Extraction and Ridge Preservation Followed by Delayed Implant Placement of a Second Mandibular Molar with Proximity to the Inferior Alveolar Nerve
Bronstein D1*, Garashi M2, Kravchenko D3
1 Associate Director of Predoctoral Periodontology, Nova Southeastern University, Fort Lauderdale, FL, USA.
2 Periodontics Resident, Nova Southeastern University, Fort Lauderdale, FL, USA.
3 Master's in Public Health, Nova Southeastern University, Fort Lauderdale, FL, USA.
*Corresponding Author
Diana Bronstein D.D.S., M.S.,M.S.,
Associate Director of Predoctoral Periodontology,
Nova Southeastern University, Fort Lauderdale, FL, USA.
Tel: 267-391-9294/954-262-7381
E-mail: db1473@nova.edu
Received: January 30, 2017; Accepted: March 04, 2017; Published: March 07, 2017
Citation: Bronstein D, Garashi M, Kravchenko D (2017) Extraction and Ridge Preservation Followed by Delayed Implant Placement of a Second Mandibular Molar with Proximity to the Inferior Alveolar Nerve. Int J Dentistry Oral Sci. 4(3), 434-438. doi: dx.doi.org/10.19070/2377-8075-1700086
Copyright: Bronstein D©2017. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
2.Sinus Augmentation
3.Block Grafts
4.Guided Bone Regeneration
5.Future Directions
6.Case Report
6.1 Clinical Presentation
6.2 Case Management
7.Clinical Outcomes
8.References
Introduction
Teeth are extracted for a variety of reasons, including trauma, pathology, and infection. Tooth loss has a direct effect on the quality of life of an individual, as it alters the capacity to speak, masticate, and socialize. After extraction of a tooth, a series of biological events takes place that results in substantial anatomical changes to the remaining bony architecture [1]. Investigators have demonstrated that post-extraction loss of bone volume is an irreversible process that has a predictable order, and results in horizontal as well as vertical deficits. Weijden et al., reported that the buccal aspect of bone resorbs first, more so in width than height, with mandibular bone resorption occurring before that of maxillary bone [2]. Disuse atrophy, which is one factor implicated in the resorption of the alveolar ridge, poses a significant problem for tooth replacement, specifically when implant therapy is planned [3]. As a result, the functional and esthetic rehabilitation of the edentulous area may be compromised.
Initial efforts aimed at preservation of the alveolar ridge following tooth extraction focused on root retention. However, this option was not always feasible due to root fracture or extensive caries [4]. Contemporary ridge preservation techniques employ the use of grafting materials of synthetic, animal, or human origin, and include autogenous chips, xenografts, allografts, and alloplasts [5]. Furthermore, the use of resorbable and non-resorbable barriermembranes can aid in covering extraction sockets and preserving alveolar ridges [6]. The aim of this article is to evaluate some of those techniques.
Sinus Augmentation
Sinus augmentation is an effective and predictable approach for increasing crestal bone volume in the deficient posterior maxilla [7]. The two techniques that have been historically described are the crestal osteotome technique and the lateral window approach. In the osteotome technique, an area of bone is exposed by creating a flap and a twist drill is used to remove bone in small increments until the floor of the maxillary sinus is approximated. The osteotomy is then widened to a size approximately 1mm less than the size of the intended implant [8]. Grafting during this technique is still a topic of much debate. One group concluded that grafting materials of human or animal origin are to be placed if optimal results are expected, however the results of that study ought to be interpreted with caution owing to its study design [9]. Perforation of the sinus floor should be avoided, and can be detected by a change in resistance or a change in tone upon tapping. The more involved procedure for sinus augmentation is known as the lateral window approach. An opening is created in the antrum of the sinus for access. The osteotomy can be prepared using traditional hand pieces or piezoelectric instruments, with the use of the latter considerably reducing the risk of perforation of the Sniderian membrane [10]. Zhang et al., looked at whether platelet rich plasma has an effect on bone regeneration during sinus augmentation using this lateral window approach. Bone formation was evaluated by measuring the amount of newly formed bone and residual bone substitute in the area of interest. The investigators found no statistically significant difference in either newly formed bone or residual bone substitute between the experimental groups that were treated with platelet rich fibrin and those without platelet rich fibrin [11].
As stated previously, the implementation of implant supported prostheses requires sufficient quantity and adequate quality of bone. Unlike in the case of single tooth loss, in individuals who suffer from severe partial or complete edentulism, bone is re-sorbed in a more intense manner and the rehabilitation of such patients requires autologous bone from intraoral or extraoral areas [12]. Because obtaining these grafts requires surgical skill, results in morbidity to the patient, and can provoke pain and inflammation, various materials are being studied that would allow surgeons to reconstruct defects in a safer and less traumatic manner. Novell et al., looked at the efficacy of allogenic bone grafts compared with autogenous bone grafts when the outcome measure is implant stability. Those investigators obtained a survival rate of 95% with the use of allografts for reconstruction of bone defects. They observed a reabsorption rate that was less than what was expected when autografts are used [13]. In this particular study implant and bone graft placement were two separate surgeries. They concluded that the use of allogenic grafts is a realistic alternative for the repair of defects in the atrophic maxilla.
However, allogenic materials are not flawless. An ideal bone substitute ought to be osteoconductive, should be capable of guiding growth and proliferation of osteoblasts, and the ideal shape of which is governed by the shape of the defect. The latter property is difficult to achieve, as allogenic grafts need to be adapted during the surgical procedure. This process is time-and operator-dependent [14]. Luongo et al., described outcomes of reconstructive efforts performed using custom-made synthetic bone grafts. In 14 patients, a CBCT of the defect was obtained and a 3D reconstruction was rendered. A 3D virtual model of the custom made scaffold was imported into CAD/CAM software and eventually milled. In all cases implants were placed and restored with single crowns. The authors reported a 100% success rate after 8 years with no late biological complications. A larger sample size and randomized design will be required to draw conclusions about the reliability and generalizability of the proposed treatment [15].
Guided Bone Regeneration
A major difficulty in the successful regeneration of bone is that fibrous connective tissue rather than bone quickly occupies the bony defect. Membranes have been used successfully to protect bony defects from invasion by fibrous connective tissue [16]. When a defect is covered by a membrane complete regeneration takes place, although the healing proceeds slowly. Traditionally resorbable nonmetallic materials have been implemented as GBR membranes, however they have lower retention, lower mechanical strength, and are more difficult to manipulate than nonresorbable mesh. The limiting factor preventing more widespread use of nonresorbable mesh is the need to remove it should it become infected. Recent efforts have been focused on the implementation of biodegradable metallic materials in medicine and dentistry [17]. In particular magnesium has received much attention owing to its biocompatibility and mechanical properties. The authors of one study set out to investigate the biocompatibility and mechanical properties of hydroxyapatite coated magnesium mesh and determine its potential as a suitable biomaterial in guided bone regeneration. The resorbable metallic mesh was applied to critical size calvarial defects in the Sprague-Dawley rat, and the animal was monitored for guided bone regeneration and biodegradation of the mesh material. The group reported that after 18 weeks, tissues above the critical size defect appeared to have caved in the control group model, but not in the resorbable mesh model. They concluded that the hydroxyapatite coated magnesium mesh had sufficient mechanical stability and biocompatibility to be used as a membrane in guided bone regeneration [18].
Future Directions
In addition to traditional methods of ridge preservation after tooth extraction, clinicians have attempted to preserve alveolar bone by employing autologous and xenogenic growth factors [19]. The use of recombinant human platelet derived growth factor (rhPDGF) and recombinant human bone morphogenic protein 2 (rhBMP2) has been described in clinical trials, with the latter having been demonstrated to show greater osteogenic potential [20]. Although rhBMP2 has shown promise as an important element in bone repair, unpleasant side effects and high cost present a barrier to therapy with rhBMP2. Various groups have attempted to demonstrate ectopic bone formation by reducing the amount of rhBMP2 and adding other factors with osteogenic activity such as fibroblast growth factor (FGF2) and prostaglandic E2 receptor agonist, however none has shown that such combinations promote alveolar ridge preservation. Arai et al., looked at whether peptide molecules that inhibit the RANK ligand, a known accelerator of osteoclast proliferation, would be effective in increasing bone formation in mice. Murine teeth were extracted and the sockets were treated with rhBMP2 and OP3-4, a peptide antagonist of the RANK ligand. The results obtained from micro CT scans showed that compared to sites that were not treated with rhBMP2 and OP3-4, the peptide-treated sites appeared to show the formation of trabecular-like structure. Furthermore, histologic analysis confirmed radiographic results, as bone formation was observed on day 21 after extraction of murine teeth. The authors were able to show that bone formation was observed in a murine tooth extraction model using OP3-4 and a minimal amount of rhBMP2. They demonstrated de novo osseous formation within extraction sockets as a means of preventing alveolar ridge resorption, suggesting a potential clinical application for alveolar ridge preservation [21].
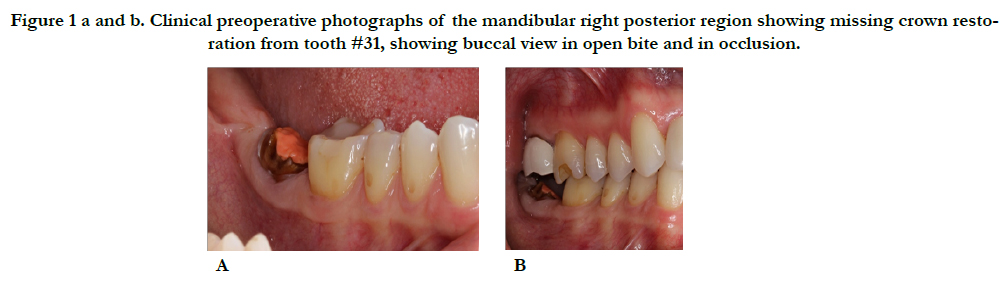
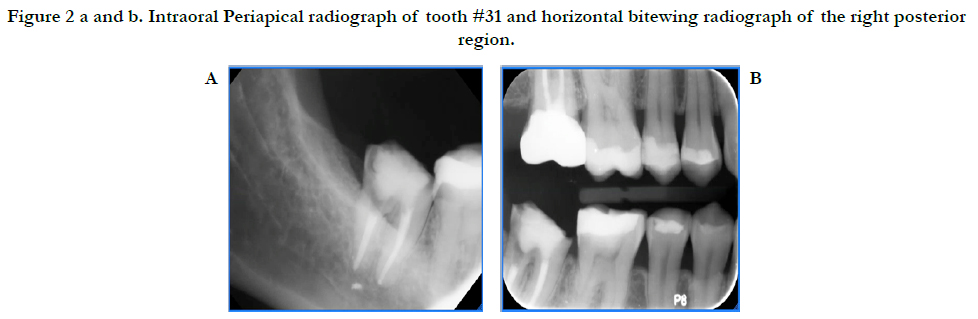
A 46-year-old Hispanic female non-smoker with non-contributory medical history with a chief complaint of “I want to replace this broken tooth with a dental implant”. Initial clinical examination revealed an intact dentition with the exception of tooth #31 which had no crown and recurrent decay around the remaining core restoration (Figure 1). The patient had good oral hygiene and opposing tooth #2 was restored with a crown. Periapical radiograph of #31 reveals its proximity to the inferior alveolar nerve (IAN) and a small radiopaque area possibly extruded Gutta Percha. Bitewing radiograph of the region reveals normal bone levels in the surround teeth (Figure 2).
Figure 1a and b. Clinical preoperative photographs of the mandibular right posterior region showing missing crown restoration from tooth #31, showing buccal view in open bite and in occlusion.
Figure 2a and b. Intraoral Periapical radiograph of tooth #31 and horizontal bitewing radiograph of the right posterior region.
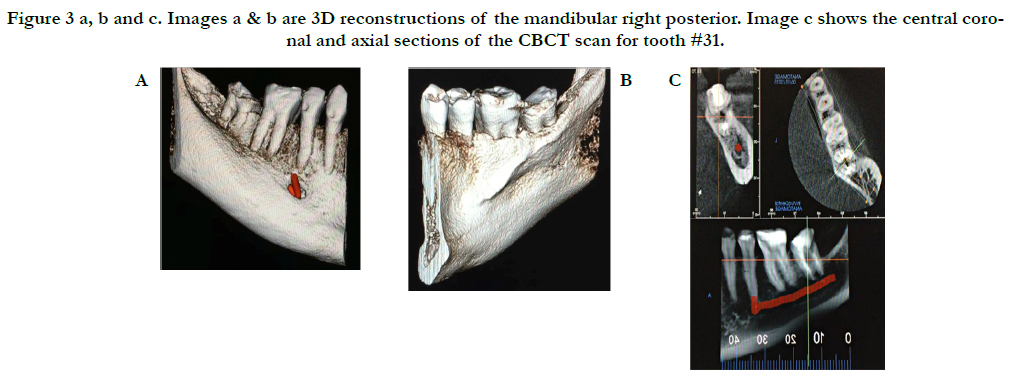
A limited view CBCT was obtained and revealed detailed information regarding tooth #31, such as proximity of the IAN, the presence of a large lingual concavity, and absence of an adequate buccal plate (Figure 3). Due to these anatomical limitations, an immediate implant was not recommended, therefore, extraction of #31 and ridge preservation followed by delayed implant placement was the chosen treatment approach. Prior to surgery, the patient was informed of this treatment plan and she provided written informed consent.
Figure 3 a, b and c. Images a & b are 3D reconstructions of the mandibular right posterior. Image c shows the central coronal and axial sections of the CBCT scan for tooth #31.
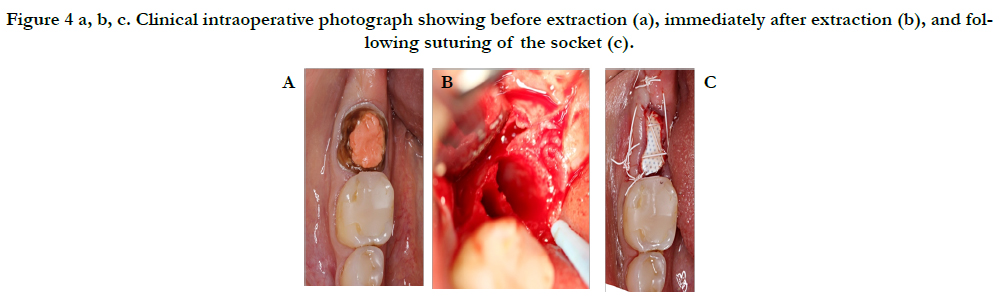
After administration of anesthesia via an inferior alveolar nerve block and long buccal injection, periotomes were used around tooth #31 and then extraction was performed with forceps. Gentle curettage of the socket and irrigation with saline was performed. Inspection of the socket revealed a very thin buccal plate. The socket was grafted with a particulate allograft and covered with a non-resorbable dense PTFE membrane which was stabilized by non-resorbable sutures and left exposed as seen in Figure 4.
Figure 4 a, b, c. Clinical intraoperative photograph showing before extraction (a), immediately after extraction (b), and following suturing of the socket (c).
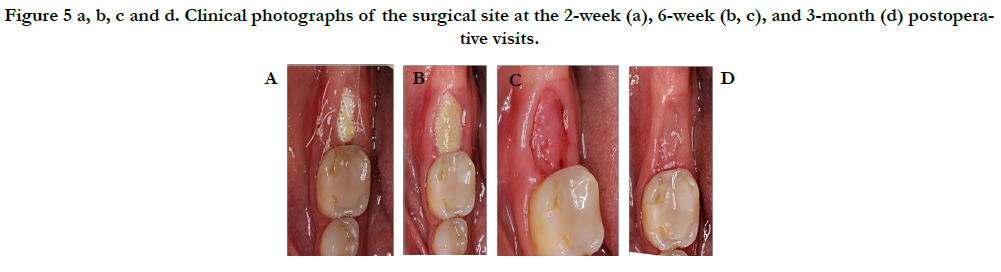
The patient was instructed to follow a cold liquid diet for the first 24 hours, followed by a soft diet for the remaining week and eat on the left side only. Patient was instructed to refrain from oral hygiene practices in the surgical site while rinsing with 0.12% chlorhexidine gluconate (three times daily) for 2 weeks, take 500 mg Amoxicillin (every 8 hours) for 7 days and 400 mg ibuprofen (every 6 hours) as needed for discomfort. At the 2-week postoperative visit, sutures were removed and the membrane was de-plaqued using a Q-tip soaked in 0.12% Chlorhexidine. The membrane was removed at the 6-week postoperative visit, and the patient returned for the final postoperative visit at 3 months as seen in Figure 5.
Figure 5 a, b, c and d. Clinical photographs of the surgical site at the 2-week (a), 6-week (b, c), and 3-month (d) postoperative visits.
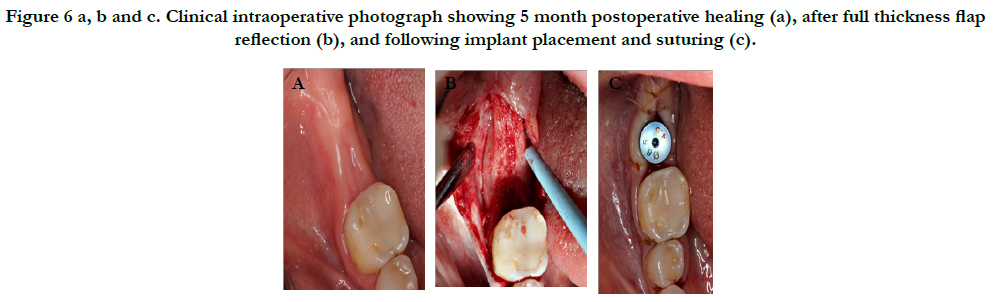
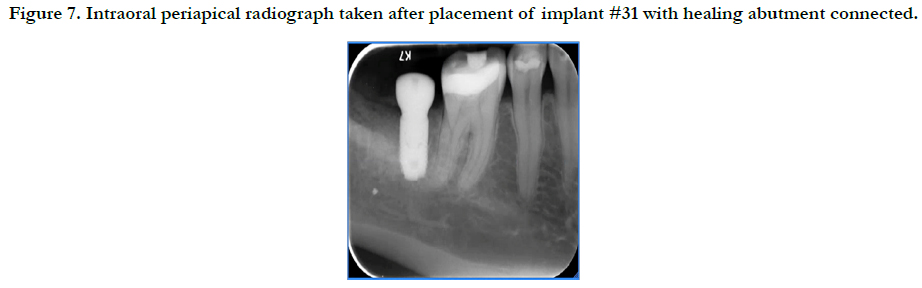
The patient returned for implant placement 5 months post-extraction and ridge preservation. After administration of anesthesia via an IANB and local infiltrations to the buccal and lingual tissues, full thickness flaps were reflected and complete healing of the ridge was evident with no loose graft particles detected. The osteotomy was prepared and implant was placed with primary stability at 35Ncm, a healing abutment was connected, and no additional grafting was necessary. Resorbable chromic gut sutures were used to approximate the flaps as seen in Figure 6. A periapical radiograph was taken as seen in Figure 7.
Figure 6 a, b and c. Clinical intraoperative photograph showing 5 month postoperative healing (a), after full thickness flap reflection (b), and following implant placement and suturing (c).
Figure 7. Intraoral periapical radiograph taken after placement of implant #31 with healing abutment connected.
The patient was instructed to follow a cold liquid diet for the first 24 hours, followed by a soft diet for the remaining week and eat on the left side only. Patient was instructed to refrain from oral hygiene practices in the surgical site while rinsing with 0.12% chlorhexidine gluconate (three times daily) for 2 weeks, take 500 mg amoxicillin (every 8 hours) for 7 days and 800 mg ibuprofen (every 8 hours) as needed for discomfort.
Clinical Outcomes
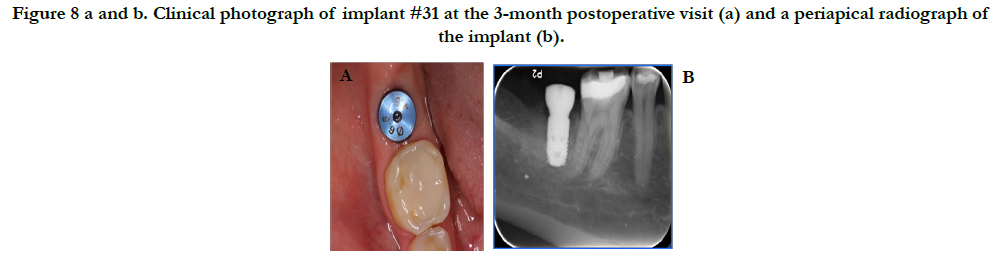
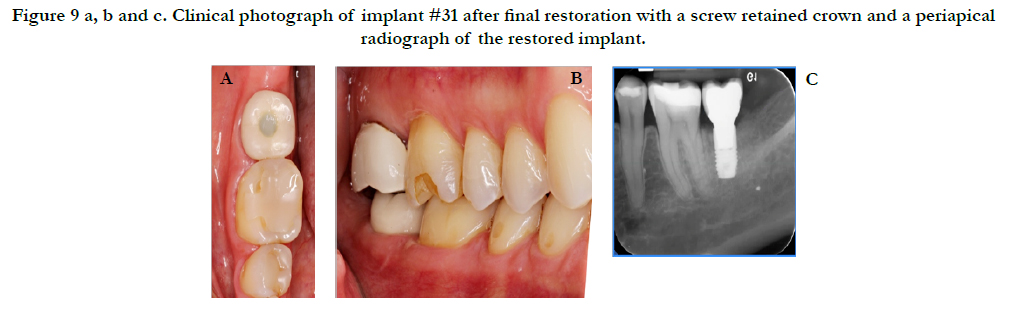
Healing was uneventful with no complications. Figure 8 reveals the final outcome at the 3-month postoperative visit. A radiograph was taken at this time and the patient was sent to the restorative department for restoration of the implant. Figure 9 shows the final restoration of implant #31 with the corresponding periapical radiograph taken by the restorative department.
Figure 8 a and b. Clinical photograph of implant #31 at the 3-month postoperative visit (a) and a periapical radiograph of the implant (b).
Figure 9 a, b and c. Clinical photograph of implant #31 after final restoration with a screw retained crown and a periapical radiograph of the restored implant.
References
- Chappuis Vivianne, Mauricio G Araújo, Daniel Buser (2000) Clinical Relevance of Dimensional Bone and Soft Tissue Alterations Post-extraction in Esthetic Sites. Periodontology. 73(1): 73-83.
- Weijden Fridus Van Der, Federico Dell'acqua, Dagmar Else Slot (2009) Alveolar Bone Dimensional Changes of Post-extraction Sockets in Humans: A Systematic Review. J Clin Periodontol. 36(12): 1048-058.
- Macmillan Hugh W (1922) The Clinical Significance of Disuse Atrophy of the Alveolar Process**Read before the Section on Periodontia at the Seventh International Dental Congress, Philadelphia, Pa., Aug. 24, 1926. J Am Dent Assoc. 14(4): 697-702.
- Garver Don G, Robert K Fenster (1980) Vital Root Retention in Humans: A Final Report. J Prosthet Dent. 43(4): 368-73.
- Pagni Giorgio, Gaia Pellegrini, William V Giannobile, Giulio Rasperini (2012) Postextraction Alveolar Ridge Preservation: Biological Basis and Treatments. Int J Dent. (2012): 1-13.
- Tomlin Elizabeth M, Shelby J Nelson, Jeffrey A Rossmann (2014) Ridge Preservation for Implant Therapy: A Review of the Literature. Open Dent J. 8(1): 66-76.
- Lee Ji-Eun, Jin SH, Ko Y, Park JB (2014) Evaluation of Anatomical Considerations in the Posterior Maxillae for Sinus Augmentation. World J Clin Cases. 2(11): 683-688.
- Chen Yonghui, ZhiyuCai, Dingguo Zheng, Pei Lin, YahuaCai, et al., (2016) Inlay Osteotome Sinus Floor Elevation with Concentrated Growth Factor Application and Simultaneous Short Implant Placement in Severely Atrophic Maxilla. Sci Rep. 6: 27348.
- Pjetursson Bjarni E, Diana Ignjatovic, Giedre Matuliene, Urs Bragger, Kurt Schmidlin, et al., (2009) Transalveolar Maxillary Sinus Floor Elevation Using Osteotomes with or without Grafting Material. Part II: Radiographic Tissue Remodeling. Clin Oral Implants Res. 20(7): 677-83.
- Wallace SS, Tarnow DP, Froum SJ, Cho C, Zadeh HH, et al., (2012) Maxillary sinus elevation by lateral window approach: evolution of technology and technique. J Evid Based Dent Pract. 12(3): 161–71.
- Zhang Yu, Stefan Tangl, Christian D Huber, Ye Lin, Lixin Qiu, et al., (2012) Effects of Choukrounâ’s Platelet-rich Fibrin on Bone Regeneration in Combination with Deproteinized Bovine Bone Mineral in Maxillary Sinus Augmentation: A Histological and Histomorphometric Study. J Cranio- Maxillofac Surg. 40(4): 321-28.
- Block Michael S, John N Kent (1993) Maxillary Sinus Grafting for Totally and Partially Edentulous Patients. J Am Dent Assoc. 124(5): 139-43.
- Novell Josep, Ferran Novell-Costa, Carlos Ivorra, Oscar Fariaas, Antonio Munilla, et al., (2012) Five-Year Results of Implants Inserted Into Freeze-Dried Block Allografts. Implant Dent. 21(2): 129-35.
- Oryan Ahmad, Soodeh Alidadi, Ali Moshiri, Nicola Maffulli (2014) Bone Regenerative Medicine: Classic Options, Novel Strategies, and Future Directions. J Orthop Surg Res. 9(1) : 18.
- Luongo Fabrizia, Francesco Guido Mangano, Aldo Macchi, Giuseppe Luongo, Carlo Mangano (2016) Custom-Made Synthetic Scaffolds for Bone Reconstruction: A Retrospective, Multicenter Clinical Study on 15 Patients. BioMed Res Int. 2016 : 5862586.
- Jamjoom Amal, Robert Cohen (2015) Grafts for Ridge Preservation. J Funct Biomater. 6(3): 833-48.
- Scheyer, Eric Todd, Rick Heard, Jim Janakievski, George Mandelaris, et al., (2016) A Randomized, Controlled, Multicentre Clinical Trial of Post-extraction Alveolar Ridge Preservation. J Clin Periodontol. 43(12): 1188-199.
- Byun Soo-Hwan, Ho-Kyung Lim, Soung-Min Kim, Sung-Mi Lee, Kim HE, et al., (2017) The Bioresorption and Guided Bone Regeneration of Absorbable Hydroxyapatite-Coated Magnesium Mesh. J Craniofac Surg. 2017: 1.
- Araújo MG, da Silva JC, de Mendonça AF, Lindhe J (2015) Ridge alterations following grafting of fresh extraction sockets in man. A randomized clinical trial. Clin. Oral Implants Res. 26(4): 407–412.
- Schliephake H (2015) Clinical efficacy of growth factors to enhance tissue repair in oral and maxillofacial reconstruction: a systematic review. Clin Implant Dent Relat Res. 17(2): 247–273.
- Arai Yuki, Kazuhiro Aoki, Yasuhiro Shimizu, Yasuhiko Tabata, Takashi Ono, et al., (2016) Peptide-induced De Novo Bone Formation after Tooth Extraction Prevents Alveolar Bone Loss in a Murine Tooth Extraction Model. Eur J Pharmacol. 782: 89-97.