Stratifying Renal Risk and Retinal Involvement in South Indian Type 2 Diabetic Patients: Using the Kdigo Classification
Anitha Rani A1, Satyavani K2, Viswanathan V3*
1 M.V. Hospital for Diabetes & Prof. M. Viswanathan Diabetes Research Centre, WHO Collaborating Centre for Research, Education and Training in Diabetes) Royapuram, Chennai, India.
2 Lab director, M.V. Hospital for Diabetes, WHO Collaborating Centre for Research, Education and Training in Diabetes) Royapuram, Chennai, India.
3 Head & Chief Diabetologist, M.V. Hospital for Diabetes & Prof. M. Viswanathan Diabetes Research Centre, WHO Collaborating Centre for Research, Education and Training in Diabetes) Royapuram, Chennai, India.
*Corresponding Author
Vijay Viswanathan,
Head & Chief Diabetologist, M.V. Hospital for Diabetes & Prof. M. Viswanathan Diabetes Research Centre,
WHO Collaborating Centre for Research, Education and Training in Diabetes, Royapuram, Chennai, India.
E-mail: drvijay@mvdiabetes.com / researchcommunication@mvdiabetes.com
Received: May 15, 2017; Accepted: June 30, 2017; Published: July 20, 2017
Citation: Anitha Rani A, Satyavani K, Viswanathan V (2017) Stratifying Renal Risk and Retinal Involvement in South Indian Type 2 Diabetic Patients: Using the Kdigo Classification. Int J Diabetol Vasc Dis Res,. 5(3), 196-201. doi: dx.doi.org/10.19070/2328-353X-1700040
Copyright: Viswanathan V© 2017. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Aim: To stratify the renal risk using estimated Glomerular Filtration Rate (eGFR) and albuminuria based on Kidney Disease Improving Global Outcome (KDIGO) classification and its association with the degree of Diabetic Retinopathy (DR) among people with Type 2 Diabetes (T2DM) in South India.
Methods: A total of 3426 (2193:1233) subjects with T2DM who underwent Fundus Photography and Fundus Fluorescent Angiography for DR, estimated Glomerular Filtration Rate (eGFR) and renal function test for assessing renal function were included in the cross - sectional study. Based on eGFR and albuminuria, patients were grouped into Low Risk (LR), Moderately increased Risk (MR), High Risk (HR) and Very High Risk (VHR) categories as per KDIGO classification. Demographic, anthropometric, and biochemical details were recorded accordingly.
Results: Among the study population (n=3426) 61.4% were in LR group followed by 17.2 % in MR, 14.3% in HR and 7.2% in VHR group respectively. Among different risk categories, significant difference (p<0.001) was observed among LR, MR, HR and VHR group in age, duration of diabetes, systolic and diastolic blood pressure, HbA1c, urea, creatinine and eGFR. eGFR significantly declined as the risk categories in KDIGO increased. Among the study population, 27.32% (n = 936) had different degree of DR. Significant difference in eGFR was observed among patients with and without DR. As the severity of the DR increases, renal risk categories based on KDIGO classification also increased. High concordance was observed between different degrees of DR and KDIGO classification.
Conclusion: In T2DM subjects, there is a significant association between DR and renal risk category using KDIGO classification. Thus, KDIGO classification can be used to stratify the renal and retinal risk in T2DM than mere albuminuria or eGFR.
2.Introduction
3.Research Design and Methods
3.1 Statistical Methods
4.Results
5.Discussion & Conclusion
6.Acknowledgements
7.References
Keywords
KDIGO; Creatinine; eGFR; DR; T2DM.
Introduction
Worldwide Diabetic nephropathy (DN) is considered as a leading cause of chronic renal failure and it is also estimated that nearly 20 % of the patients with Type 2 Diabetes Mellitus (T2DM) reach End Stage Renal Disease (ESRD) during their lifetime [1]. DN is the significant long term complication with respect to morbidity and mortality for the patients with diabetes. Estimated glomerular filtration rate (eGFR) is traditionally considered as the best marker for overall function of the kidney and is therefore used to classify patients into different stages of Chronic Kidney Disease (CKD). Earlier studies have emphasized the importance of albuminuria, in predicting the outcomes and it is a key marker for kidney damage due to glomerular permselectivity barrier [2, 3]. The Kidney Disease Outcomes Quality Initiative (KDOQI) classification of CKD was based only on the eGFR [4]. The realization that albuminuria is an independent predictor for end stage renal disease and cardiovascular mortality and morbidity, leads to the inclusion of the categories of albuminuria in the new classification scheme. The new classification of CKD, Kidney Disease Improving Global Outcome (KDIGO) guideline is based on both eGFR and albuminuria category [5].
In the south Indian population the prevalence of macroalbuminuria and microalbuminuria was 6.9%, and 2.5% respectively [6]. Among T2DM patients the common micro vascular complications are DN, diabetic neuropathy and Diabetic Retinopathy (DR) [7]. In India, data on the prevalence of DR among general population was limited. All Type 1 Diabetes children and two third of T2DM patients were expected to develop DR over a period of time [8-10]. A population-based study in India showed that the prevalence of overt nephropathy and microalbuminuria was 2.2 and 26.9% [11]. A recent Indian study showed, 21.7% prevalence of DR among T2DM [12]. The prevalence of DR among diabetic population in rural South India was 10.5% [13] and urban Chennai was 17.6% [14]. Earlier studies showed high prevalence of proteinuria among patients with proliferative retinopathy [15] and further it was also reported that DR is more severe in patients with severe DN. Overt albuminuria in the T2DM appears to be a powerful predictor of DR [16].
KDIGO based CKD classification depends on eGFR and albuminuria categories, which provides the major prognostic information, and also influences the treatment plan. Both albuminuria and eGFR provides independent information on the progression of the risk of CKD, cardiovascular disease and mortality. Different colour codes in KDIGO classification, indicates the risk based on the degree of albuminuria and the levels of eGFR. Earlier studies showed that there exist a close relationship between the development and the progression of DR and DN. However there is a paucity of data which highlights the relationship between these two complications using KDIGO classification [17] among Indian T2DM population. Against this background, this cross sectional study was aimed to stratify the renal risk using eGFR and albuminuria based on Kidney Disease Improving Global Outcome (KDIGO) classification and its association with the degree of DR among people with Type 2 Diabetes in South India.
Research Design and Methods
The cross sectional study was conducted among T2DM patients, who attended the outpatient Diabetes clinic during the study period of June 2015 to November 2015 in a tertiary care hospital in India. All patients included in the study had undergone Fundus Photography (FFP) and Fundus Fluorescent Angiography (FFA) in certain cases decided by the ophthalmologist for DR as a part of their diabetes work up and eGFR and albumin creatinine ratio for assessing renal function. The study population included all patients aged above 25 years and were screened for laboratory investigations, medications, demographic and anthropometric details. All the subjects were on treatment with oral hypoglycemic agents, some were on insulin, and few were known hypertensive’s and were on antihypertensive medication. Patients with Type 1 Diabetes and gestational diabetes and those with incomplete laboratory data were excluded from the study.
Demographic, anthropometric details such as age, gender, duration of diabetes, family history of diabetes were recorded. Body Mass Index (BMI) was calculated. Biochemical parameters such as fasting and postprandial glucose, HbA1c, lipid profile, urea, creatinine, urinary protein and urinary albumin values were recorded. All biochemical parameters were estimated using BS400 biochemistry auto analyzer, HbA1c was measured using HPLC method using variant turbo equipment (Bio-Rad). Creatinine was estimated by Jaffe’s kinetic method, urinary albumin was estimated by immunoturbidimetric procedure. Urinary protein was determined using pyrogallol method.
The eGFR was calculated based on CKD - EPI equation, which gives the best estimation of GFR. This equation was developed in 2009 [18] and intended to be more generalizable across various clinical settings. The CKD-EPI equation expressed as a single equation as:
eGFR= 141 × min ((Scr/к), 1)α × max((Scr/к), 1)-1.209 × 0.993Age × 1.018 [if female] × 1.159 [if black]
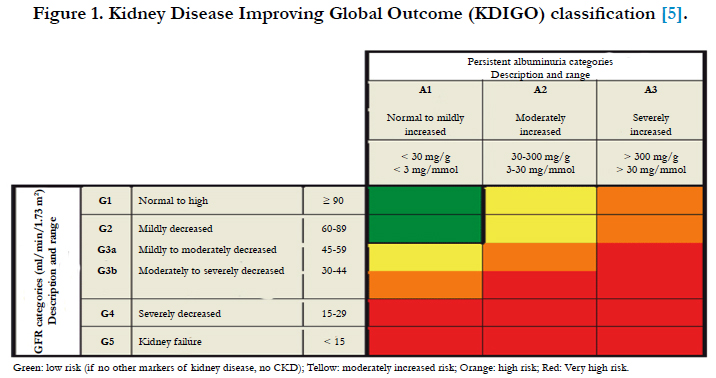
Study population was grouped into Low Risk (LR) (Green colour), Moderate Risk (MR) (Yellow colour), High Risk (HR) (orange colour) and Very High Risk (VHR) (red colour) categories according to KDIGO classification, based on eGFR and urinary albumin values. LR includes, A1G1, A1G2, MR includes A1G3a, A2G1, A2G2, HR includes A1G3b, A2G3a, A3G1, A3G2, VHR includes, A1G4, A1G5, A2G3b, A2G4, A2G5, A3G3a, A3G3b, A3G4, A3G5 accordingly (5) (cf. Figure 1).
Figure 1. Kidney Disease Improving Global Outcome (KDIGO) classification [5].
Through trained ophthalmologist retinal examination was performed in all patients who were included in the study. Fundus Photography was taken to document DR followed by FFA to confirm findings in those cases as advised by the ophthalmologist. Classification of DR was based on evidence based approach of Early Treatment Diabetic Retinopathy Study (ETDRS) [19]. Retinopathy was classified as non-proliferative (NPDR) (microaneurysms, intra-retinal hemorrhages, hard exudates without new vessels) or proliferative (PDR) (newly formed blood vessels and/or growth of fibrous tissue into the vitreous cavity or scars of photocoagulation). Subjects were divided into groups based on the severity of DR as Mild NPDR, Moderate NPDR, Severe NPDR, early PDR and High risk PDR. The study was approved by the Institutional Research committee.
Data was analyzed using SPSS (version 16.0, Illinois, USA) software. Mean and standard deviation for continuous variables and percentages for categorical variables are reported accordingly. Chi-square test, ANOVA or t-test were performed as applicable for comparing the variables between different groups, and a P value < 0.05 was considered to be statistically significant. To measure the association of the ordinal variables (Severity of DR and different study groups [LR, MR, HR and VHR] based on KDIGO classification), Kendall's tau-c correlation coefficient test was performed to show the probability of concordance minus the probability of discordance pairs.
Results
A total of 3426 (M: F, 2193:1233) patients were included in the study with mean age of 52.7 years and mean duration of diabetes of 13.8 years. A total of 3426 subjects were grouped into LR, MR, HR and VHR according to eGFR value and albuminuria based on KDIGO classification. Among the study population 61.4% were in LR group [A1 G1 - 25.16%; A1G2 - 36.22%]; followed by 17.2 % in MR [A2G1 - 4.87% ; A2G2 - 8.61%; A1G3a - 3.70%]; 14.3% in HR [A3G1 - 3.76%; A3G2 - 7.85%; A2G3a - 2.072; A1G3b - 0.58%] and 7.2% in VHR [A3G3a - 2.86%; A2G3b - 0.81%; A3G3b - 1.92% ; A1G4 - 0.145; A2G4 -0.08% ; A3G4 - 0.93%; A1G5 - 0; A2G5 - 0; A3G5 - 0.379%] group respectively.
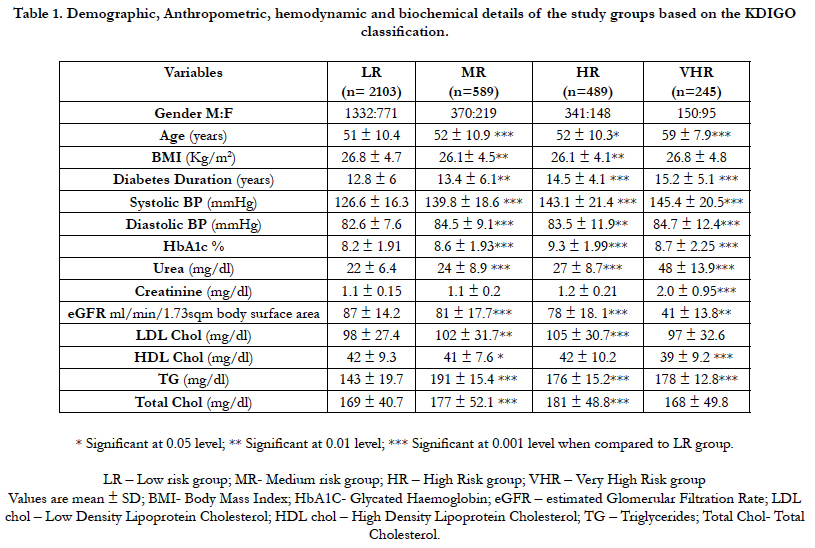
Table 1 shows the demographic, anthropometric and clinical details of the study groups as per the grades of KDIGO classification. Among these four groups (LR, MR, HR, VHR), significant difference was observed in age, duration of diabetes, systolic and diastolic blood pressure, HbA1c, urea, creatinine, eGFR, LDL chol, HDL chol, TG and Total Chol levels. When comparing the MR vs HR group Statistical significance (p<0.001) was observed in DM duration, HbA1c, Urea, eGFR and TG respectively. In comparing HR vs VHR groups significant difference (p<0.001) was observed in age, BMI, duration of diabetes, systolic BP, HbA1c, Urea, creatinine, eGFR, LDL Chol, HDL Chol, TG and Total cholesterol. There was a significant decreasing trend in eGFR with increase in the risk category (LR [87 ± 14.19]; MR [81 ± 17.78]; HR [78 ± 18.11] and VHR [41 ± 13.81]) (Table 1).
Table 1. Demographic, Anthropometric, hemodynamic and biochemical details of the study groups based on the KDIGO classification.
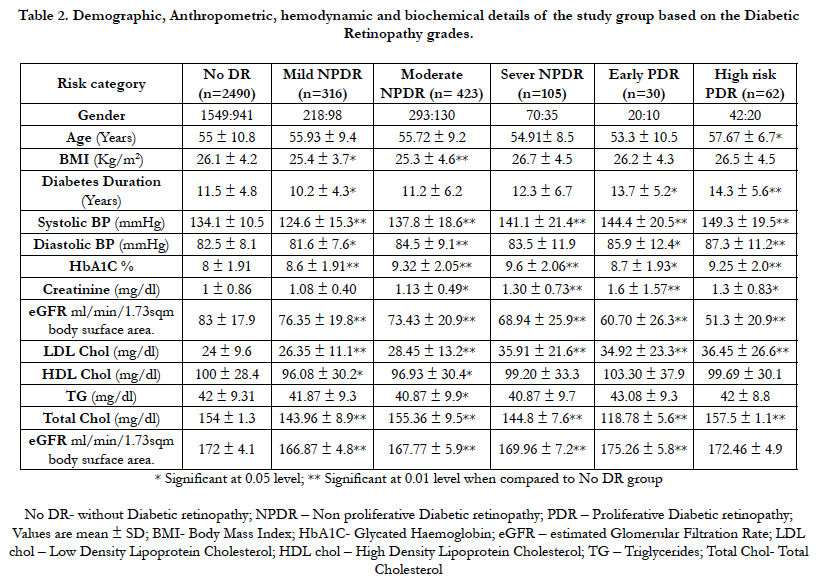
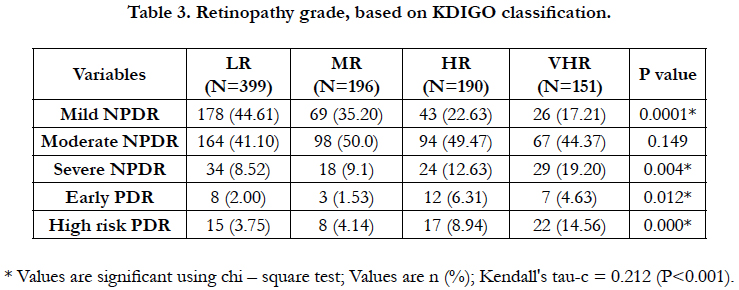
Out of 3426 patients, 27.3% (n = 936) had Diabetic Retinopathy. As the severity of the DR increased, the levels of creatinine, urea increased and eGFR reduced gradually. Likewise, as the severity of the DR increases, the number of patients decreased with decreasing eGFR (Table 2). Significant statistical association was observed between different degrees of KDIGO risk categories and different degrees of DR such as mild NPDR (P<0.001), severe NPDR (P<0.004), early PDR (P<0.012) and high risk PDR (P<0.001) respectively (Table 3). In LR category majority of the patients had mild NPDR, whereas in MR, HR and VHR category majority of the patients had moderate NPDR. As the renal risk category increased the presence of PDR also increased. The Kendall’s tau (0.0212), showed significant (P<0.001) concordance between DR and diabetic nephropathy based on KDIGO classification; suggests that there is a significant positive association between different degrees of DR and different stages of CKD based on KDIGO classification. Thus the findings highlighted that there was a significant association between the different degrees of DR with the degree of CKD based on KDIGO classification.
Table 2. Demographic, Anthropometric, hemodynamic and biochemical details of the study group based on the Diabetic Retinopathy grades.
Discussion & Conclusion
Diabetes mellitus is the commonest metabolic disorder and its prevalence is high in India. It is evident that the patients with diabetes are at the high risk of developing diabetic micro vascular complications [20]. Few studies show the association of Diabetic nephropathy and DR among T2DM and the severity of renal impairment is associated with the severity of DR [21, 22]. Progression of retinopathy and development of nephropathy, both increases the risk for incidence of each other [23]. The current study findings showed an association between the different degrees of DR and different degree of Diabetic nephropathy based on KDIGO classification in south Indian T2DM population.
HbA1c level was higher in HR group when compared to other groups, similar finding was found in the earlier studies, which highlighted that the risk of progression of nephropathy increases significantly with HbA1c variability [24, 25]. It was reported that approximately one fifth of the people with diabetes are affected by multiple complications and the frequency increases with increasing age and duration of diabetes [26, 27]. The study subjects in the present study were older with long duration of diabetes, implying that increased age and diabetes duration were responsible for the development of the DR and nephropathy complication.
Evidence suggests a strong correlation between DR and DN, and a potential role for DR in predicting DN among T2DM patients with renal disease [28, 29]. T2DM patients with proliferative DR more often showed renal involvement, such as urinary albumin excretion [30]. In the study population the mean eGFR was found to be decreased significantly with increasing severity of DR, which is in line with the previous studies. However the present study findings were based on KDIGO classification, whereas the findings of the earlier studies were not based on the KDIGO classification. Furthermore unlike other studies CKDEPI formula was used to calculate the eGFR. Thus the findings highlighted that there was a significant association between the different degrees of Diabetic retinopathy with the degree of CKD based on KDIGO classification and the declining eGFR was also found to be associated with the different stages of DR.
The comparison of the different degree of CKD according to KDIGO classification, which is based on eGFR and albumin, was the major strength of the study. The cross sectional nature of the study was the major limitation, thus a prospective follow up study has to be carried out to find the relationship between renal and retinal involvement. A prospective longitudinal study focused on the comprehensive interventions is recommended based on the risk factors and its association with the progression from moderate risk category to higher risk categories need to be evaluated further. The present study highlights the need for screening the patients periodically to look for diabetic complications. All physicians treating diabetes patients at all levels, including primary care setting should be aware of the risk factor of diabetic complications and also educate the patients about the potential risk factors. The periodical evsaluation of the renal status should be recommended, in the presence of DR or PDR. In conclusion, this cross sectional observation, in South Indian population with T2DM has focused on stratifying the renal risk using KDIGO classification instead of merely looking at eGFR or albuminuria. It also revealed a positive association between the DR and different degree of CKD according to KDIGO classification.
Acknowledgement
We thank Mr. Selvakumar D, IT Department, for help in data collection and Mr. Sriram in statistical help.
References
- Ayodele OE, Alebiosu CO, Salako BL (2004) Diabetic nephropathy--a review of the natural history, burden, risk factors and treatment. J Natl Med assoc. 96(11): 1445.
- Hemmelgarn BR, Manns BJ, Lloyd A, James MT, Klarenbach S, et al., (2010) Relation between kidney function, proteinuria, and adverse outcomes. Jama. 303(5): 423-429.
- Astor BC, Hallan SI, Miller ER, Yeung E, Coresh J (2008) Glomerular filtration rate, albuminuria, and risk of cardiovascular and all-cause mortality in the US population. Am j epidemiol. 167(10): 1226-1234.
- National KF (2002) K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am j kidney dis. 39(2): S1.
- Kidney Disease: Improving Global Outcomes Work Group T (2013) KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney intl Suppl. 2013(3): 1–150.
- Mohan V, Meera R, Premalatha G, Deepa R, Miranda P, et al., (2000) Frequency of proteinuria in type 2 diabetes mellitus seen at a diabetes centre in southern India. Postgrad med j. 76(899): 569-573.
- West KM, Erdreich LJ, Stober JA (1980) A detailed study of risk factors for retinopathy and nephropathy in diabetes. Diabetes. 29(7): 501-508.
- Wild S, Roglic G, Green A, Sicree R, King H (2004) Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 27(5): 1047–1053.
- World Health Organization (2006) Prevention of blindness from diabetes mellitus: report of a WHO consultation in Geneva, Switzerland, 9-11 November 2005.
- Guidelines for the Comprehensive Management of Diabetic Retinopathy in India. A VISION 2020: The Right to Sight INDIA Publication, July 2008.
- Unnikrishnan R, Rema M, Pradeepa R, Deepa M, Shanthirani CS, et al., (2007) Prevalence and risk factors of diabetic nephropathy in an urban south Indian population: the chennai Urban rural epidemiology study(CURE S 45). Diabetes care. 30(8): 2019-2024.
- Gadkari SS, Maskati QB, Nayak BK (2016) Prevalence of diabetic retinopathy in India: The all India ophthalmological society diabetic retinopathy eye screening study 2014. Indian J ophthalmol. 64(1): 38.
- Nirmalan PK, Katz J, Robin AL, Tielsch JM, Namperumalsamy P, et al., (2004) Prevalence of Vitreoretinal Disorders in a Rural Population of Southern India: The Aravind Comprehensive Eye Study. Arch ophthalmol. 122(4): 581-586.
- Rema M, Premkumar S, Anitha B, Deepa R, Pradeepa R, et al., (2005) Prevalence of diabetic retinopathy in urban India: the Chennai Urban Rural Epidemiology Study (CURES) eye study, I. Invest ophthalmol vis sci. 46(7): 2328-2333.
- Kornerup T (1958) Retinopathia diabetica proliferans. Acta ophthalmologica. 36(1); 87-101.
- Estacio RO, McFarling E, Biggerstaff S, Jeffers BW, Johnson D, e al., (1998) Overt albuminuria predicts diabetic retinopathy in Hispanics with NIDDM. Am j kidney dis. 31(6): 947-953.
- Viswanathan, V, Kumpatla S, Tilak P, Kuppusamy A (2013) Relationship Between Retinal-Renal Complications Among Type 2 Diabetic Subjects in India. Int J Diabetol Vasc Dis Res. 1(2): 8-14.
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, et al., (2009) A new equation to estimate glomerular filtration rate. Ann intern med. 150(9): 604-612.
- Wilkinson CP, Ferris FL, Klein RE, Lee PP, Agardh CD, et al., (2003) Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmol. 110(9): 1677-1682.
- American Diabetes Association. (2003) Economic costs of diabetes in the US in 2002. Diabetes care. 26(3): 917-932.
- Rani PK, Raman R, Gupta A, Pal SS, Kulothungan V, et al., (2011) Albuminuria and diabetic retinopathy in type 2 diabetes mellitus sankara nethralaya diabetic retinopathy epidemiology and molecular genetic study (SNDREAMS, report 12). Diabetol metab syndr. 3(1): 9.
- Looker HC, Krakoff J, Knowler WC, Bennett PH, Klein R, et al., (2003) Longitudinal studies of incidence and progression of diabetic retinopathy assessed by retinal photography in Pima Indians. Diabetes Care. 26(2): 320-326.
- Kramer CK, Retnakaran R (2013) Concordance of retinopathy and nephropathy over time in Type 1 diabetes: an analysis of data from the Diabetes Control and Complications Trial. Diabet Med. 30(11): 1333-1341.
- Hernandez D, Espejo-Gil A, Bernal-Lopez MR, Mancera-Romero J, Baca-Osorio AJ, et al., (2013) Association of HbA1c and cardiovascular and renal disease in an adult Mediterranean population. BMC nephrol. 14(1): 151.
- Rodríguez‐Segade S, Rodríguez J, García López JM, Casanueva FF, Camiña, F (2012) Intrapersonal HbA1c variability and the risk of progression of nephropathy in patients with Type 2 diabetes. Diabetic Med. 29(12): 1562-1566.
- Morgan C, Currie CJ, Stott NCH, Smithers M, Butler CC, et al., (2000) The prevalence of multiple diabetes‐related complications. Diabet med. 17(2): 146-151.
- Sue Kirkman M, Briscoe VJ, Clark N, Florez H, Haas LB, et al., (2012) Diabetes in older adults: a consensus report. J Am Geriatr Soc. 60(12): 2342- 2356.
- Kotlarsky P, Bolotin A, Dorfman K, Knyazer B, Lifshitz T, Levy J (2015) Link between retinopathy and nephropathy caused by complications of diabetes mellitus type 2. Intl ophthalmol. 35(1): 59-66.
- He F, Xia X, Wu XF, Yu XQ, Huang FX (2013) Diabetic retinopathy in predicting diabetic nephropathy in patients with type 2 diabetes and renal disease: a meta-analysis. Diabetologie. 56(3): 457-466.
- Boelter MC, Gross JL, Canani LH, Costa LA, Lisboa HR, et al., (2006) Proliferative diabetic retinopathy is associated with microalbuminuria in patients with type 2 diabetes. Braz j med biol res. 39(8): 1033-1039.