Long - Term Effects of Rapid Maxillary Expansion on Upper Airway Structures
Tai Ching PS, Savoldi F, Tsoi JKH, Gu M*
Orthodontics, Faculty of Dentistry, The University of Hong Kong, Hong Kong S.A.R., P.R. China.
*Corresponding Author
Dr. Gu Min,
Orthodontics, 2/F, Prince Philip Dental Hospital,
34 Hospital Road, Hong Kong S.A.R., P.R. China.
Tel: +852 2859 0258
E-mail: drgumin@hku.hk
Received: August 27, 2018; Accepted: October 22, 2018; Published: October 22, 2018
Citation:Tai Ching PS, Savoldi F, Tsoi JKH, Gu M. Long - Term Effects of Rapid Maxillary Expansion on Upper Airway Structures. Int J Dentistry Oral Sci. 2018;S1:02:003:12-17. doi: dx.doi.org/10.19070/2377-8075-SI02-01003
Copyright: Gu M©2018. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
2.Introduction
3.Rapid Maxillary Expansion (RME) Appliance
4.Incidence and Pathophysiology of Obstructive Sleep Apnoea (OSA)
5 Anatomy of Upper Airway Structures
6 Diagnostic Methods on Upper Airway Changes
7 Discussion
7.1 Literatures on The Effects of RME on Upper Airway Structures
7.2 The Effects on Airway and Nasal Breathing
7.3 Effects on Paediatric Obstructive Sleep Apnoea
7.4 Effects on Dental and Hyoid Bone and Tongue Posture
7.5 Future Scope
5.Conclusion
6.References
Abbreviations
OSA: Obstructive Sleep Apnea; RME: Rapid Maxillary Expansion; RPE: Rapid Palatal Expansion. SDB: Sleep-Disordered Breathing; PSG: Polysomnography; CBCT: Cone Beam Computed Tomography; CT: Computed Tomography; MRI: Magnetic Resonance Imaging; AHI: Apnoea-Hypopnoea Index.
Introduction
Maxillary expansion is the routine treatment modality for treating patients with transverse maxillary deficiency [1]. It is also proposed to treat paediatric obstructive sleep apnea (OSA), which is characterized by narrowing or obstruction of upper airways. The two terms rapid maxillary expansion (RME) and rapid palatal expansion (RPE) are often confused and used interchangeably. However, they are technically referred to two different treatments. RME is referred to expansion of the entire maxilla surgically, thus, RPE is expanding the palate only using a tooth- or tooth-tissueborne palatal expander [2]. The distribution forces that generated by RPE indeed do extend to the entire maxilla and the contributory effect on the suture at sites distant from the palate, therefore all expansion treatments will be referred to as RME in this study.
The goal of RME is to enlarge the airway and reduce its collapsibility, resulting in a more anteriorly positioned tongue in the oral cavity [3]. Evaluation of the upper airway structures and its changes after treatment have become important diagnostic tests for both clinical and research purposes. The purpose of this article is to review the literatures and to investigate the long-term effects of RME on upper airway structures, airway and nasal breathing, paediatric obstructive sleep apnea, and a brief discussion on dental, hyoid bone and tongue posture.
Rapid Maxillary Expansion (RME) Appliance
RME is routinely used in orthodontics to treat children with transverse maxillary deficiency. It is also an emerging treatment modality in management of paediatric OSA [4, 5]. Angell described the first clinical use of RME in the 1860s [6]. It is a nonsurgical maxillary expansion technique commonly used for the correction of maxillary width deficiency and posterior crossbite by increasing the width of dental arch [7]. It opens the midpalatal suture and employs a buccal rotational force on the maxillary alveolar shelves [8, 9].
Maxillary expansion is proposed to create positive effects on increasing the airway volume and hence the tongue will be positioned more anteriorly in the oral cavity [3]. There are growing number of studies suggested that RME can be considered for patients not only with OSA but also with nose breathing, nocturnal enuresis and conductive hearing loss [10, 11].
Incidence and Pathophysiology of Obstructive Sleep Apnoea (OSA)
OSA is a common morbidity affecting approximately 2-3% of children and its highest incidence occurs between 2 and 8 years of age [12]. Transverse maxillary deficiency not only results in occlusal disharmony but it is also associated with a vast number of craniofacial abnormalities, which predispose a person to have narrowing of upper airway dimensions. These craniofacial abnormalities include retrognathic mandible, shorter anteroposterior face length, reduced distance from the posterior nasal spine to the posterior pharyngeal wall, lower position of the hyoid bone, larger soft palate, smaller pharynx, larger tongue, obesity and combinations of these have been recognized as part of the pathophysiology of OSA [13]. Maxillary constriction associated with low tongue posture that may contribute to the oropharynx airway narrowing which may also play a role in the pathophysiology of OSA [14].
Anatomy of Upper Airway Structures
The upper airway structures refer to the ‘pharynx and its surrounding structures’, including the nose, nasal cavity, sinuses, larynx and upper trachea [15-17]. The pharynx is a muscular tube posterior to the nasal cavity, oral cavity and larynx, and anterior to the cervical vertebrae. It situates from the skull base to the lower border of the cricoid cartilage [15, 16]. The pharynx can be divided into three segments along its path: the nasopharynx, oropharynx, laryngopharynx (hypopharynx) [18]. The oropharynx airway is subdivided into the retropalatal (from the level of the hard palate to the caudal margin of the soft palate) and retroglossal (from the caudal margin of the soft palate to the base of epiglottis) regions [19]. It is suggested that potential narrowing or obstruction of the upper airway can contribute to OSA or sleepdisordered breathing (SDB).
Diagnostic Methods on Upper Airway Changes
Different diagnostic modalities including acoustic rhinometry, polysomnography (PSG), two-dimensional radiographic, threedimensional imaging technique such as cone beam computed tomography (CBCT), computed tomography (CT) and magnetic resonance imaging (MRI) have been used to reveal the upper airway changes of RME [20]. The International Classification of Sleep Disorders describes that the diagnosis of OSA involves specific questionnaires, clinical history, physical examination and classical PSG [21]. The gold standard for diagnosing OSA is yet the PSG examination. However, the cephalometric radiograph is traditionally used as a screening tool to evaluate the airway obstruction and craniofacial structures in Orthodontics [22, 23]. The well-known limitations of a 3D object being projected onto a 2D plane: magnification distortion, geometric distortion, superimposed structures, and inconsistent head position during imaging [24]. CBCT has become a popular method to assess the airway patency not only because of its significantly lower radiation than medical CT, but it provides a higher contrast between the hard and soft tissues, greater spatial resolution than medical CT, lower cost and easier access and availability to dentists [23, 25, 26]. However, the complexity of the 3D anatomy, the superimposition of bilateral structures as well as magnification differences and difficulties in landmark identification limits the accuracy of an evaluation. Other functional measurement methods such as PSG, rhinomanometry, acoustic rhinometry could provide an objective information on the respiratory status and quantify the airway patency for comparison before and after RME treatment.
Apart from the amount of maxillary width increase [27, 28], it has been well accepted in the literature that RME has other beneficial effects on upper airway structures. These include the reduction of nasal resistance [1, 29, 30], increase in intranasal capacity [31- 35], increase in pharyngeal airway volume [3, 36], raises tongue posture [37] and changes in hyoid bone position [34]. However, there are studies demonstrating controversial results, which will be discussed further.
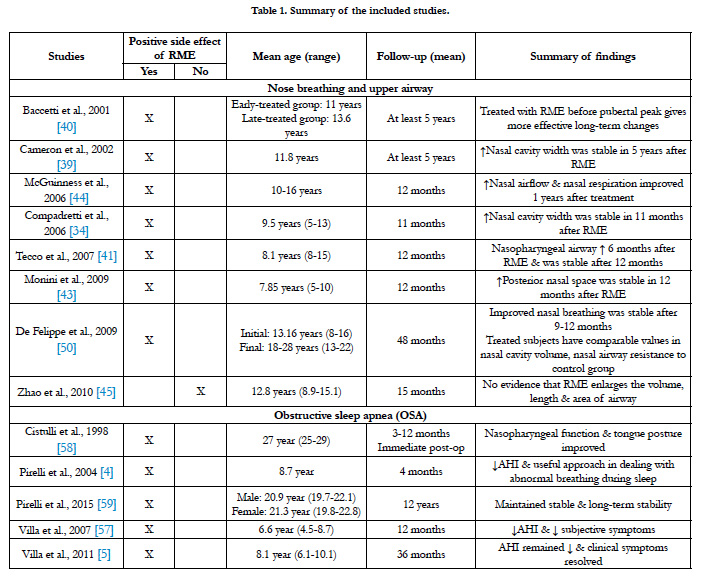
Baratieri et al., [38] demonstrated the evidence of long-term effects of RME on airway dimensions and functions in their systematic review. There were only eight articles which met their inclusion criteria and were of moderate methodological quality. In these studies, airway dimensions were either measured twodimensionally using posteroanterior or lateral cephalometric radiographs, or three-dimensionally using a CBCT. Cameron et al., [39] found a significant enlargement in nasal cavity width which remained stable for five years after RME. Baccetti et al., [40] indicated a larger increase in the early treatment group (cervical vertebral stage 1-3) compared to the late treatment group (cervical vertebral stage 4-6). This study recommended RME treatment before the pubertal peak can achieve more effective long-term changes. There is also a significant increase in nasopharyngeal airway adequacy showed in other studies [41-43] using a two-dimensional measurement method. However, two of these studies did not include a control group. McGuinness and McDonald investigated the cranio-cervical angle on lateral radiographs and found that there is an increase in nasal airflow and improvement in nasal respiration one year after maxillary expansion [44].
Zhao et al., [45] had taken CBCT images of patients at a supine position and elucidated that the retropalatal airway was significantly smaller in children with constricted maxilla compared to controls. In contrast to the previous two-dimensional findings, they concluded that there were no significant differences in airway volume between RME treated and control group. This result is consistent to Malkoç et al., [46], which evaluated the pharyngeal airway dimensions using lateral and posteroanterior cephalometric radiographs. They reported that RME does not clinically significantly affect pharyngeal airway dimensions. Their hypothesis is that RME might be counteracted by reflex mechanisms, which acts to preserve the airway patency.
It also remains controversial whether a lateral cephalometric radiograph should be taken in an upright or a supine position to screen for airway obstruction. However, it is suggested that the state of consciousness might be a more important factor affecting upper airway muscle tone rather than the head position [47]. In addition, Zhao et al., [45] did not control the subjects’ tongue positions and the nasal ventilation conditions when the CBCT images were taken. Tongue posture is an important anatomic factor that affects the shape and size of oropharyngeal airway volume. Iwasaki et al., [3] had asked the subjects to hold their breath while taking the CBCT images to ensure a static pharyngeal airway size. Maxillary constriction with nasal obstruction can cause low tongue posture resulting in retroglossal narrowing [48]. Iwasaki et al., [3] concluded that RME not only improves the lower tongue posture, but also eases the nasal obstruction and enlarges the pharyngeal airway. It can also increase the intraoral volume to improve the lowered tongue posture in patient with OSA. Nasal volume was shown to be increased and consequently increasing the nasal permeability and improving the nasal respiration after RME [49]. RME has been demonstrated to increase the retropalatal volume, nasal cavity dimension, improve breathing, and has been also theorized to produce change in the upper airway dimensions [32].
Functional measurement methods such as rhinomanometry and acoustic rhinometry showed improvement in nasal breathing that remained stable after RME [34]. De Felippe et al., [50] revealed improvement in nasal breathing for the treated group and the values in nasal cavity volume and nasal cavity resistance were comparable to the control group which did not undergo any treatment. Baratieri et al., [38] concluded in their systematic review that there is a low to moderate evidence that RME improves the conditions for nasal breathing and that the results can be expected to be stable for at least 11 months. In a recent systematic review and metaanalysis study, Buck et al., [51] investigated the volumetric changes in the upper airway spaces following RME in growing subjects by means of acoustic rhinometry, three-dimensional radiography and digital photogrammetry. RME seems to be associated with an increase in the nasal cavity volume in the short and long term. However, the overall quality of evidence was judged to be very low. Caprioglio et al., [20] using PSG examination indicated a significant increase in all functional parameters (such as, AHI and peripheral capillary oxygen saturation - SpO2), but this study showed no correlation when compared it with the increased airway volume, which was measured using CBCT. Because of the uncertainties in these literatures, RME is not advisable to treat patients having breathing problems with normal developed maxillary arches.
Many of the children with OSA is between 2 to 8 years of age [12]. This is the period when children have the high potential of rapid growth. Because the relative size of lymphoid tissue to airway diameter is smaller in children, the prevalence of OSA could be increased with rising obesity in children and adolescents. Factors like adenotonsillar hypertrophy or obesity, and upper airway neuromotor tone can cause imbalances between upper airway structural load, thus leading to upper airway collapse during sleep in OSA patients [52]. Untreated OSA can lead to a significant health burden for patients. It is associated with a number of significant morbidities such as growth failure, systemic and pulmonary hypertension, and endothelial dysfunction [52-54]. Contrary to popular belief, a meta-analysis of the current literature demonstrated that paediatric sleep apnoea is often not cured by tonsillectomy and adenoidectomy [55]. A success rate of 66.3% indicates that a large number of children have residual disease. Although complete resolution is not achieved in most cases, tonsillectomy and adenoidectomy still offer significant improvements in AHI, making it a valuable first-line treatment for paediatric OSA. Villa et al., [56] determined that both RME and adenotonsillectomy displayed significant decrease in AHI and hence both treatments help improve OSA. Villa et al., [57] elucidated the impact of RME on OSA children with high and narrow palate. They found a significant decrease in the AHI, hypopnea obstructive index, arousal index and a decrease of the subjective symptoms in the 12-months follow-up. In the 3-year follow-up, the hypopnea index remained stable and the clinical symptoms resolved [5]. However, the studies done by Villa et al., [57] only included treatment group of 14 and 10 patients respectively without comparing with a control group. These studies represent a low level of evidence and must be considered with caution. Other studies [4, 58] reported a general improvement of the nasopharyngeal function and a new tongue posture. Pirelli et al., [4] followed thirty-one children with OSA and reviewed them up to 4 months after RME treatment. All of them had a decreased AHI with a mean maxillary cross-sectional widths expanded to about 4.5mm. In their recent 12-year follow-up study, Pirelli et al., [59] evaluated the long-term efficacy of RME in these thirty-one patients using PSGs and CT images. They demonstrated a longterm stability of RME in these patients. When the RME opens the midpalatal suture, the lateral wall of nasal cavity also displays laterally and thus increases the nasal volume and decreases the upper airway resistance. This increase in intranasal cavity after RME might be the explanation for the increase in total pharyngeal and retropalatal airway volumes in children treated with RME [3, 36]. However, because of low patient numbers and missing control groups, these studies represent a low level of evidence.
Lagravère et al., [60] conducted a meta-analysis on the immediate changes of RME and found a number of dental and skeletal improvements. However, the author provided very little detail of the methods they used, such as how the data was extracted for the review, or how many reviewers performed the extraction. Again, these studies represent a low level of evidence.
Seto et al., [48] suggested that a constricted maxillary arch and a low hyoid position are the characteristics of OSA. There is a relationship between low hyoid bone, long tongues, OSA and snoring in both adults and children. Hyoid bone normally descends during maturation. Phoenix et al., [37] evaluated cephalograms of RME and control group (138 and 148 patients, respectively) and suggested that RME tends to normalize hyoid bone position, which has its clinical importance as the hyoid bone is an anatomic structure that could be easily identified on lateral cephalograms.
RME is predominantly recommended to treat young patients at the developmental age until the closure of midpalatal suture. Few studies have showed improvement on OSA using RME not only in children and adolescents but also in adult patients [58, 61]. However, different kinds of treatment approaches such as surgically assisted RME or segmental Le Fort I surgery have been suggested as the midpalatal suture has closed in adults [62]. It would be interesting to explore the long-term effects on upper airway structures in these treated adult patients with OSA.
It is recently postulated that ankyloglossia could be a risk factor for maxillary hypoplasia [63]. Restricted tongue mobility was associated with narrowing of the maxillary arch and elongation of the soft palate. These findings suggested that variations in tongue mobility may affect maxillofacial development [63]. Thus there might be a relationship between ankyloglossia in paediatric OSA. More studies are required to elucidate the role of ankyloglossia in the etiology of transverse maxillary deficiency, the correlation of ankyloglossia in paediatric OSA patients, the efficacy of treating ankyloglossia in children with transverse maxillary deficiency and the long-term effects of RME in upper airway structures of these children.
Adenoids and palatine tonsils hypertrophy is common in paediatric OSA. Therefore, data indicating airway enlargement by RME of children with these problems will be useful. Future studies should also take CBCT in supine position during sleep to match the usual clinical examination with the consideration of the tongue posture. More studies evaluating actual respiratory status are needed in the future. Most studies compared with the results taken during the first 3-4 months review period and recorded them immediately after expansion was completed to isolate the effects of RME on upper airway structures. Few studies demonstrated a long-term follow-up of RME treatment on dental, skeletal and upper airway structures. Furthermore, more studies investigating on the long-term effects and stability of RME on the upper airway structures will be beneficial for our clinical understanding and the prevalence of relapse throughout life and the extent of relapse should be studied. Thus, this information may be useful for the clinicians to ensure that RME is accurately prescribed to patients with certain abnormalities in upper airway and skeletal structures.
Conclusion
It has been well recognized in the literature that RME induces a number of positive influences on upper airway structures. Thus, it might aid in improving the quality of life in children at high risk for OSA particularly those with maxillary constriction. As there are only a few studies with high level evidence on the long-term effects of RME on upper airway structures and the impact of RME on general health, their results must be considered with caution. Further longitudinal studies with larger sample sizes including the breathing evaluation would be necessary to estimate the real effects of RME on the upper airway. It should be taken in consideration that there are technical limitations in any radiology-based craniofacial morphology studies in both 2D or 3D approach, due to the structural complexity of a human skull in a living subject. Following the universally accepted ‘as low as reasonably achievable’ principle, it is not possible to take annual or semi-annual 3D radiographs for ethical reasons on the same subject to answer any research questions regarding craniofacial growth.
References
- Schmidt-Nowara W, Lowe A, Wiegand L, Cartwright R, Perez-Guerra F, Menn S. Oral appliances for the treatment of snoring and obstructive sleep apnea: a review. Sleep. 1995 Jul;18(6):501-10. PubMed PMID: 7481421.
- Kapila SD. Cone beam computed tomography in orthodontics: Indications, insights, and innovations. John Wiley & Sons; 2014 Aug 29.
- Iwasaki T, Saitoh I, Takemoto Y, Inada E, Kakuno E, Kanomi R, et al. Tongue posture improvement and pharyngeal airway enlargement as secondary effects of rapid maxillary expansion: a cone-beam computed tomography study. Am J Orthod Dentofacial Orthop. 2013 Feb;143(2):235-45. doi: 10.1016/j.ajodo.2012.09.014. PubMed PMID: 23374931.
- Pirelli P, Saponara M, Guilleminault C. Rapid maxillary expansion in children with obstructive sleep apnea syndrome. Sleep. 2004 Jun 15;27(4):761- 6. PubMed PMID: 15283012.
- Villa MP, Rizzoli A, Miano S, Malagola C. Efficacy of rapid maxillary expansion in children with obstructive sleep apnea syndrome: 36 months of follow-up. Sleep Breath. 2011 May;15(2):179-84. doi: 10.1007/s11325-011-0505-1. PubMed PMID: 21437777.
- Angell EC. Treatment of irregularities of the permanent or adult teeth. Dent. Cosmos. 1860;1:599 - 600.
- Haas AJ. Palatal expansion: just the beginning of dentofacial orthopedics. Am J Orthod. 1970 Mar;57(3):219-55. PubMed PMID: 5263785.
- Lee H, Ting K, Nelson M, Sun N, Sung SJ. Maxillary expansion in customized finite element method models. Am J Orthod Dentofacial Orthop. 2009 Sep;136(3):367-74. doi: 10.1016/j.ajodo.2008.08.023. PubMed PMID: 19732671.
- Gautam P, Valiathan A, Adhikari R. Stress and displacement patterns in the craniofacial skeleton with rapid maxillary expansion: a finite element method study. Am J Orthod Dentofacial Orthop. 2007 Jul;132(1):5.e1-11. PubMed PMID: 17628242.
- Eichenberger M, Baumgartner S. The impact of rapid palatal expansion on children’s general health: a literature review. Eur J Paediatr Dent. 2014 Mar;15(1):67-71. PubMed PMID: 24745597.
- Fagundes NC, Rabello NM, Maia LC, Normando D, Mello KC. Can rapid maxillary expansion cause auditory improvement in children and adolescents with hearing loss? A systematic review. Angle Orthod. 2017 Nov;87(6):886-896. doi: 10.2319/021517-111.1. PubMed PMID: 28885035.
- Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008 Feb 15;5(2):242-52. doi: 10.1513/pats.200708- 135MG. PubMed PMID: 18250218.
- McCrillis JM, Haskell J, Haskell BS, Brammer M, Chenin D, Scarfe WC, et al. Obstructive sleep apnea and the use of cone beam computed tomography in airway imaging: A review. Semin Orthod; 2009. p. 63–9.
- Subtelny JD. The significance of adenoid tissue in orthodontia. Angle Orthod. 1954;24:59-69.
- Gu M, McGrath CP. Hägg U, Wong RWK, Yang Y. Anatomy of the upper airway and its growth in childhood. J Dent Oral Biol. 2016;1:1005.
- Scanlon VC, Sanders T. Essentials of anatomy and physiology. 6 th ed. Philadelphia: F.A. Davis Company; 2003.
- Shier D, Butler J, Lewis R. Hole’s human anatomy & physiology. 12 th ed. New York: McGraw-Hill; 2010.
- Kheirandish-Gozal L, Gozal D. Sleep disordered breathing in children: a comprehensive clinic guide to evaluation and treatment. New York: Humana Press; 2012.
- Schwab RJ. Upper airway imaging. Clin Chest Med. 1998;19:33-54. PubMed PMID: 9554216.
- Caprioglio A, Meneghel M, Fastuca R, Zecca PA, Nucera R, Nosetti L. Rapid maxillary expansion in growing patients: correspondence between 3-dimensional airway changes and polysomnography. Int J Pediatr Otorhinolaryngol. 2014 Jan;78(1):23-7. doi: 10.1016/j.ijporl.2013.10.011. PubMed PMID: 24231036.
- American Academy of Sleep Medicine. The International Classification of Sleep Disorders: diagnostic and coding manual. 2nd ed. USA: American Academy of Sleep Medicine; 2005. p. 1-297.
- Major MP, Flores-Mir C, Major PW. Assessment of lateral cephalometric diagnosis of adenoid hypertrophy and posterior upper airway obstruction: a systematic review. Am J Orthod Dentofacial Orthop. 2006;130:700-8. PubMed PMID: 17169731.
- Pirila-Parkkinen K, Lopponen H, Nieminen P, Tolonen U, Paakko E, Pirttiniemi P. Validity of upper airway assessment in children: a clinical, cephalometric, and MRI study. Angle Orthod. 2011 May;81(3):433-9. doi: 10.2319/063010-362.1. PubMed PMID: 21261486.
- Malkoc S, Usumez S, Nur M, Donaghy CE: Reproducibility of airway dimensions and tongue and hyoid positions on lateral cephalograms. Am J Orthod Dentofacial Orthop. 2005 Oct;128(4):513-6. PubMed PMID: 16214635.
- Tso HH, Lee JS, Huang JC, Maki K, Hatcher D, Miller AJ: Evaluation of the human airway using cone-beam computerized tomography. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009 Nov;108(5):768-76. PubMed PMID: 19716716.
- Ludlow JB, Davies-Ludlow LE, Brooks SL. Dosimetry of two extraoral direct digital imaging devices: NewTom cone beam CT and Orthophos Plus DS panoramic unit. Dentomaxillofac Radiol. 2003 Jul;32(4):229-34. PubMed PMID: 13679353.
- Haas AJ. The Treatment of Maxillary Deficiency by Opening the Midpalatal Suture. Angle Orthod. 1965 Jul;35:200-17. PubMed PMID: 14331020.
- Biederman W. A hygienic appliance for rapid expansion. JPO J Pract Orthod. 1968 Feb;2(2):67-70. PubMed PMID: 5241062.
- Hershey HG, Stewart BL, Warren DW. Changes in nasal airway resistance associated with rapid maxillary expansion. Am J Orthod. 1976 Mar;69(3):274-84. PubMed PMID: 766643.
- Hartgerink DV, Vig PS, Abbott DW. The effect of rapid maxillary expansion on nasal airway resistance. Am J Orthod Dentofacial Orthop. 1987 Nov;92(5):381-9. PubMed PMID: 2445196.
- Hilberg O, Jackson AC, Swift DL, Pedersen OF. Acoustic rhinometry: evaluation of nasal cavity geometry by acoustic reflection. J Appl Physiol (1985). 1989 Jan;66(1):295-303. PubMed PMID: 2917933.
- Enoki C, Valera FC, Lessa FC, Elias AM, Matsumoto MA, Anselmo-Lima WT. Effect of rapid maxillary expansion on the dimension of the nasal cavity and on nasal air resistance. Int J Pediatr Otorhinolaryngol. 2006 Jul;70(7):1225-30. PubMed PMID: 16497390.
- Bicakci AA, Agar U, Sokucu O, Babacan H, Doruk C. Nasal airway changes due to rapid maxillary expansion timing. Angle Orthod. 2005 Jan;75(1):1-6. PubMed PMID: 15747808.
- Ceroni Compadretti G, Tasca I, Alessandri-Bonetti G, Peri S, D'Addario A. Acoustic rhinometric measurements in children undergoing rapid maxillary expansion. Int J Pediatr Otorhinolaryngol. 2006 Jan;70(1):27-34. PubMed PMID: 15955568.
- da Silva Filho OG, Boas MC, Capelozza Filho L. Rapid maxillary expansion in the primary and mixed dentitions: a cephalometric evaluation. Am J Orthod Dentofacial Orthop. 1991 Aug;100(2):171-9. PubMed PMID: 1867168.
- Chang Y, Koenig LJ, Pruszynski JE, Bradley TG, Bosio JA, Liu D. Dimensional changes of upper airway after rapid maxillary expansion: a prospective cone-beam computed tomography study. Am J Orthod Dentofacial Orthop. 2013 Apr;143(4):462-70. PubMed PMID: 23561406.
- Phoenix A, Valiathan M, Nelson S, Strohl KP, Hans M. Changes in hyoid bone position following rapid maxillary expansion in adolescents. Angle Orthod. 2011 Jul;81(4):632-8. PubMed PMID: 21306225.
- Baratieri C, Alves M, Jr, de Souza MM, de Souza Araujo MT, Maia LC. Does rapid maxillary expansion have long-term effects on airway dimensions and breathing? Am J Orthod Dentofacial Orthop. 2011 Aug;140(2):146-56. PubMed PMID: 21803251.
- Cameron CG, Franchi L, Baccetti T, McNamara JA, Jr. Long-term effects of rapid maxillary expansion: a posteroanterior cephalometric evaluation. Am J Orthod Dentofacial Orthop. 2002 Feb;121(2):129-35. PubMed PMID: 11840125.
- Baccetti T, Franchi L, Cameron CG, McNamara JA Jr. Treatment timing for rapid maxillary expansion. Angle Orthod. 2001 Oct;71(5):343-50. PubMed PMID: 11605867.
- Tecco S, Caputi S, Festa F. Evaluation of cervical posture following palatal expansion: a 12-month follow-up controlled study. Eur J Orthod. 2007 Feb;29(1):45-51. PubMed PMID: 16957058.
- Compadretti GC, Tasca I, Bonetti GA. Nasal airway measurements in children treated by rapid maxillary expansion. Am J Rhinol. 2006 Jul-Aug;20(4):385-93. PubMed PMID: 16955765.
- Monini S, Malagola C, Villa MP, Tripodi C, Tarentini S, Malagnino I,et al.Rapid maxillary expansion for the treatment of nasal obstruction in children younger than 12 years. Arch Otolaryngol Head Neck Surg. 2009 Jan;135(1):22-7. PubMed PMID: 19153303.
- McGuinness NJ, McDonald JP. Changes in natural head position observed immediately and one year after rapid maxillary expansion. Eur J Orthod. 2006 Apr;28(2):126-34. PubMed PMID: 16157633.
- Zhao Y, Nguyen M, Gohl E, Mah JK, Sameshima G, Enciso R. Oropharyngeal airway changes after rapid palatal expansion evaluated with cone-beam computed tomography. Am J Orthod Dentofacial Orthop. 2010 Apr;137(4 Suppl):S71-8. PubMed PMID: 20381764.
- Malkoc S, Usumez S, Iseri H. Long-term effects of symphyseal distraction and rapid maxillary expansion on pharyngeal airway dimensions, tongue, and hyoid position. Am J Orthod Dentofacial Orthop. 2007 Dec;132(6):769-75. PubMed PMID: 18068595.
- Pracharktam N, Hans MG, Strohl KP, Redline S. Upright and supine cephalometric evaluation of obstructive sleep apnea syndrome and snoring subjects. Angle Orthod. 1994;64(1):63-73. PubMed PMID: 8172396.
- Seto BH, Gotsopoulos H, Sims MR, Cistulli PA. Maxillary morphology in obstructive sleep apnoea syndrome. Eur J Orthod. 2001 Dec;23(6):703-14. PubMed PMID: 11890066.
- Haralambidis A, Ari-Demirkaya A, Acar A, Kucukkeles N, Ates M, Ozkaya S. Morphologic changes of the nasal cavity induced by rapid maxillary expansion: a study on 3-dimensional computed tomography models. Am J Orthod Dentofacial Orthop. 2009 Dec;136(6):815-21. PubMed PMID: 19962604.
- De Felippe NL, Bhushan N, Da Silveira AC, Viana G, Smith B. Long-term effects of orthodontic therapy on the maxillary dental arch and nasal cavity. Am J Orthod Dentofacial Orthop. 2009 Oct;136(4):490.e1-8. PubMed PMID: 19815146.
- Buck LM, Dalci O, Darendeliler MA, Papageorgiou SN, Papadopoulou AK. Volumetric upper airway changes after rapid maxillary expansion: a systematic review and meta-analysis. Eur J Orthod. 2017 Oct 1;39(5):463-473. PubMed PMID: 27440774.
- Marcus CL, Katz ES, Lutz J, Black CA, Galster P, Carson KA. Upper airway dynamic responses in children with the obstructive sleep apnea syndrome. Pediatr Res. 2005 Jan;57(1):99-107. PubMed PMID: 15557113.
- Gozal D, Kheirandish-Gozal L, Serpero LD, Sans Capdevila O, Dayyat E. Obstructive sleep apnea and endothelial function in school-aged nonobese children: effect of adenotonsillectomy. Circulation. 2007 Nov 13;116(20):2307-14.PubMed PMID: 17967978.
- Miman MC, Kirazli T, Ozyurek R. Doppler echocardiography in adenotonsillar hypertrophy. Int J Pediatr Otorhinolaryngol. 2000 Aug 11;54(1):21-6. PubMed PMID: 10960692.
- Friedman M, Wilson M, Lin HC, Chang HW. Updated systematic review of tonsillectomy and adenoidectomy for treatment of pediatric obstructive sleep apnea/hypopnea syndrome. Otolaryngol Head Neck Surg. 2009 Jun;140(6):800-8. PubMed PMID: 19467393.
- Villa MP, Castaldo R, Miano S, Paolino MC, Vitelli O, Tabarrini A, et al. Adenotonsillectomy and orthodontic therapy in pediatric obstructive sleep apnea. Sleep Breath. 2014 Sep;18(3):533-9. PubMed PMID: 24277354.
- Villa MP, Malagola C, Pagani J, Montesano M, Rizzoli A, Guilleminault C, et al. Rapid maxillary expansion in children with obstructive sleep apnea syndrome: 12-month follow-up. Sleep Med. 2007 Mar;8(2):128-34. PubMed PMID: 17239661.
- Cistulli PA, Palmisano RG, Poole MD. Treatment of obstructive sleep apnea syndrome by rapid maxillary expansion. Sleep. 1998 Dec 15;21(8):831-5. PubMed PMID: 9871945.
- Pirelli P, Saponara M, Guilleminault C. Rapid maxillary expansion (RME) for pediatric obstructive sleep apnea: a 12-year follow-up. Sleep Med. 2015 Aug;16(8):933-5. PubMed PMID: 26141004.
- Lagravere MO, Heo G, Major PW, Flores-Mir C. Meta-analysis of immediate changes with rapid maxillary expansion treatment. J Am Dent Assoc. 2006 Jan;137(1):44-53. PubMed PMID: 16456998.
- Bach N, Tuomilehto H, Gauthier C, Papadakis A, Remise C, Lavigne F, et al. The effect of surgically assisted rapid maxillary expansion on sleep architecture: an exploratory risk study in healthy young adults. J Oral Rehabil 2013 Nov;40(11):818-25. PubMed PMID: 24138678.
- Vinha PP, Eckeli AL, Faria AC, Xavier SP, de Mello-Filho FV. Effects of surgically assisted rapid maxillary expansion on obstructive sleep apnea and daytime sleepiness. Sleep Breath. 2016 May;20(2):501-8. PubMed PMID: 26092279.
- Yoon AJ, Zaghi S, Ha S, Law CS, Guilleminault C, Liu SY. Ankyloglossia as a risk factor for maxillary hypoplasia and soft palate elongation: A functional - morphological study. Orthod Craniofac Res. 2017 Nov;20(4):237-244. PubMed PMID: 28994495.