increased level of circulating GDF- 15, was also observed in acute myocardial infarction, shows that there exist a strong link between GDF-15 and inflammation [25, 28]. As GDF-15 plays an important role in various diseases, current study assessed the level of GDF-15 among T2DM patients with CAD and CKD. The mean level of GDF-15 in T2DM subjects was lower when compared to other groups. The findings highlighted that elevated levels of GDF-15 is associated with both CKD and CAD. A recent study also showed that the expression of GDF-15 is up regulated in CKD and represents a novel independent serum marker of mortality [11].
Further, GDF-15 is also patented for the diagnosis of kidney injury after surgery, prediction of kidney failure after heart surgery and detecting the prognosis of CKD [29]. Our study finding is consistent with the earlier study, which suggested that higher levels of GDF-15 are a precursory marker of cardiovascular disease in patients with diabetic nephropathy in addition to other well known cardiovascular risk factors like NTproBNP and glomerular filtration rate (GFR) [30, 31]. Among other biomarkers, GDF-15 is found to be one of the promising biomarkers which predicts cardiovascular outcome and many studies have suggested that higher plasma GDF-15 levels are seen in patients with cardiovascular pathologies such as CAD or chronic heart failure [23, 32]. Present study findings are in line with the earlier findings that the GDF-15 is closely associated with cardiovascular and kidney disease [23, 28, 32-34]. Thus GDF-15 could be an important precursory biomarker for T2DM patients.
The association of injured tissue response to stress and increased GDF-15 levels confirmed that the GDF-15 expression is increased with the severity of the underlying disease condition [19]. Previous studies strongly supports GDF 15 as a independent biomarker for cardiovascular disease [29, 35]. Current study findings showed higher plasma GDF-15 levels in T2DM with CAD and CKD (Group II, III and IV) as compared to T2DM subjects. However, when compared to CKD, the mean level of GDF-15 is higher in CAD with T2DM subjects. This may suggested that the GDF-15 is superior marker for CAD. Hence the level of GDF-15 increased with the severity of the underlying disease. Thus our data suggested that plasma GDF-15 had a good capability in predicting CAD and CKD, and it can be used as an adjunct in distinguishing CAD and CKD.
Limitations
Current study has various limitations, firstly because of the cross sectional nature of the study, we could not able to clearly establish a causal relationship between plasma GDF-15 levels and the development of CAD and CKD. Secondly, the interaction of drugs which increases the levels of plasma GDF-15 has not been studied and identified. Thirdly, the sample size used in this study was relatively small. Fourthly, current study is a single centre study, which lacks generalizablity of the findings. Thus further multicentre study with large sample size is required to make these associations clear.
Conclusion
In conclusion, our study highlighted that the level of GDF-15 was significantly increased in T2DM patients with both CKD and CAD, when compared to CAD, CKD and T2DM. Thus GDF- 15 would be a promising diagnostic marker for both CAD and CKD. Further research is essential before considering GDF-15 as therapeutic intervention and prevention of diabetic complications.
Acknowledgement
We acknowledge the help rendered by Dr. Zenith Khashim in sample collection and preparation.
References
- International Diabetes Federation Diabetes Atlas, 6th ed. 2013 May 27. 160.
- Rossing P, Hougaard P, Borch-Johnsen K, Parving HH. Predictors of mortality in insulin dependent diabetes: 10 year observational follow up study. BMJ. 1996 Sep 28;313(7060):779–784.
- van Dokkum RP, Eijkelkamp WB, Kluppel AC, Henning RH, van Goor H, Citgez M, et al. Myocardial infarction enhances progressive renal damage in an experimental model for cardio-renal interaction. J Am Soc Nephrol. 2004 Dec;15(12):3103–3110.
- Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003 Nov;42(5):1050-65.
- Hellemons ME, Mazagova M, Gansevoort RT, Henning RH, De ZD, Bakker SJ, et al. Growth-differentiation factor 15 predicts worsening of albuminuria in patients with type 2 diabetes. Diabetes Care. 2012 Nov;35(11):2340–2346.
- Kempf T, Zarbock A, Widera C, Butz S, Stadtmann A, Rossaint J, et al. GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat Med. 2011 May;17(5):581–588.
- Lajer M, Jorsal A, Tarnow L, Parving HH, Rossing P. Plasma growth differentiation factor-15 independently predicts all-cause and cardiovascular mortality as well as deterioration of kidney function in type 1 diabetic patients with nephropathy. Diabetes Care. 2010 Jul;33(7): 1567–1572.
- Zimmers TA, Jin X, Hsiao EC, McGrath SA, Esquela AF, Koniaris LG. Growth differentiation factor-15/macrophage inhibitory cytokine-1 induction after kidney and lung injury. Shock. 2005 Jun;23(6):543–548.
- Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta super family. Proc Natl Acad Sci USA. 1997 Oct 14;94(21):11514 –9.
- Schlittenhardt D, Schober A, Strelau J, Bonaterra GA, Schmiedt W, Unsicker K, et al. Involvement of growth differentiation factor-15/macrophage inhibitory cytokine-1 (GDF-15/MIC-1) in oxLDL-induced apoptosis of human macrophages in vitro and in arteriosclerotic lesions. Cell Tissue Res. 2004; 318: 325–333.
- Breit SN, Carrero JJ, Tsai VW, Yagoutifam N, Luo W, Kuffner T, et al. Macrophage inhibitory cytokine-1 (MIC-1/GDF15) and mortality in end stage renal disease. Nephrol Dial Transplant. 2012 Jan;27(1):70-5.
- Belair MF, Heudes D, Bruneval P, Doucet A, Jean Paul Duong Van Huyen, Lydie Cheval, et al. GDF15 triggers homeostatic proliferation of acid-secreting collecting duct cells. J Am Soc Nephrol. 2008;19(10):1965–1974.
- Simonson MS, Tiktin M, Debanne SM, Rahman M, Berger B, Hricik D, et al. The renal transcriptome of db/db mice identifies putative urinary biomarker proteins in patients with type 2 diabetes: a pilot study. Am J Physiol Renal Physiol. 2012;302(7):F820–F829.
- Lindahl B. The story of growth differentiation factor 15: another piece of the puzzle. Clin Chem. 2013 Nov;59(11):1550–1552.
- Wallentin L, Hijazi Z, Andersson U, Alexander JH, De Caterina R, Hanna M , et al. Growth differentiation factor-15, a marker of oxidative stress and inflammation, for risk assessment in patients with atrial fibrillation: insights from the apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation (ARISTOTLE) trial. Circulation. 2014 Nov;130 (21):1847–1858.
- Damman P, Kempf T, Windhausen F, van Straalen JP, Guba-Quint A, Fischer J, et al. Growth-differentiation factor 15 for long-term prognostication in patients with non-ST-elevation acute coronary syndrome: an Invasive versus Conservative Treatment in Unstable coronary Syndromes (ICTUS) substudy. Int J Cardiol. 2014 Mar 15;172(2):356–363.
- Ago T, Sadoshima J. GDF15, a cardioprotective TGF-beta superfamily protein. Circ Res. 2006 Feb 17;98(3):294–297.
- Bauskin AR, DA Brown, S Junankar, Rasiah KK, Hunter M, Liu T, et al. The propeptide mediates formation of stromal stores of PROMIC-1: role in determining prostate cancer outcome. Cancer Res. 2005 Mar 15;65(6):2330–2336.
- Ferrari N, Pfeffer U, R Dell’Eva, C Ambrosini, DM Noonan, A Albini. The transforming growth factor-β family members bone morphogenetic protein-2 and macrophage inhibitory cytokine-1 as mediators of the antiangiogenic activity of N-(4-hydroxyphenyl) retinamide. Clin Cancer Res. 2005;11(12):4610-4619.
- Eggers KM, Kempf T, Wallentin L, Wollert KC, Lind L. Change in growth differentiation factor 15 concentrations over time independently predicts mortality in community-dwelling elderly individuals. Clin. 2013 Jul;59(7):1091-1098.
- Fairlie WD, Moore AG, Bauskin AR, Russell PK, Zhang HP, Breit SN. MIC-1 is a novel TGF-beta superfamily cytokine associated with macrophage activation. J Leukoc Biol. 1999 Jan;65(1):2-5.
- J Jurczyluk, D Brown, KK Stanley. Polarised secretion of cytokines in primary human microvascular endothelial cells is not dependent on N-linked glycosylation. Cell Biol Int. 2003;27(12):997–1003.
- B Berm´udez, S L´opez, YM Pacheco, Villar J, Muriana FJ, Abia R, et al. Influence of postprandial triglyceride-rich lipoproteins on lipid mediated gene expression in smooth muscle cells of the human coronary artery. Cardiovasc Res. 2008 Jul 15;79(2):294–303.
- Lok B, Winkens R, Goldschmeding R, Nous FM, Van Kuik J, Lahpor JR, et al. Circulating growth differentiation factor-15 correlates with myocardialfibrosis in patients with non-ischaemic dilated cardiomyopathy decreases rapidly after left ventricular assist device support. Eur J Heart Fail. 2012 Nov;14(11):1249–1256.
- Kempf T, Horn-Wichmann R, Brabant G, Peter T, Allhoff T, Klein G, et al. Circulating concentrations of growth-differentiation factor 15 in apparently healthy elderly individuals and patients with chronic heart failure as assessed by a new immunoradiometric sandwich assay. Clin Chem. 2007;53(2):284–291.
- Ho JE, Mahajan A, Chen MH, Larson M, Elizabeth L, Kai C, et al. Clinical and genetic correlates of growth differentiation factor 15 in the community. Clin Chem. 2012;58(11):1582–1591.
- Kempf T, Björklund E, Olofsson S, Lindahl B, Allhoff T, Peter T, et al. Growth-differentiation factor-15 improves risk stratification in ST-segment elevation myocardial infarction. Eur Heart J. 2007 Dec;28(23):2858–2865.
- Wollert KC, Kempf T, Lagerqvist B, Allhoff t, Peter T, Venge P, et al. Growth differentiation factor 15 for risk stratification and selection of an invasive treatment strategy in non ST-elevation acute coronary syndrome. Circulation. 2007 Oct 2;116(14):1540– 1548.
- Paul WX Foley, Berthold Stegemann, Kelvin Ng, Sud Ramachandran, Anthony Proudler, Michael P Frenneaux, et al., Growth differentiation factor- 15 predicts mortality and morbidity after cardiac resynchronization therapy. Eur Heart J. 2009 Nov;30(22):2749–2757.
- Xu X, Li Z, Gao W. Growth differentiation factor 15 in cardiovascular diseases: from bench to bedside. Biomarkers. 2011 Jul 1;16(6):466–475.
- Kempf T, von Haehling S, Peter T, Allhoff T, Cicoira M, Doehner W, et al. Prognostic utility of growth differentiation factor-15 in patients with chronic heart failure. J Am Coll Cardiol. 2007 sep 11;50(11):1054–1060.
- Khan SQ, Ng K, Dhillon O, Kelly D, Quinn P, Squire IB, et al. Growth differentiation factor-15 as a prognostic marker in patients with acute myocardial infarction. Eur Heart J. 2009 May;30(9):1057–1065.
- Rohatgi A, Patel P, Das SR, Ayers CR, Khera A, Martinez-Rumayor A, et al. Association of growth differentiation factor-15 with coronary atherosclerosis and mortality in a young, multiethnic population: observations from the Dallas Heart Study. Clin Chem. 2012 Jan;58(1):172-182.
- Vila G, Riedl M, Anderwald C, Resl M, Handisurya A, Clodi M, et al. The relationship between insulin resistance and the cardiovascular biomarker growth differentiation factor-15 in obese patients. Clin Chem. 2011 Feb;57(2):309–316.
- Wollert KC. Growth-differentiation factor-15 in cardiovascular disease: from bench to bedside, and back. Basic Res Cardiol. 2007 Jun 05;102(5):412– 415.