Evaluation of Antioxidant Activity of Ocimum Sanctum-An In Vitro Study
R.Saravanan1, Jaiganesh Ramamurthy2*
1 Department of Periodontics, Saveetha dental college and Hospitals, Saveetha Institute of Medical and Technical Sciences (SIMATS), Saveetha University,
Chennai, India.
2 Professor and Head, Department of Periodontics, Saveetha dental college and Hospitals, Saveetha Institute of Medical and Technical Sciences (SIMATS), Saveetha University, Chennai, India.
*Corresponding Author
Jaiganesh Ramamurthy,
Professor and Head, Department of Periodontics, Saveetha dental college and Hospitals, Saveetha Institute of Medical and Technical Sciences (SIMATS), Saveetha University,
Chennai, India.
Tel: +91-9840443463
E-mail: jaiganeshr@saveetha.com
Received: July 21, 2021; Accepted: November 10, 2021; Published: November 12, 2021
Citation: R.Saravanan, Jaiganesh Ramamurthy. Evaluation of Antioxidant Activity of Ocimum Sanctum-An In Vitro Study. Int J Dentistry Oral Sci. 2021;8(11):5001-5005. doi: dx.doi.org/10.19070/2377-8075-210001007
Copyright: Jaiganesh Ramamurthy©2021. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Introduction: Antioxidants are substances that can prevent or slow damage to cells caused by free radicals, unstable molecules
that the body produces as a reaction to environmental and other pressures. They are sometimes called “free-radical
scavengers.” The sources of antioxidants can be natural or artificial.Periodontitis results from the loss of balance between
microbial virulence factors and a proportionate host response.Antioxidant is a substance that is present at low concentrations
which significantly delays or prevents oxidation of that substrate.Ocimum sanctum which is a plant extract had a medicinal
value and it had been used in asian countries to treat various disease.Ocimum sanctum has an antioxidant agent.
Materials and Methods: Ocimum Sanctum commercially available powders are used to analyze antioxidant potential. In
which three radical scavenging activity analyzed DPPH radical scavenging,Superoxide anion radical scavenging, Nitric oxide
radical scavenging.
Results: The results from obtained from the reagents shows DPPH radical scavenging activity shows tulsi shows higher activity
compared with vitamin C,Superoxide anion radical scavenging,Nitric oxide radical scavenging activity shows vitamin c has
higher activity compared with tulsi.
Conclusion: The above results of the antioxidant activity shows that vitamin c activity is higher in Superoxide anion radical
scavenging,Nitric oxide radical scavenging activity of each concentration of tulsi 100µg/ml,200µg/ml,300µg/ml,400µg/ml,
500µg/ml.
2.Introduction
3.Materials and Methods
3.Results
4.Discussion
5.Conclusion
5.References
Keywords
Ocimumsanctum; DPPH Radical Scavenging; Superoxide Anion Radical Scavenging; Nitric Oxide Radical Scavenging.
Introduction
Tulsi is a basil family Lamiaceae (tribe ocimeae) is native to the Indian
subcontinent, China, and Southeast Asia and widespread as
a cultivated plant throughout the Southeast Asian tropic [1]. Tulsi
is known as “Mother Medicine of Nature” with its medicinal
properties [2]. Within India, tulsi has been adopted into medicinal
value and lifestyle practices that provide the health benefits that
are just beginning with modern science. The science on tulsi, in
ancient Ayurvedic suggests that tulsi is a tonic for the body, mind
and spirit that offers solutions to many modern health problems.
Tulsi provides a better lifestyle approach to health. Tulsi which
penetrate the deep tissues, dry tissue secretions. Consumption of
tulsi gives sweetness to the voice, intelligence, stamina and a calm
emotional disposition [3-6]. Tulsi has the properties, which including
anxiety, cough, asthma, diarrhea, fever, dysentery, arthritis, eye
diseases, otalgia, indigestion, hiccups, vomiting, gastric, cardiac
and genitourinary disorders, back pain, skin diseases, ringworm,
insect, snake and scorpion bites and malaria [7].The seeds, leaves
and roots of holy basil traditionally have been ascribed a powerful
medicinal value. It is used both internally and externally. Tulsi has
the effect of antiseptic and analgesic properties and relieves swelling.
The leaves when chewed mitigate gum infections. Fresh juice
of the tulsi leaves is an effective domestic remedy for earaches.
Tea made with leaves of holy basil is common for cold, cough and mild indigestion [8]. Periodontitis is a prevalent inflammatory
disease, affecting 10% of people worldwide [9]. It can result in the
destruction of teeth,pdl and alveolar bone loss that ends up with
a loss of teeth. In addition, periodontitis also has associations
with several systemic diseases, e.g. cardiovascular disease, diabetes,
and adverse pregnancy outcomes . Current concept suggests
that this inflammatory disease is initiated by bacterial infection
and subsequently progressed by aberrant host response, which
mainly contributes to periodontal tissue destruction [10]. In recent
years, reactive oxygen species (ROS) have gained more and
more attention, because of their central role to the progression
of many inflammatory diseases [11]. ROS are described as oxygen
free radicals and other non-radical oxygen derivatives involved in
oxygen radical production [12]. In which they are involved in normal
cellular metabolism. Another category of substances called
antioxidants exist in the cells and can effectively delay or inhibit
ROS-induced oxidation [13]. ROS are effectively neutralized by
antioxidants.When inflammation occurs in the tissues ROS production
is drastically increased mainly due to cells of the innate
immune system, e.g., neutrophils and macrophages during the
process of phagocytosis and respiratory burst [14]. Subsequently,
high levels or activities of ROS cannot be balanced by the antioxidant
defense system, which leads to oxidative stress and tissue
damage [11] . ROS causes tissue damage, involving lipid peroxidation,
DNA damage, protein damage, and oxidation of important
enzymes; meanwhile, they can function as signaling molecules or
mediators of inflammation [15]. Neutrophils have several selective
mechanisms for controlling bacterial invasion, including both
intracellular oxidative and non-oxidative killing mechanisms. The
oxidative killing mechanism of neutrophils and phagocytes involves
the formation of reactive oxygen species. ROS generates
the neutrophils and requires a minimum oxygen tension of about
1% and a pH of 7.0–7.5. Cells require adequate levels of Antioxidants
in order to prevent tissue damage caused by excessive
production of reactive oxygen species [16]. The aim of this study
is to evaluate antioxidant activity of Ocimum sanctum.
Materials And Methodology
The commercially available Ocimum sanctum powder were used
to identify the antioxidant activity. In which three radical scavenging
activity analyzed DPPH radical scavenging,Superoxide anion
radical scavenging, Nitric oxide radical scavenging.
DPPH free radical scavenging activity of plant extract
Scavenging of 2, 2-Diphenyl-1-picrylhydrazyl (DPPH) radicals
was assessed by the method of Hatano et al. (1989).
Principle
The scavenging reaction between DPPH and an antioxidant of
the sample (H-A) can be written as:
DPPH + (H-A) DPPH-H + (A) (Purple) (Antioxidant) (Yellow)
Antioxidants of the sample react with DPPH which is a stable
free radical and gets reduced to the DPPH-H and as a consequence
the absorbance decreases from the DPPH radical to the
DPPH-H form. The degree of discoloration indicates the scavenging
potential of the antioxidant compounds of the extracts in
terms of hydrogen donating ability.
Reagents
1. Methanolic solution of DPPH (0.1mM): DPPH (19.7mg) was
dissolved in 500ml of analytical grade methanol.
2. Ascorbic acid (1%): Ascorbic acid (1g) was dissolved in 100 ml
of methanol.
3. Extract preparation (Stock): Each extracts (50mg) were dissolved
in 50 ml of analytical grade methanol. The required concentrations
of the extracts were diluted accordingly from the
stock.
4. Extract preparation (working) [Eg. 5µl/ml]:
The extract of 0.005ml (5µl) was made up to 1ml (1000µl) by the
addition of 995 µl of water.
Procedure
DPPH solution (1.0 ml) was added to 1.0 ml of plant extract different
concentrations (100-500µg/ml). The mixture was kept at
room temperature for 50 minutes and the activity was measured
at 517nm. Ascorbic acid at various concentrations thulsi chooranam
(100-500µg/ml) was used as standard. The percentage of
free radical inhibition was calculated as IC50. It denotes the concentration
of the sample required to scavenge 50% of DPPH free
radical. The capability to scavenge the DPPH radical was calculated
using the following formula,
DPPH radical scavenging (%) =Control OD-Sample OD/Control
ODX100
Nitric oxide radical scavenging activity
Scavenging of nitric oxide radical was assayed by the method of
Garrat, (1964).
Principle
Sodium nitroprusside in aqueous solution at physiological pH
spontaneously generates nitric oxide which interacts with oxygen
to produce nitrite ions that can be estimated using Griess reagent
scavengers of nitric oxide which compete with oxygen, leading to
reduced production of nitrite ions.
Reagents
1. Sodium nitroprusside (10 mM):
Sodium nitroprusside (29.79mg) was dissolved in 100 ml of double
distilled water.
2. Phosphate buffer saline (0.1M, pH 7.4):
Sodium chloride (0.8g), 0.2g potassium chloride (KCl), 1.44g sodium
orthophosphate (NaHPO4) and 0.024 g of potassium dihydrogen
phosphate (KH2PO4) were dissolved in 80ml of double
distilled water and the pH was adjusted to 7.4 and was made up to
100ml with double distilled water.
3. Sulfanilic acid (0.33% w/v):
Sulfanilic acid (330mg) was dissolved in 100 ml of 20% acetic
acid.
4. Naphthyl ethylenediamine dihydrochloride (0.1%, w/v):
Naphthyl ethylenediamine dihydrochloride (100mg) was dissolved
in 100ml of double distilled water.
5. Extract preparation (Stock):
Each extracts (100mg) were dissolved in 100ml of analytical grade
methanol. The required concentrations of the extracts were diluted
accordingly from the stock.
6. Extract preparation (working) [Eg. 100µl/ml]:
The extract of 0.1ml was made up to 1ml by the addition of 900
µl of water.
Procedure
The reaction mixture (3ml) containing sodium nitroprusside
(10mM, 2 ml), phosphate buffer saline (0.5 ml) and different concentrations
of extracts of tulsi Chooranam (100-500µg/ml) were
incubated at 25C for 150 minutes. After incubation, 0.5 ml of the
reaction mixture containing nitrite was pipetted out and mixed
with 1 ml of sulfanilic acid reagent (0.33% in 20% acetic acid)
and allowed to stand for 5 minutes for completing diazotization.
Then, 1 ml of naphthyl ethylenediamine dihydrochloride was
added, mixed and allowed to stand for 30 minutes at 25°C. A pink
colored chromophore is formed in diffused light. Ascorbic acid
at various concentrations (100-500µg) were used as standard. The
activity was measured at 550 nm and the results were expressed as
% of scavenging using the following formula,
Nitric oxide radical scavenging (%) = Control OD-Sample OD/
Control ODX100
Superoxide anion scavenging activity
Scavenging of superoxide anion activity was assessed by the
method of Liu et al. (1997).
Principle
Superoxide anion is generated by the Phenazine methosulphate-
NADH (PMS-NADH) system by oxidation of NADH and is assessed
by the reduction of nitrobluetetrazolium (NBT).
Reagents
1. Tris-Hcl buffer (16µM, pH 8.0):
Tris-HCl (126.08) was dissolved in 40 ml of double distilled water.
pH was adjusted to 8.0 and then made up to 50 ml with double
distilled water.
2. Nitrobluetetrazolium (NBT) (50µM):
Nitrobluetetrazolium (408.82 mg) was dissolved in 10 ml of double
distilled water.
3. Phenazine methosulphate (PMS) (10µM):
Phenazine methosulphate (30.63 mg) was dissolved in 10ml of
double distilled water.
4. NADH (78µM) for 10 ml:
NADH (517.48 mg) was dissolved in 10ml of double distilled
water.
5. Extract preparation (Stock):
Each extracts (100mg) were dissolved in 100ml of analytical grade
methanol. The required concentrations of the extracts were diluted
accordingly from the stock.
6. Extract preparation (working) Eg. [100µl/ml]:
The extract of 0.1ml was made up to 1ml by the addition of 900
µl of water.
Procedure
Superoxide anions were chemically generated in a mixture of
phenazine methosulphate (PMS) and NADH. The reaction was
quantified by coupling superoxide generation to the reduction
of nitrobluetetrazolium (NBT). In this experiment, the superoxide
radicals were generated in 3ml of Tris-Hcl buffer (16mM,
pH 8.0) containing 1ml of NBT (50 µM), 1ml of NADH (78
mM) and 1ml of various concentrations (100- 500 µg/ml) of
A.cepa varieties extracts. Ascorbic acid at various concentrations
(100,200,300,400 and 500µg) were used as standard. The reaction
mixture was incubated at 25°C for 5 minutes and the activity was
measured at 560nm. Results were expressed as % of scavenging
using the following formula,
Superoxide anion scavenged (%) = Control OD-Sample OD/
Control ODX100
Statistical Analysis
Antioxidant activity of tulsi was calculated in SPSS 2.0 version in
which ANOVA analysis of variances has been detected in DPPH
radical scavenging, Nitric oxide radical scavenging activity and Superoxide
anion scavenging activity.
Results
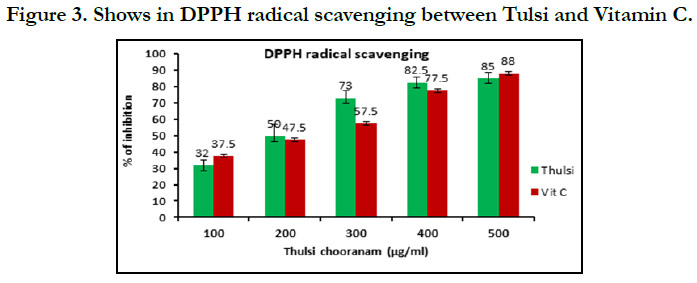
The results from the antioxidant activity of tulsi of DPPH radical
scavenging (Figure 3 ) shows that in 100µg/ml shows vitamin c
has higher concentration compared with tulsi, 200µg/ml, 300µg/
ml, 400µg/ml, 500µg/ml shows tulsi has higher concentration
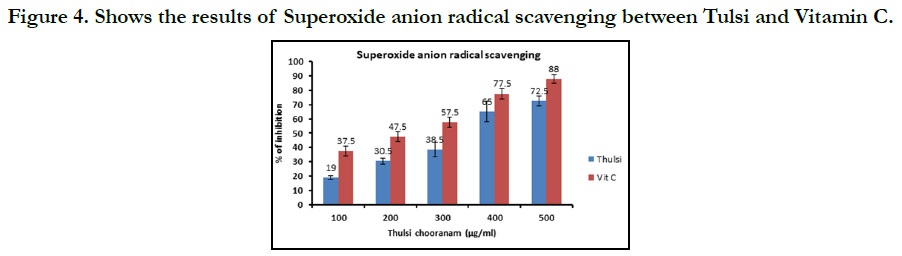
compared with vitamin C.In superoxide anion radical scavenging
(Figure 4) 100µg/ml, 200µg/ml, 300µg/ml, 400µg/ml, 100µg/ml
show that Vitamin C has higher concentration compared with tulsi.
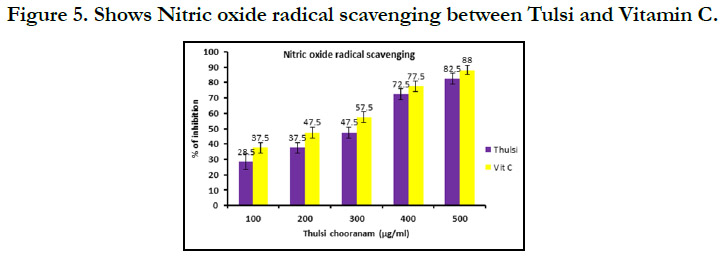
In Nitric oxide radical scavenging (Figure 5) shows that 100µg/
ml, 200µg/ml, 300µg/ml, 400µg/ml, 100µg/ml show that Vitamin
C has higher concentration compared with tulsi.The results
of the study shows that DPPH radical scavenging show the tulsi
has highest antioxidant activity compared with Superoxide anion
scavenging and Nitric oxide radical scavenging.
Discussion
Medicinal plants are the sources of natural antioxidants and
represent the discovery of new drugs in the therapeutic disease.
Most members of the Lamiaceae family have exhibited interesting
biological effects due to their antioxidant compounds [17] .Ocimum
sanctum has various properties such as antistress, antiseptic,
analgesic, anti-inflammatory, antimicrobial, immunomodulatory,
hypoglycemic, hypotensive, cardioprotective and antioxidant [18].
Leaves of Ocimum sanctum contain water-soluble phenolic compounds
and various other constituents, such as eugenol, methyl
eugenol and caryophyllene that may act as an immunostimulant.
Saponins act as antihyperlipidemic, hypotensive and cardio depressive
properties .The accumulations of free radicals in organs
or tissues are strongly associated with oxidative damages in biomolecules
and cell membranes. This can lead to many chronic diseases,
such as inflammatory, cancer, diabetes, aging, cardiac dysfunction,
and other degenerative diseases [19]. The relationship
between oxidative stress and periodontal disease is quite strong
and can be a two-way path. Periodontal inflammation increases
the number of oxidative stress markers, and it tends to potentiate aspects of periodontal destruction [20]. Ocimum sanctum has
antioxidant activity through the analysis and DPPH radical scavenging
analysis. It is concluded that there is a good antioxidant
potential of Ocimum sanctum with ethanolic Soxhlet extraction
[21]. Superoxide is a reactive oxygen species that can damage cells
and DNA, leading to various diseases. This assay was determined
by NBT assay and the value ranges from 12.04% to 60.160%
methanol leaves extracted respectively at a concentration 10-500
µg/mL. While that of the control, ascorbic acid the inhibition
percentage ranges from 10µg/mL to 500µg/mL.Themethanolic
leaves extracts of O. sanctum had strong antioxidant activity
against all the free radicals.The DPPH radical is widely used in
assessing free radical scavenging activity was 65.75% in methanol
respectively at a concentration of 500µg/mL leaves extracts. In
vitro, antioxidant effects of O. basilicum were tested using DPPH
and CATALASE methods. The extract O. basilicum expressed
the strongest antioxidant activity. The extracts of O. basilicum
leaves showed good free radical scavenging activity. The broad
range of antioxidant activity of this extract indicates the potential
of the plant as a source of natural antioxidants with potential
application to reduce oxidative stress and consequent health benefits.
DPPH radicals are widely used in the model system to investigate
the scavenging activities of several natural compounds.
In this analysis, the scavenging behavior of the ethanolic extract
was similar to that of ascorbic acid. The DPPH radical scavenging
activity of ascorbic acid and ethanolic extracts increased in a
dose-dependent manner. At a concentration of 100ug/ ml both
ethanolic extract and standard ascorbic acid showed 81.25% and
98.10% antioxidant activity by DPPH radicals scavenging assay.
Conclusions
Within the limitation of the study we are able to identify the antioxidant
activity of ocimumsanctum.From the aqueous extract
of ocimum sanctum antioxidant activity were analyzed by DPPH
free radical scavenging ,Nitric oxide radical scavenging and Superoxide
anion radical scavenging.We have found that Vitamin C has
higher activity compared with tulsi.
Further studies has to be done before using this novel product as
mouthwash in patients with periodontal disases.
Acknowledgement
The authors are thankful to the Director of academics,Chancellor
and Dean of Saveetha Dental College and Hospitals for providing
a platform to do research activities.
References
-
[1]. Bast F, Rani P, Meena D. Chloroplast DNA phylogeography of holy basil
(Ocimumtenuiflorum) in Indian subcontinent. ScientificWorldJournal.
2014 Jan 2;2014:847482. doi: 10.1155/2014/847482. PMID: 24523650.
[2]. Singh A, Assistant Professor Amity University Lucknow Campus, Lucknow., et al. GOODS AND SERVICES TAX: THE NEW ECONOMIC REFORM, CHALLENGES AND OPPORTUNITIES IN IMPLICATION AND THE WAY AHEAD. International Journal of Advanced Research 2017; 5: 1851–1858.
[3]. Mahajan N, Rawal S, Verma M, Poddar M, Alok S. A phytopharmacological overview on Ocimum species with special emphasis on Ocimum sanctum. Biomedicine & Preventive Nutrition. 2013 Apr 1;3(2):185-92.
[4]. Bhat ZF, Kumar S, Kumar L. Effect of Ocimum sanctum Linn (Tulsi) on the oxidative stability and storage quality of chicken sausages. Nutrition & Food Science 2015; 45: 510–523.
[5]. Pattanayak P, Behera P, Das D, Panda SK. Ocimum sanctum Linn. A reservoir plant for therapeutic applications: An overview. Pharmacogn Rev. 2010 Jan;4(7):95-105. PubMed PMID: 22228948.
[6]. Mondal S, Varma S, Bamola VD, Naik SN, Mirdha BR, Padhi MM, et al. Double-blinded randomized controlled trial for immunomodulatory effects of Tulsi (Ocimum sanctum Linn.) leaf extract on healthy volunteers. J Ethnopharmacol. 2011 Jul 14;136(3):452-6. PubMed PMID: 21619917.
[7]. Wangcharoen W, Phimphilai S. Chlorophyll and total phenolic contents, antioxidant activities and consumer acceptance test of processed grass drinks. J Food Sci Technol. 2016 Dec;53(12):4135-4140. PubMed PMID: 28115753.
[8]. Kumar BR, Yuvaraj S, Srivastava A, et al. CoMFA Study, Syntheses, Antitubercular and Anticancer Activity of Some Novel 1,4-Dihydropyridines. Letters in Drug Design & Discovery 2008; 5: 7–14.
[9]. Richards D. Review finds that severe periodontitis affects 11% of the world population. Evid Based Dent. 2014 Sep;15(3):70-1. PubMed PMID: 25343387.
[10]. Bartold PM, Van Dyke TE. Periodontitis: a host-mediated disruption of microbial homeostasis. Unlearning learned concepts. Periodontol 2000. 2013 Jun;62(1):203-17. PubMed PMID: 23574467.
[11]. Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014 Mar 1;20(7):1126-67. PubMed PMID: 23991888.
[12]. Lushchak VI. Free radicals, reactive oxygen species, oxidative stress and its classification. ChemBiol Interact. 2014 Dec 5;224:164-75. PubMed PMID: 25452175.
[13]. Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997 Mar;82(2):291-5. PubMed PMID: 9129943.
[14]. Chapple IL, Matthews JB. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol 2000. 2007;43:160-232. PubMed PMID: 17214840.
[15]. Roos D, van Bruggen R, Meischl C. Oxidative killing of microbes by neutrophils. Microbes Infect. 2003 Nov;5(14):1307-15. PubMed PMID: 14613774.
[16]. Bansal N, Gupta ND. Role of dietary antioxidants in periodontitis: A preventive approach. IOSR Journal of Dental and Medical Sciences. 2014;13(9):81-4.
[17]. Schofield P, Mbugua DM, Pell AN. Analysis of condensed tannins: a review. Animal Feed Science and Technology 2001; 91: 21–40. [18]. Williamson EM, Dabur Research Foundation, DaburAyurvet Limited. Major Herbs of Ayurveda. 2002.
[19]. Wang S, Konorev EA, Kotamraju S, Joseph J, Kalivendi S, Kalyanaraman B. Doxorubicin induces apoptosis in normal and tumor cells via distinctly different mechanisms. intermediacy of H(2)O(2)- and p53-dependent pathways. J Biol Chem. 2004 Jun 11;279(24):25535-43. PubMed PMID: 15054096.
[20]. da Silva JC, Muniz FWMG, Oballe HJR, Andrades M, Rösing CK, Cavagni J. The effect of periodontal therapy on oxidative stress biomarkers: A systematic review. J ClinPeriodontol. 2018 Oct;45(10):1222-1237. PubMed PMID: 30076616.
[21]. Xia KZ, Perveen N, Khan NH. Phytochemical analysis, antibacterial and antioxidant activity determination of Ocimum sanctum. Pharm PharmacolInt J. 2018;6(6):490-7.