Dental Pulp Stem Cell Biomarkers for Cellular Viability Following Long-Term Cryopreservation
Tomlin A1, Nelson B2, Kingsley K3*
1 Department of Advanced Education in Orthodontics and Dentofacial Orthopedics, University of Nevada, Las Vegas, School of Dental Medicine, West Charleston, Las Vegas, Nevada, USA.
2 Department of Clinical Sciences, University of Nevada, Las Vegas, School of Dental Medicine, Shadow Lane, Las Vegas, Nevada, USA.
3 Department of Biomedical Sciences, University of Nevada, Las Vegas, School of Dental Medicine, Shadow Lane, Las Vegas, Nevada, USA.
*Corresponding Author
Karl Kingsley PhD, MPH,
Professor of Biomedical Sciences, University of Nevada,
Las Vegas – School of Dental Medicine, 1001 Shadow Lane,
Las Vegas, Nevada 89106 USA.
Tel: 702-774-2623
Fax: 702-774-2721
E-mail: Karl.Kingsley@unlv.edu
Received: July 10, 2017; Accepted : April 30, 2018; Published: April 30, 2018
Citation: Tomlin A, Nelson B, Kingsley K. Dental Pulp Stem Cell Biomarkers for Cellular Viability Following Long-Term Cryopreservation. Int J Cell Syst Dev Biol. 2018;1(1):1-6.
Copyright: Karl Kingsley© 2018. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Many studies have demonstrated clinical applications for the use of dental pulp stem cells (DPSC) for the treatment of various conditions, which have driven medical and scientific interest in the collection, isolation and banking of DPSC tissues for research into these potential therapies. Few studies to date have evaluated the viability of DPSC following long-term cryopreservation. Based upon the paucity of information regarding long-term viability and biological markers for DPSC, the current aims of this study were to characterize and evaluate the effects of long-term cryopreservation, as well as to identify biomarkers that may be useful for future potential screening and applications. Using previously collected DPSC isolates, growth and viability over a period of four years were examined, revealing an overall decline in viability at each time point that did not appear to be linear. In addition, the analysis of specific intracellular biomarkers, including Nestin, NANOG, Sox-2 and Oct4 revealed that Oct4 and Sox-2 may be the most important variable factors associated with both DPSC growth rate and viability during cryopreservation. This information may be useful for future applications and therapies that could screen and sort DPSC using predetermined biomarkers to improve both efficiency and feasibility.
2.Introduction
3.Materials and Methods
3.1 Human subjects
3.2 Collection and isolation
3.3 Culture and Cryopreservation
3.4 Cellular viability
3.5 RNA isolation and RT-PCR
3.6 Statistics
4.Discussion
5.Discussion
6.Conclusion
7.Acknowledgements and Declarations
7.References
Keywords
Biomarkers; Human Dental Pulp-Derived Stem Cells; Cryopreservation.
Introduction
Many recent studies using animal models have demonstrated clinical applications for the use of dental pulp stem cells (DPSC) for the treatment of various conditions, including oral and maxillofacial reparation, retinal disorders, neuropathies and central nervous system disorders [1-4]. New evidence has elucidated several potential mechanisms for inducing DPSC differentiation prior to implantation or clinical use, including induction into neural, osteogenic and odontoblastic precursors [5-7]. These developments have led to considerable scientific interest in DPSC and their potential to generate novel and innovative treatments for common, as well as intractable, disease states [8-10].
These advances have driven broad medical and scientific interest in the collection, isolation and banking of DPSC tissues for research into these potential therapies [11-13]. For example, studies from this institution have demonstrated the feasibility and potential for the collection, isolation and in vitro mechanisms for culture-induced differentiation and de-differentiation of DPSCs [14-16]. However, despite these achievements, much remains unknown regarding the parameters, including biological characteristics and biomarkers that influence not only differentiation, but long-term viability following extended cryopreservation [17-19].
Although some prior efforts have evaluated the effects of cryopreservation on DPSC, the majority of these studies have evaluated only short-term effects - less than six months [2, 12, 20, 21]. The few studies that have investigated the effects of long-term cryopreservation and storage are providing critical knowledge towards the advancement and ultimate development of DPSCbased therapies [19, 22, 23]. Based upon the paucity of information regarding long-term viability and biological markers for DPSC, the current aims of this study were to characterize and evaluate the effects of long-term cryopreservation, as well as to identify biomarkers that may be useful for future potential screening and applications.
Original approval for the collection, isolation and storage of dental pulp stem cells (DPSC) from teeth was granted for protocol OPRS#0907-3148 “Isolation of Non-Embryonic Stem Cells from Dental Pulp” in February, 2010 [14]. Approval for the current study to analyze retrospectively collected biological specimens was granted for protocol OPRS#763012-1 in August, 2015. In brief, adult patients that were scheduled for an extraction in the clinic were asked to provide Informed Consent in order to participate. The majority of patient participants had healthy, vital intact teeth extracted prior to Orthodontic treatment [24]. Patients having teeth extracted due to injury (fracture) or compromised dental pulp, including pulp infection or disease, were excluded.
The original protocol for the collection and isolation of DPSC from vital, intact teeth involved isolation of the dental pulp from the pulp chamber following extraction. In brief, this involved cross sectioning of the tooth at the cemento-enamel junction (CEJ), following by extraction of the dental pulp with an endodontic broach which was then placed into sterile 1.5 microcentrifuge tubes with phosphate buffered saline (PBS) for transfer to the biomedical laboratory for culture.
The original study protocol allowed for the isolation of dental pulp stem cells (DPSC) using the direct outgrowth method [14, 25]. In brief, cells were allowed to grow for ten passages and the rate of growth or doubling time (DT) was evaluated and assessed as the interval between 1:4 passaging and achieving confluence, as previously described [14, 16, 22]. This allowed for the identification of three distinct classes of DPSC, those with rapid doubling times (rDT) less than three days, those with relatively slow doubling times (sDT) of greater than one week (8-10 days), and a smaller subset with intermediate doubling times (iDT). These phenotypes were noted for each isolate prior to cryopreservation at (-80°C) using OptiFreeze Cryopreservation media from Fisher Scientific (Fair Lawn, NJ), as previously described [14-16, 22-24].
Upon thawing at each time point (1 week, 1 month, 6 months, 12 months, 24 months, 36 months and 48 months), viability was assessed using the Trypan Blue exclusion assay as previously described [22-24]. In brief, thawed cells were centrifuged and resuspended with cell culture media RPMI-1640 with 2 mM L-Glutamine containing 4.5 g/L glucose, 1.5 g/L sodium bicarbonate, 10 mM HEPES, 1.0 mM sodium Pyruvate, and 1% Penicillin- Streptomycin (10,000 unit/mL). Aliquots of 20uL cell suspension were then mixed with Trypan Blue and placed into hemocytometer counting slides for analysis using a BioRad TC20 automated cell counter (Hercules, CA) using the protocol recommended by the manufacturer. These data include total cell number, total live cells (used to calculate viability) and percentage of viable cells. Three measurements were taken for each DPSC isolate for statistical analysis and averaging.
RNA was isolated from an aliquot of each DPSC isolate using 1.0 x 107 cells at each of the previous time points, including baseline (T0) prior to cryopreservation, and at each of the subsequent one year time points (T1-T4). RNA was isolated using the total RNA isolation reagent (TRIR) from Molecular Research Center, Inc. (Cincinnati, OH) using the protocol recommended by the manufacturer. RNA quality and quantity was assessed using spectrophotometric analysis of each sample at 260 and 280 nm. The ratio of A260:A280 measurements provided a measurement of RNA purity (acceptable range between 1.7 – 2.0) and a general estimate of quantity.
All isolates with sufficient quality (A260:A280 > 1.7) and quantity (> 1 ng/uL) were processed and screened for DPSC biomarker expression as previously described [14-16, 22-24]. Mesenchymal stem cell (MSC) and DPSC biomarkers used in this screening included several previously validated cell surface (CD24, CD44 and CD133) and intracellular markers (Nestin, NANOG, Sox-2, Oct4) [26-28], as well as the housekeeping gene GAPDH (glyceraldehyde 3-phosphate dehydrogenase) or GAPDH, as follows:
CD24 FORWARD: ACTCTCACTTGAAATTGGGC;
CD24 REVERSE: GCACATGTTAATTACTAGTAAAGG;
CD44 forward primer, 5’-GAAAGGCATCTTATGGATGTGC-3’
CD44 reverse primer, 5’-CTGTAGTGAAACACAACACC-3’
\
CD133 forward primer, 5’-CTCATGCTTGAGAGATCAGGC-3’
CD133 reverse primer, 5’-CGTTGAGGAAGATGTGCACC-3’
Nestin FORWARD: CGTTGGAACAGAGGTTGGAG;
Nestin REVERSE: TCCTGAAAGCTGAGGGAAG;
NANOG forward primer, 5’-GCTGAGATGCCTCACACGGAG-3’
NANOG reverse primer, 5’-TCTGTTTCTTGACTGGGACCTTGTC-3’
Oct4 forward primer, 5’-TGGAGAAGGAGAAGCTGGAGCAAAA-3’
Oct4 reverse primer, 5’-GGCAGATGGTCGTTTGGCTGAATA-3’
Sox2 forward primer, 5’-ATGGGCTCTGTGGTCAAGTC-3’
Sox2 reverse primer, 5’-CCCTCCCAATTCCCTTGTAT-5’
GAPDH FORWARD: ATCTTCCAGGAGCGAGATCC;
GAPDH REVERSE: ACCACTGACACGTTGGCAGT
In brief, all reactions were standardized using 1ng/uL of extracted RNA and then processed using ABgene Reverse-iT One-Step RT-PCR protocol and reagents, as previously described [22-24]. Per standard procedures, reverse transcript was performed for 30 minutes at 47°C and then 30 amplification cycles were run, which included denaturation of 20 seconds, annealing of 30 seconds at the optimal temperature for each primer set, and five minutes of final extension at 72°C. Results were visualized using gel electrophoresis and ethidium bromide in a Kodak Gel Logic 100 Imaging System and 1D Image Analysis Software (Rochester, NY).
Basic descriptive statistics for viability were derived from the viability averages and reported in tables. DPSC from different categories of growth rates (rDT, iDT, sDT) were aggregated to create overall averages for these groups. Differences in viability at all time points between DPSC-rDT, -iDT, and -sDT were evaluated using two-tailed t-tests, which provide robust analysis even for samples with moderate sizes (n~20) [29, 30].
Results
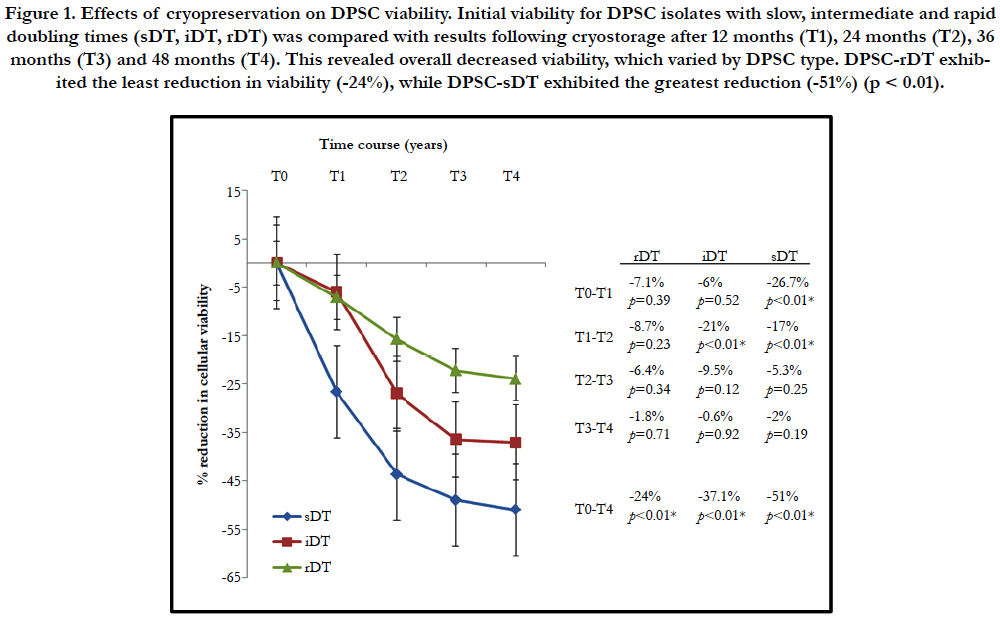
All DPSC were cultured for a minimum of ten passages to establish their growth rate, which varied within the range of 2.0 and 10.3 days. The doubling times were then used to group the DPSC into rapid doubling times (rDT < 3 days), intermediate doubling times (4-6 days) or comparatively slow doubling times (sDT > 8-10 days) - as previously established (Tomlin, 2016). Baseline viability was measured prior to the initial storage and cryopreservation following the initial ten passages. An aliquot from each DPSC line was retrieved from cryostorage at each of four time intervals and placed into cell culture (Figure 1). The analysis of cellular viability at each of the four time points (12 months - 48 months, T1 - T4) revealed an inverse relationship between the duration of DPSC cryopreservation and cellular viability upon thawing.
Figure 1. Effects of cryopreservation on DPSC viability. Initial viability for DPSC isolates with slow, intermediate and rapid doubling times (sDT, iDT, rDT) was compared with results following cryostorage after 12 months (T1), 24 months (T2), 36 months (T3) and 48 months (T4). This revealed overall decreased viability, which varied by DPSC type. DPSC-rDT exhibited the least reduction in viability (-24%), while DPSC-sDT exhibited the greatest reduction (-51%) (p < 0.01).
More specifically, DPSC with a rapid doubling time (rDT) exhibited an average decrease in cellular viability of -7.1% following 12 months in cryostorage, while DPSC with an intermediate doubling time (iDT) decreased an average of 6% over this time interval. DPSC with the slowest doubling time (sDT) exhibited the greatest decrease at this initial time point of -26.7%, which was statistically significant (p < 0.01). At each successive time point (T2-T4) all DPSC isolates exhibited decreasing viability, with the most significant declines observed between T1 and T2 - while the smallest occurred between T3 and T4.
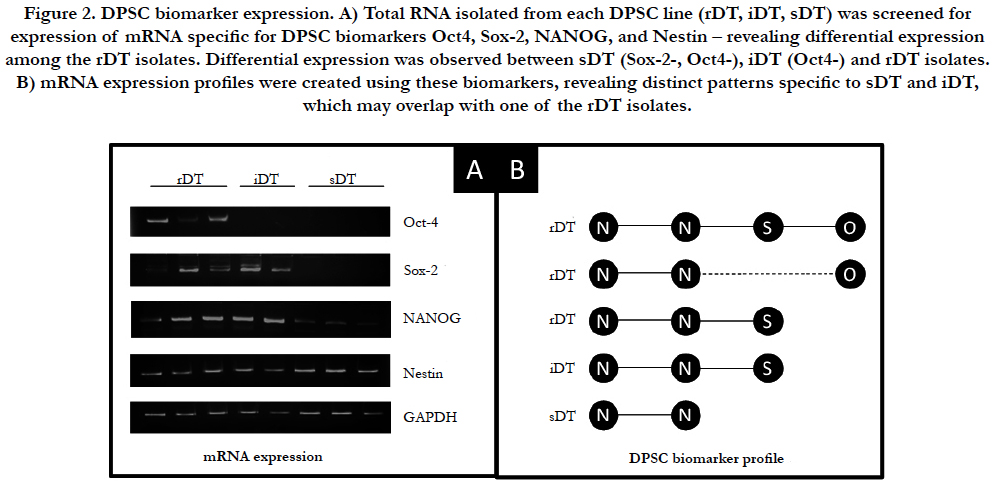
At each time point and determination of cellular viability, mRNA expression was assessed for multiple specific DPSC biomarkers. Some DPSC biomarkers examined (ABCG, CD24, CD44, CD133) were not included in this analysis as they exhibited no differences in mRNA expression (data not shown) (Figure 2). Intracellular mesenchymal stem cell markers Oct-4, Sox-2, NANOG and Nestin did exhibit differences in mRNA expression and were examined (Figure 2A). This analysis revealed differential expression of mRNA among the three groups DPSC-rDT cell lines. For example, although all three groups were observed to express mRNA for Nestin and NANOG, only one DPSC-rDT expressed both Sox-2 and Oct4. The remaining DPSC-rDT exhibited differential expression of either Oct4 or Sox-2 but not both (Figure 2B). Both of the DPSC-iDT exhibited similar mRNA expression profiles, which included Nestin, NANOG and Sox-2 but not Oct4. However, all of the DPSC-sDT exhibited similar expression of Nestin and, to a limited extent, NANOG.
Figure 2. DPSC biomarker expression. A) Total RNA isolated from each DPSC line (rDT, iDT, sDT) was screened for expression of mRNA specific for DPSC biomarkers Oct4, Sox-2, NANOG, and Nestin – revealing differential expression among the rDT isolates. Differential expression was observed between sDT (Sox-2-, Oct4-), iDT (Oct4-) and rDT isolates. B) mRNA expression profiles were created using these biomarkers, revealing distinct patterns specific to sDT and iDT, which may overlap with one of the rDT isolates.
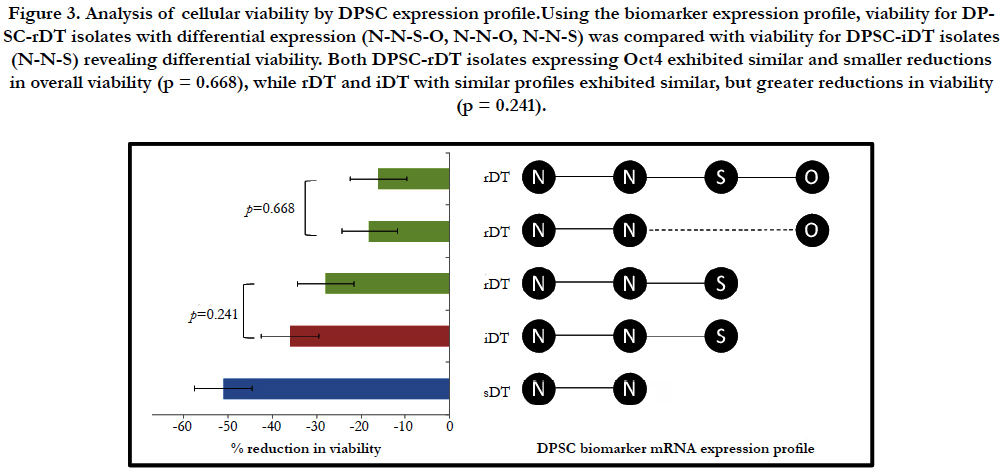
Due to the resulting overlap in the DPSC biomarker expression profiles of the DPSC-iDT and one of the rDT isolates, viability of each DPSC isolate was further evaluated based upon the individual expression profile result (Figure 3). Disaggregating the rDT isolates in this analysis revealed that the rDT isolates expressing Nestin (N), NANOG (N), Sox-2 (S) and Oct4 (O) (NN- S-O) were virtually indistinguishable from the rDT isolates that expressed Nestin, NANOG and Oct4, but not Sox-2 (N-N-O) (p = 0.668). In addition, the overall reduction in viability for the rDT isolates that expressed Oct4 (regardless of Sox-2) expression was significantly lower than the reductions in viability among the rDT isolates that expressed Nestin, NANOG, and Sox-2 (N-N-S) but not Oct4.
Figure 3. Analysis of cellular viability by DPSC expression profile.Using the biomarker expression profile, viability for DPSC-rDT isolates with differential expression (N-N-S-O, N-N-O, N-N-S) was compared with viability for DPSC-iDT isolates (N-N-S) revealing differential viability. Both DPSC-rDT isolates expressing Oct4 exhibited similar and smaller reductions in overall viability (p = 0.668), while rDT and iDT with similar profiles exhibited similar, but greater reductions in viability (p = 0.241).
Analysis of viability from the DPSC-rDT and iDT isolates with similar biomarkers expression profiles of Nestin, NANOG and Sox-2 (N-N-S) revealed similar reductions in viability at most time points, but were statistically indistinguishable from one another (p=0.241). Finally, the analysis of DPSC-sDT isolates, which only expressed Nestin and NANOG (N-N) revealed the greatest reduction in cellular viability at each interim time and the largest reduction overall between T0 and T4. These findings were significantly different from those of the DPSC-iDT and DPSC-rDT isolates evaluated.
Discussion
This study is among the first to provide evidence that long-term cryopreservation has significant effects on the viability of DPSC [22]. It is important to note that although previous studies have evaluated some of the biological effects of cryopreservation on DPSC, most evaluated these effects after a period of six months or less [2, 12, 21]. If clinical and therapeutic applications are to be a viable option for patients, more studies regarding the basic biology and feasibility of storage and cryopreservation will be needed to further elucidate the parameters that govern these observations and findings.
More importantly, this study may be the first to provide evidence that the reduced viability and long-term effects of cryopreservation may not be strictly dose-dependent. For example, although some studies evaluated and analyzed viability and growth following a short time interval (usually one to two weeks) compared with a longer time interval, such as six months [2, 21], this study may represent the first evidence to demonstrate that the declines in viability appear to be most striking within the first two years, with smaller changes observed in following years and almost no change in viability between years three and four - regardless of DPSC phenotype (sDT, iDT, rDT). Moreover, the magnitude of these changes in viability appeared to correlate with cellular phenotype or growth rate - the more rapidly growing DPSC-rDT exhibiting the smallest reduction in viability at all time points and the slowest growth DPSC-sDT exhibiting the largest overall reduction.
To more fully examine these observations, the evaluation of biomarkers from each DPSC isolate revealed similar expression of cell surface markers (CD24, CD44, CD133) but striking differential expression of key intracellular biomarkers (NANOG, Sox-2, Oct4) [22, 27, 28]. For example, although all sDT and iDT isolates had similar expression profiles to one another (N-N and N-NS, respectively), the three rDT isolates exhibited differential expression (N-N-S-O, N-N-O, N-N-S). Interestingly, when the viability of each individual isolate was analyzed independently, this revealed that the rDT and iDT isolates with similar biomarker profiles (N-N-S) had similar viability following cryopreservation, which was lower and distinct from the rDT that also expressed Oct4 (N-N-S-O, N-N-O). This may suggest that Oct4 but not Sox-2, both associated with pluripotency in mesenchymal and dental pulp stem cells, may also be associated with (or an indicator of) one or more biological pathways involved in the regulation of cellular viability [27, 28].
Despite the significance of these findings, it is important to note that there are several limitations which must also be considered. First, this is a retrospective examination of previously collected DPSC isolates - therefore, the initial conditions of isolation, culture and storage were outside the parameters of this study and could not be subjected to change or experimentation. Also, this study was conducted using patients from a public Universitybased dental school patient population, which may be significantly different from the traditional orthodontic patient populations seeking treatment and potential DPSC cryopreservation [23]. Finally, differing methods or materials for cryopreservation were not studies - which may have influenced the outcomes observed in this study.
Conclusion
This study is among the first to provide evidence that long-term storage and cryopreservation of DPSC varies non-linear over time. This study is also among the first to provide evidence that phenotypic behaviors, such as doubling time or growth, may be one of the most important factors that determines long-term DPSC viability. Finally, this study also revealed that Oct4 and Sox-2 are among the most important variable factors that are associated with both growth and viability, which may be useful for future applications and therapies that could screen and sort DPSC using predetermined biomarkers.
Acknowledgements and Declarations
The authors would like to thank the Department of Advanced Education in Orthodontics and Dentofacial Orthopedics and the Office of Research at the University of Nevada, Las Vegas - School of Dental Medicine for funding support.
References
- Aurrekoetxea M, Garcia-Gallastegui P, Irastorza I, et al. Dental pulp stem cells as a multifaceted tool for bioengineering and the regeneration of craniomaxillofacial tissues. Front Physiol. 2015 Oct 16;6:289. PubMed PMID: 26528190.
- Hata M, Omi M, Kobayashi Y, Nakamura N, et al. Transplantation of cultured dental pulp stem cells into the skeletal muscles ameliorated diabetic polyneuropathy: therapeutic plausibility of freshly isolated and cryopreserved dental pulp stem cells. Stem Cell Res Ther. 2015 Sep 7;6:162. PubMed PMID: 26345292.
- Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Concise review: dental pulp stem cells: a novel cell therapy for retinal and central nervous system repair. Stem Cells. 2017 Jan;35(1):61-67. PubMed PMID: 27273755.
- Nagpal A, Kremer KL, Hamilton-Bruce MA, Kaidonis X, Milton AG, Levi C, Shi S, Carey L, Hillier S, Rose M, Zacest A. TOOTH (The Open study Of dental pulp stem cell Therapy in Humans): Study protocol for evaluating safety and feasibility of autologous human adult dental pulp stem cell therapy in patients with chronic disability after stroke. Int J Stroke. 2016 Jul;11(5):575-85. PubMed PMID: 27030504.
- Ajlan SA, Ashri NY, Aldahmash AM, Alnbaheen MS. Osteogenic differentiation of dental pulp stem cells under the influence of three different materials. BMC Oral Health. 2015 Oct 28;15:132. PubMed PMID: 26510991.
- Zhang J, Lu X, Feng G, Gu Z, Sun Y, et al. Chitosan scaffolds induce human dental pulp stem cells to neural differentiation: potential roles for spinal cord injury therapy. Cell Tissue Res. 2016 Oct;366(1):129-42. PubMed PMID: 27147262.
- Liu G, Xu G, Gao Z, Liu Z, et al. Demineralized dentin matrix induces odontoblastic differentiation of dental pulp stem cells. Cells Tissues Organs. 2016;201(1):65-76. PubMed PMID: 26569105.
- Collart-Dutilleul PY, Chaubron F, De Vos J, Cuisinier FJ. Allogenic banking of dental pulp stem cells for innovative therapeutics. World J Stem Cells. 2015 Aug 26;7(7):1010-21. PubMed PMID: 26328017.
- Chen YJ, Zhao YH, Zhao YJ, et al. Potential dental pulp revascularization and odonto-/osteogenic capacity of a novel transplant combined with dental pulp stem cells and platelet-rich fibrin. Cell Tissue Res. 2015 Aug;361(2):439-55. PubMed PMID: 25797716.
- Mead B, Berry M, Logan A, Scott RA, Leadbeater W, Scheven BA. Stem cell treatment of degenerative eye disease. Stem Cell Res. 2015 May;14(3):243- 57. PubMed PMID: 25752437.
- Eubanks EJ, Tarle SA, Kaigler D. Tooth storage, dental pulp stem cell isolation, and clinical scale expansion without animal serum. J Endod. 2014 May;40(5):652-7. PubMed PMID: 24767559.
- Lindemann D, Werle SB, Steffens D, et al. Effects of cryopreservation on the characteristics of dental pulp stem cells of intact deciduous teeth. Arch Oral Biol. 2014 Sep;59(9):970-6. PubMed PMID: 24949827.
- Wu W, Zhou J, Xu CT, Zhang J, et al. Derivation and growth characteristics of dental pulp stem cells from patients of different ages. Mol Med Rep. 2015 Oct;12(4):5127-34. PubMed PMID: 26239849.
- Alleman M, Low E, Truong K, Huang E, et al. Dental pulp-derived stem cells (DPSC) differentiation in vitro into odontoblast and neuronal progenitors during cell passaging is associated with alterations in cell survival and viability. IJBMR. 2013;2(2):133-41.
- Burnett A, Kumar R, Westphal JD, Kingsley K. Dichloroacetate (DCA) Promotes a De-Differentiated Phenotype in Dental Pulp-Derived Stem Cells in vitro. Int J Biol Sci. 2015;2(3):25-32.
- Loveland K, Young A, Khadiv M, Culepper M, Kingsley K. Dental Pulp Stem Cell (DPSC) Pluripotency Enhanced by Transforming Growth Factor (TGF-β1) in Vitro may be Inhibited by Differentiation-Inducing Factors Laminin-5 and Dexamethasone. Int J Biol Sci. 2014;1(3):55.
- Arora V, Arora P, Munshi AK. Banking stem cells from human exfoliated deciduous teeth (SHED): saving for the future. J Clin Pediatr Dent. 2009 Summer;33(4):289-94. PubMed PMID: 19725233.
- Gioventù S, Andriolo G, Bonino F, et al. A novel method for banking dental pulp stem cells. Transfus Apher Sci. 2012 Oct;47(2):199-206. PubMed PMID: 22795998.
- Ma L, Makino Y, Yamaza H, Akiyama K, et al. Cryopreserved dental pulp tissues of exfoliated deciduous teeth is a feasible stem cell resource for regenerative medicine. PloS one. 2012 Dec 14;7(12):e51777. PubMed PMID: 23251621.
- Perry BC, Zhou D, Wu X, Yang FC, et al. Collection, cryopreservation, and characterization of human dental pulp–derived mesenchymal stem cells for banking and clinical use. Tissue Eng Part C Methods. 2008 Jun;14(2):149-56. PubMed PMID: 18489245.
- Woods EJ, Perry BC, Hockema JJ, et al. Optimized cryopreservation method for human dental pulp-derived stem cells and their tissues of origin for banking and clinical use. Cryobiology. 2009 Oct;59(2):150-7. PubMed PMID: 19538953.
- Tomlin A, Sanders MB, Kingsley K. The effects of cryopreservation on human dental pulp-derived mesenchymal stem cells. IEEE J Biomed Health Inform. 2016;3(2):103-112.
- Young A, Kingsley K. Dental Pulp Stem Cell: A review of factors that influence the therapeutic potential of stem cell isolates. BME. 2015;2(2):61-9.
- Hung E, Lee S, Fitzgerald B, Hill CK, Kingsley K. Dental pulp-derived stem cell (DPSC) survival and viability may correlate with specific patient demographics. FDSRI Winter. 2013;1(3):14-21.
- Bakopoulou A, Leyhausen G, Volk J, et al. Assessment of the impact of two different isolation methods on the osteo/odontogenic differentiation potential of human dental stem cells derived from deciduous teeth. Calcif Tissue Int. 2011 Feb;88(2):130-41. PubMed PMID: 21153807.
- Camilleri ET, Gustafson MP, Dudakovic A, et al. Identification and validation of multiple cell surface markers of clinical-grade adipose-derived mesenchymal stromal cells as novel release criteria for good manufacturing practice-compliant production. Stem Cell Res Ther. 2016 Aug 11;7(1):107. PubMed PMID: 27515308.
- Ferro F, Spelat R, D'Aurizio F, Puppato E, et al. Dental pulp stem cells differentiation reveals new insights in Oct4A dynamics. PloS one. 2012 Jul 23;7(7):e41774. PubMed PMID: 22844522.
- Liu L, Wei X, Ling J, Wu L, Xiao Y. Expression pattern of Oct-4, Sox2, and c-Myc in the primary culture of human dental pulp derived cells. J Endod. 2011 Apr;37(4):466-72. PubMed PMID: 21419292.
- Glaser AN. 20040 High-Yield Biostatistics. 3rd Ed. Lippincott Williams Wilkins.
- Jekel, Katz, Elmore.Epidemiology, Biostatistics and Preventive Medicine, 2nd Ed. 2001.