Behaviour of Streptococcus mutans on Enamel Surface Coated with Initial Biofilm, In Vivo
Arzu Erol1,2*
1 Clinic of Operative Dentistry, Periodontology and Preventive Dentistry, Saarland, Germany (host).
2 Molecular Biology and Genetic, Faculty of Science, Bulent Ecevit University, Zonguldak, Turkey.
*Corresponding Author
Arzu Erol,
Molecular Biology and Genetic, Faculty of Science,
Bulent Ecevit University, Zonguldak, Turkey.
Tel/Fax: 00905320688394
E-mail: erol.arzu@yahoo.com
Received: April 22, 2017; Accepted: May 23, 2017; Published: May 30, 2017
Citation: Arzu Erol (2017) Behaviour of Streptococcus mutans on Enamel Surface Coated with Initial Biofilm, In Vivo. Int J Dentistry Oral Sci. S7:002, 8-14. doi: dx.doi.org/10.19070/2377-8075-SI070002
Copyright: Arzu Erol© 2017. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Adherence of oral microorganisms to the surface of dental materials is viewed as an essential stride in the advancement of auxiliary caries and periodontal illness. This research was to look at the underlying bacterial attachment on enamel surface in vivo. The point of this analysis was to explore and think about the adherence of various Streptococcus mutans to initial biofilm on enamel surface until 48h keeping in mind the end goal to discover conceivable contrasts.
Bovine enamel samples were incubated with two different Streptococcus mutans as UA159 and ATCC25175 for 2h, 4h, 6h, 24h, 48h. There were two negative controls, the samples incubated in saliva and bacterial culture without any Streptococcus mutans. Other their case, first enamel samples incubated with mutans in BHI medium, second enamel samples were incubated with mutans in sterile saliva and the last samples coated with human saliva proteins then incubated with mutans in BHI medium. The adherent microorganisms were evaluated and envisioned utilizing checking electron microscopy (SEM) and live/dead recolouring.
The lowest bacterial count of Streptococcus mutans (UA159) was detected on initial biofilm on enamel 4h and the highest bacterial count of Streptococcus mutans (UA159) 24h. Uncoated enamel samples surface exhibited no anti-adhesive properties in BHI with UA159. Further improvement of the surface characteristics is essential for Streptococcus mutans. Streptococcus mutans (ATCC25175) were detected to release the secretion 6h on initial biofilm, while Streptococcus mutans (ATCC25175) were adhesive force. Compared to different Streptococcus mutans; coated and uncoated enamel in BHI and saliva exhibited various adhesive properties.
2.Introduction
3.Materials and Methods
3.1 Preparation of Specimens
3.2 Production of Dentinal Specimens
3.3 Bacterial Growth Condition
3.4 Saliva Disinfection
3.5 Assessment of Bacterial Adhesion
3.6 SEM Analysis
3.7 Mechanism of BacLightTMviability Assay
4.Results
5.Conclusion
6.Acknowledgements
7.References
Keywords
Initial Biofilm; Bacterial Adhesion; Live/Dead Staining, SEM, Streptococcus mutans.
Introduction
Oral bacterial biofilms remain one of the greatest challenges in dental research [13]. Despite extensive research, there is still a strong demand for fundamental investigation of bacterial adherence to dental surfaces and research of coated enamel surface with initial biofilm, in vivo and in situ [8, 10, 11].
Microbial group creating on the interfaces between strong surfaces and organic liquids produce biofilms. The initial biofilm is vital importance for bacterial adherence. Biofilms comprise of a grid of exopolysaccharides, proteins, and nucleic acids [9, 14]. A considerable measure of infections in the oral hole, for example, caries, stomatitis, periodontal, and peri-embed maladies, are connected with biofilms [17]. Test and clinical reviews exhibited that peri-embed and periodontal tissues respond also to oral biofilms [2, 6, 12, 16]. Likewise, the expulsion of natural pollution from dental and embed surfaces is a standout amongst the most essential elements influencing the mending of periodontal and peri-embed tissues (Mombelli et al., 1992).
Streptococci alone frame the greatest relationship in the oral cavity [3]. S.mutans produces an extracellular polysaccharide from sucrose which causes dental caries. This extracellular substance has α (1-3) glucose linkage which helps in the connection of the bacterium. Moreover, this polysaccharide points in providing vitality amid insufficiency of any unessential sugar. S. mutans is not just the fundamental bacterium occupied with the improvement of plaque additionally for the initiation of dental caries [18]. Past beginning adherence, it gives the idea that an assortment of qualities is required for the adjustment of S. mutans and other oral streptococci in biofilms. Cells existing in the biofilm have phenotypic attributes, which are unmistakable from those of their planktonic partners, presumably went with critical changes in the examples of quality expression (Costerton, 1987; [19]).
The results of the study show that initial biofilm on enamel of S. mutans differ from their planktonic counterparts and the strains of S. mutans tested behave somewhat differently.
Non-damaged bovine permanent lower incisors of 2-y old cattle were used in this study. The crowns of the freshly extracted bovine teeth were separated from the root at the cementumenamel junction under water cooling using a diamond cutting disc (Schott Diamantwerkzeuge GmbH, Stadtoldendorf, Germany), which was conducted with a saw (Conrad Apparatebau Clausthal GmbH, Clausthal-Zellerfeld, Germany). Then the roots and pulp tissues were carefully discarded. From each crown, samples with a surface area size of 4 x 4 mm2 (for BacLighTMviability assay and for SEM) were cut from the middle third of the labial surfaces in order to standardize the test pieces. The samples were then stored in 0.1% thymol solution at 4°C.
To expose the sound dentin, the enamel was ground away under permanent water cooling by a polishing machine (Buehler, Düsseldorf, Germany), using silicon carbide grinding paper (P600-P2.500, FEPA-P, waterproof silicon carbide paper, Buehler, Düsseldorf, Germany). Then the dentinal surface was ground plan-parallelly and polished by wet grinding with abrasive paper down to P-grit size of ‘4.000’ (according to Federation of European Producers of Abrasives (FEPA) standard, mean grain size = 5 μm). The samples were 1 mm thick. Subsequently, a light-microscopic examination (Motic Deutschland GmbH, Wetzlar, Germany) with 10-fold magnification was performed on the surface area of each dentinal specimen. Samples with any microscopically visible discolorations, demineralization or surface in homogeneity were discarded and excluded from the study.
According to Hannig et al., [7], pre-treatment with NaOCl and disinfection with 70% ethanol was adopted. Polished dentinal specimens (n = 240) were firstly cleaned using a 3% NaOCl (Hedinger, Stuttgart, Germany) solution for 30 s to remove any residues from the polishing procedure. Next, the specimens were washed five times in distilled water (Ecotainer; B. Braun Melsungen AG, Melsungen, Germany), followed by disinfection in 70% ethanol (Hedinger, Stuttgart, Germany) for 20 min and finished with another five washes in double distilled water. Before expriment, rehydration of specimens took place by storage in distilled water for 24 h.
A strain of Streptococcus mutans (UA159 and ATCC25175) was obtained from the Culture Collection of the University of Saarland and used for the in vitro adhesion tests. S. mutans was cultured in Brain Heart Infusion (BHI, Difco, CA, USA) supplemented with 10% (v/v) heat-inactivated horse blood serum to improve its growth. The culture of S. mutans was statically incubated for 16 hours at 37°C under aerobic conditions. This culture, used as source for the experiments, was reduced at a finaldensity of 1*1010 cells/mL as determined by comparing the OD600 of the sample with a standard curve relating OD600 to cell number.
Saliva was gathered from solid female volunteer who was not on any solution. The Subject did not brush their teeth for no less than 2h before accumulation and in a similar period they didn't expend any hard sustenance or treat that could possibly prompt to gashes of the oral mucosa or the gums. None of the volunteer had gingivitis on the other hand periodontitis. For all the gathered specimens, no undeniable sullying of gathered spit with hints of blood was watched.
Saliva were pooled together and centrifuged at 2,600 g for 10 minutes to turn down extensive garbage and eukaryotic cells. The supernatant was alluded to as pooled salivation and utilized all through this review and after that sifted through a 0.22 μm pore low-protein restricting channel and solidified at - 20°C until utilize. Spit was defrosted quickly before utilize and centrifuged at 1430 × g for 5 min to evacuate any hasten that may have shaped amid solidifying and defrosting. Sans cell spit was likewise utilized for covering veneer tests preceding becoming the biofilms.
After extensive washing of each samples, 100 μL of an overnight growth culture (107 bacteria/mL) was seeded onto each sample test placed at the bottom of a 6-well plate and incubated in static conditions at 37°C for 2h, 4h, 6h, 24h and 48 hours. The choice of this time of incubation is due to the fact that biofilm formation in the oral cavity normally occurs in 2 to 4 hours. After incubation, loosely adhering bacteria were removed by gently washing the samples tests with PBS. The same experiment was performed with the control.
The samples with fixed biofilm were dehydrated in an ascending series of ethanol from 50% to 100% and then dried in hexamethyldisilazane (HMDS, [(CH3)3Si]2NH) solution (98.5%, ABCR GmbH & Co. KG, Karlsruhe, Germany) overnight. After critical point drying, specimens were sputtered and coated by a thin layer of gold-palladium (SC 7640 High Resolution Sputter Coater, Quorum Technologies Ltd., U.K.).
Dentinal specimens were examined by a scanning electron microscope XL 30 ESEM FEG (FEI, Eindhoven, The Netherlands) operating at 5 kV under a magnification ranging from 2.000 - to 20.000-fold to investigate the surface and fractured surface with the purpose of a thorough description of bacterial adherence. The settings for the scanning electron microscope, such as tilt angle, spot size, scanning mode, were kept constant for all samples.
LIVE/DEAD BacLightTMBacterial Viability Kit (Art. No. L7012, Invitrogen, Molecular probes, Eugene, Oregon, USA) was used to distinguish and quantify live and dead bacteria in bacterial population, based on the membrane integrity of cells. Cells with a compromised membrane that are considered to be dead were stained red, whereas cells with an intact membrane were stained green. Two dyes, SYTO 9 green-fluorescent nucleic acid stain and propidium iodide (C27H34I2Nv4, PI), the red-fluorescent nucleic acid stain, were utilized. These two dyes differ both in their spectral characteristics and in their ability to penetrate healthy bacterial cells. When used alone, the SYTO 9 stains all bacteria in a population, whereas PI penetrates only bacteria with compromised membranes. When both dyes are used, PI reduces the SYTO 9 dye fluorescence. A clear and reliable distinction of the bacterial viability can be easily achieved with an appropriate mixture of SYTO 9 and propidium iodide stains. The total number of adherent bacteria and the correlation between the viable (green) and damaged (rot) bacteria can hence be evaluated [4].
Results
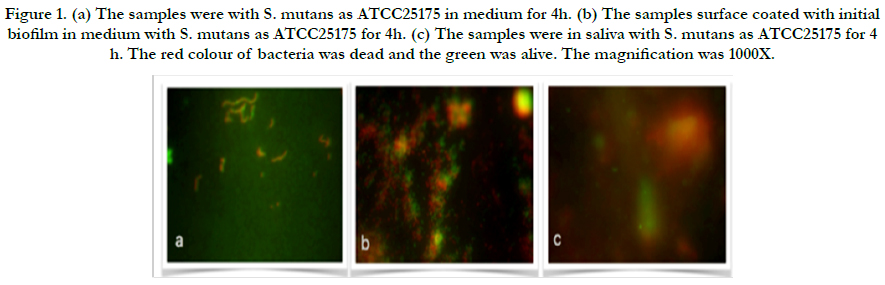
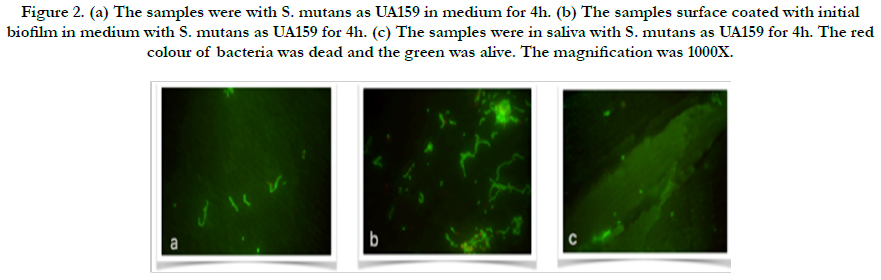
In the present study, adherent Streptococcus mutans on bovine enamel were visualized successfully using SEM and Florescence microscope after exo-oral incubated for 2-48 h. All bacterial species (UA159 and ATCC25175) tested were detectable on bovine enamel samples exposed to the aseptic saliva which collected from the same subject for initial biofilm composition. Representative images of the initial bacterial colonization for each type for first 4h are given in Figure 1-2. A high level of variability was recorded between each species. While single bacteria as well as monolayered (Figure 1a) chains or multi-layered (Figure 1b) and three-dimensional aggregates of bacteria were observed for ATCC25175, confirming the semi-planktonic nature of bacterial populations on coated with initial biofilm enamel surface. It is understood that the proportions of dead and live bacteria are close to each other. However, only single bacteria (Figure 2a and b) were observed in the UA159 strain. It is confirmed that the initial biofilm structure facilitates bacterial adhesion. S. mutans were not seen in the samples without medium in both species, show in Figure 1c and 2c.
Figure 1. (a) The samples were with S. mutans as ATCC25175 in medium for 4h. (b) The samples surface coated with initial biofilm in medium with S. mutans as ATCC25175 for 4h. (c) The samples were in saliva with S. mutans as ATCC25175 for 4 h. The red colour of bacteria was dead and the green was alive. The magnification was 1000X.
Figure 2. (a) The samples were with S. mutans as UA159 in medium for 4h. (b) The samples surface coated with initial biofilm in medium with S. mutans as UA159 for 4h. (c) The samples were in saliva with S. mutans as UA159 for 4h. The red colour of bacteria was dead and the green was alive. The magnification was 1000X.
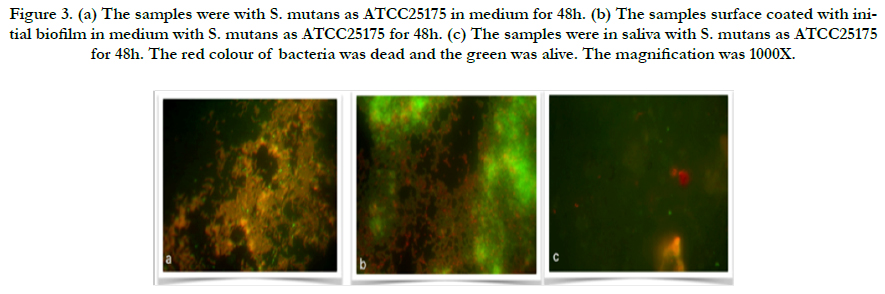
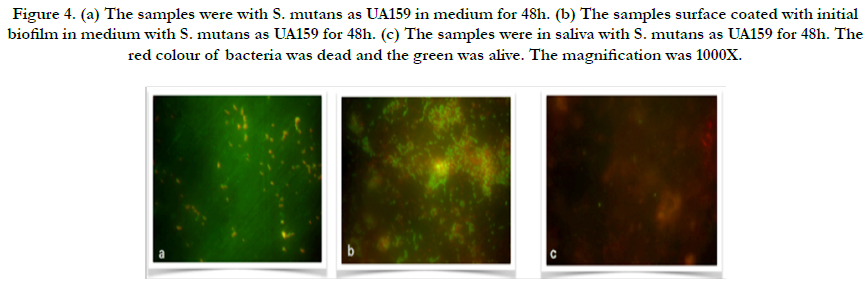
Representative images of the initial bacterial colonization for each type for first 48h are given in Figure 3-4. A high level of variability was recorded between each species. Compared to initial biofilm-coated and non-coated surface, ATCC25175 strains that proliferated in the same manner after 48 hours appear to have a higher proportion of living bacteria on initial biofilm-coated surfaces (Figure 3a and b), confirms that the initial biofilm-coated structure helps keep bacteria on the surface easier, as well as help them stay alive. The single bacteria as well as monolayered (Figure 4a) and more than chains of bacteria (Figure 4b) were observed for UA159, which confirms that the mutans of lives on initial biofilm-covered surfaces. S. mutans were not seen in the samples without medium in both species, show in Figure 3c and 4c.
Figure 3. (a) The samples were with S. mutans as ATCC25175 in medium for 48h. (b) The samples surface coated with initial biofilm in medium with S. mutans as ATCC25175 for 48h. (c) The samples were in saliva with S. mutans as ATCC25175 for 48h. The red colour of bacteria was dead and the green was alive. The magnification was 1000X.
Figure 4. (a) The samples were with S. mutans as UA159 in medium for 48h. (b) The samples surface coated with initial biofilm in medium with S. mutans as UA159 for 48h. (c) The samples were in saliva with S. mutans as UA159 for 48h. The red colour of bacteria was dead and the green was alive. The magnification was 1000X.
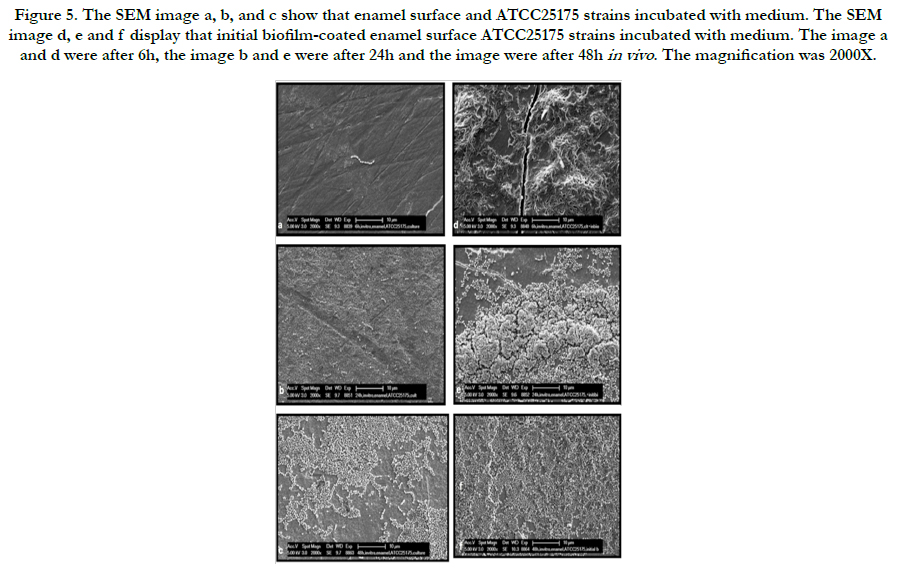
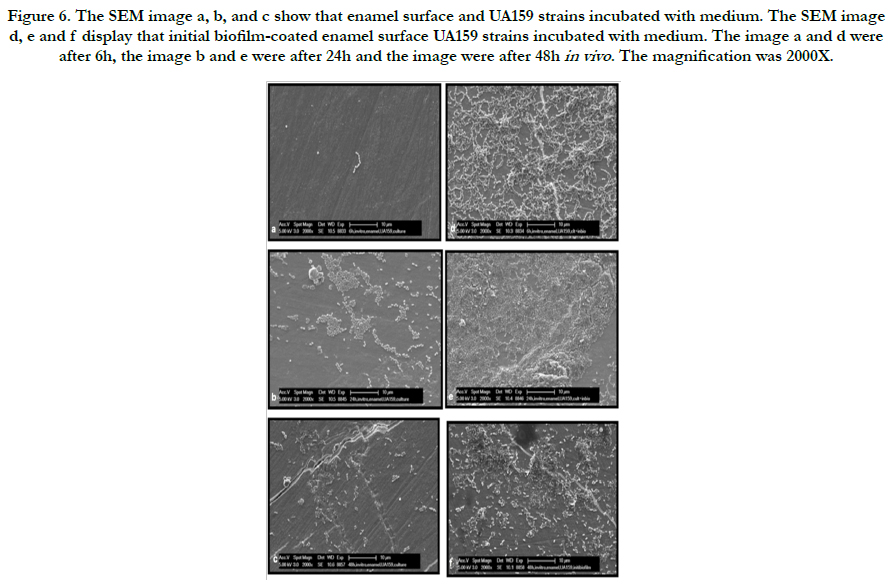
Representative electron microscopic micrographs for each strains are given in Figure 4-6. The SEM micrograph shows complete initial biofilm-coated of the enamel surface by a bacterial monolayer with several multi-layered micro-colonies between 6 and 48 hours for ATCC25175 strains (Figure 5 d, e and f). It is observed that the bacteria adherence was less in the samples of enamel surface that were not covered with initial biofilm (Figure 5 a, b and c). The Figure 6 represent that compared to the initial biofilm-coated and non-coated surfaces, initial biofilm-coated enamel surface has a greater UA159 adhesion and proliferation than enamel surfaces at 6, 24 and 48 hours.
Figure 5. The SEM image a, b, and c show that enamel surface and ATCC25175 strains incubated with medium. The SEM image d, e and f display that initial biofilm-coated enamel surface ATCC25175 strains incubated with medium. The image a and d were after 6h, the image b and e were after 24h and the image were after 48h in vivo . The magnification was 2000X.
Figure 6. The SEM image a, b, and c show that enamel surface and UA159 strains incubated with medium. The SEM image d, e and f display that initial biofilm-coated enamel surface UA159 strains incubated with medium. The image a and d were after 6h, the image b and e were after 24h and the image were after 48h in vivo . The magnification was 2000X.
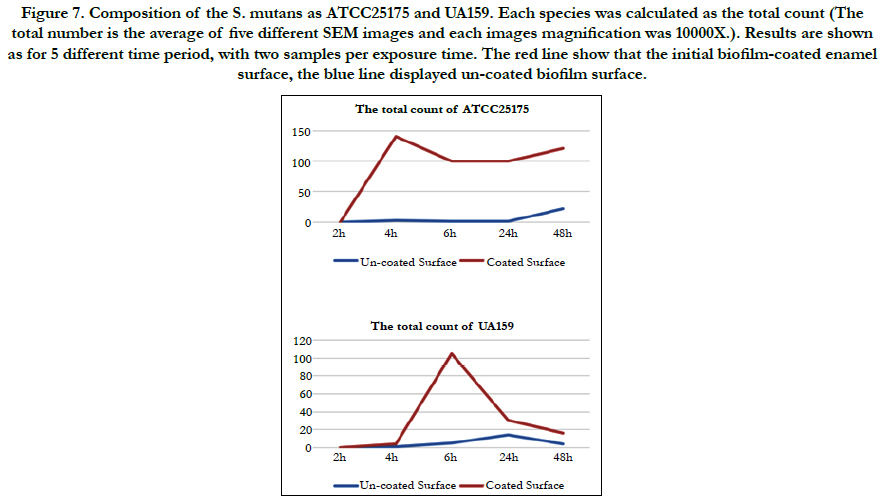
In this study, the covering grade of the substratum was calculated. Although it is of great relevance, this parameter is often neglected in studies concerning the oral biofilm [1]. When the bacterial counts for both species are calculated, ATCC 25175 appears to have reached the highest number after 48 hours on the uncoated surface, with the coated surface showing a surface that could not be counted after 4 hours. (Bacteria were counted by hand as the numbers were few.) Looking at UA159, it is observed that at the end of 24 hours the number reached to the highest number on the uncoated surface and decreased in the number of hours (48h). On the coated surface; It seems to cover the surface until it could not be counted in the 6th hour, and the number is rapidly decreasing in the following hours. Compared to the number of these two mutans, ATCC 25175 species showed a great increase in the biofilm structure (Figure 7).
Figure 7. Composition of the S. mutans as ATCC25175 and UA159. Each species was calculated as the total count (The total number is the average of five different SEM images and each images magnification was 10000X.). Results are shown as for 5 different time period, with two samples per exposure time. The red line show that the initial biofilm-coated enamel surface, the blue line displayed un-coated biofilm surface.
The behaviour of the ATCC 25175 mutant appears to be maximal at 6th hour (Figure 8). On the initial biofilm-coated enamel surface, the release agent was clearly visible for attachment to the surface or for a different purpose. Nevertheless, when look closely at the uncoated enamel surface, there were no difference compared to previous times. When look at the behaviour of the mutant at 24 hours, see the areas of attachment that we have established with the surface on the initial biofilm-coated surface. The effect of the ATCC 25175 mutant causing decay; initial biofilm proteins (saliva proteins) have a structure that positively affects the adhesion to the surface. ATCC 25175 has an advantageous genetic difference(Zhuang P., 2016). When the UA159 mutation is examined at high magnification, the Figure 8 shows that the biofilm structure does not provide this mutant vital advantage, as surface morphology does not have a different morphology.
Figure 8. The high magnification images of ATCC 25175 and UA159, at 6 hours and 24 hours, in viv o. The magnification was 2000X. time period was at 6h and 24h. the surfaces were un-coated surface as raw enamel surface and coated surface as enamel surface coated with initial biofilm. The samples Streptococcus mutans were ATCC25175 and UA159.
Conclusion
Whether enamel surfaces were not covered by initial biofilm, there were very few proliferations of both species on enamel surface. Coating the surface with biofilm causes the streptococcus mutans to cling to the surface and cause damage to the disease and caries there. The greatest factor in the surface damage of Streptococcus mutans were the covering of the enamel surface with saliva.
This study demonstrated that the ATCC25175 mutant effectively increased in the presence of initial biofilm on the surface. In contrast, UA159 mutant increased in maximum amount in the first six hours on the biofilm-coated surface. Later it lost its effect. This means that when mutans were compared, the ATCC25175 mutant was more effective in natural mouth flora.
Although it is known that both mutans are associated with tooth decay, there is not much genetic information in their rights such as genomic sequence, proteins synthesize and attachment proteins. This information gives, the genome which makes both mutans different, will be effective in elucidating the structure of membrane proteins that facilitate attachment on the initial biofilm.
Acknowledgment
This work was conducted under the supervision of a Prof. M. Hannig at Clinic of Operative Dentistry, Periodontology and Preventive Dentistry, Saarland.
References
- Al-Ahmad A, Follo M, Selzer A, Hellwig E, Hannig M, et al., (2009) Bacterial colonization of enamel in situ investigated using fluorescence in situ hybridization. J Med Microbiol. 58(10): 1359–1366.
- Berglundh T, Lindhe J, Marinello C, Ericsson I, Liljenberg B (1992) Soft tissue reaction to de novo plaque formation on implants and teeth. An experimental study in the dog. Clin Oral Implants Res. 3(1): 1-8.
- Bhatia R, Ichhpujani RL (2003) Microbiology for Dental Students. (3rd edn), Jaypee Brothers.
- Boulos L, Prevost M, Barbeau B, Coallier J, Desjardins R, et al., (1999) LIVE/DEAD Backlight: application of a new rapid staning method for direct enumeration of viable and total bacteria in drinking water. J Microbiol Methods. 37(1): 77-86.
- Costerton JW (1995) Overview of microbial biofilms. J Ind Microbiol. 15(3): 137-140.
- Ericsson I, Berglundh T, Marinello C, Liljenberg B, Lindhe J (1992) Longstanding plaque and gingivitis at implants and teeth in the dog. Clin Oral Implants Res. 3(3): 99-103.
- Hannig C, Hannig M, Attin T (2005) Enzymes in the acquired enamel pellicle. Eur J Oral Sci. 113(1): 2–13.
- Hannig C, Hannig M, Rehmer O, Braun G, Hellwig E, et al., (2007a) Fluorescence microscopic visualization and quantification of initial bacterial colonization on enamel in situ. Arch Oral Biol. 52(11): 1048–1056.
- Hannig C, Spitzmu¨ ller B, Hannig M (2009) Characterization of lysozyme activity in the in situ pellicle using a fluorimetric assay. Clin Oral Investig. 13(1): 15–21.
- Hannig C, Spitzmu¨ ller B, Al-Ahmad A, Hannig M (2008a) Effects of Cistus-tea on bacterial colonization and enzyme activities of the in situ pellicle. J Dent. 36(7): 540–545.
- Hannig M, Kriener L, Hoth-Hannig W, Schmidt H (2007b) Influence of nano-composite surface coating on biofilm formation in situ. J Nanosci Nanotechnol. 7(12): 4642–4648.
- Lindhe J, Meyle J (2008) Peri-implant diseases: Consensus Report of the sixth European Workshop on Periodontology. J Clin Periodontol. 35(8): 282-285.
- Marsh PD, Bradshaw DJ (1995) Dental plaque as a biofilm. J Ind Microbiol. 15(3): 169–175.
- Marsh PD (2003) Are dental diseases examples of ecological catastrophes? Microbiology. 149(2): 279-294.
- Mombelli A, Marxer M, Gaberthüel T, Grander U, Lang NP (1995) The microbiota of osseointegrated implants in patients with a history of periodontal disease. J Clin Periodontol. 22(2): 124–130.
- Pontoriero R, Tonelli MP, Carnevale G, Mombelli A, Nyman SR, et al., (1994) Experimentally induced peri-implant mucositis. A clinical study in humans. Clin Oral Implants Res. 5(4): 254-259.
- Renvert S, Samuelsson E, Lindahl C, Persson GR (2009) Mechanical nonsurgical treatment of peri-implantitis: a double-blind randomized longitudinal clinical study. I: Clinical results. J Clin Periodontol. 36(7): 604-609.
- Todar K (2015) The Normal Bacterial Flora of Humans. Online textbook of Bacteriology.
- Whiteley M, Bangera MG, Bumgarner RE, Parsek MR, Teitzel GM, et al., (2001) Gene expression in Pseudomonas aeruginosa biofilms. Nature . 413(6858): 860-864.