Optical Spectroscopy and Prevention of Deleterious Brain- damaging Cerebral Vascular Effects of Cocaine by Magnesium Ions: Effects on Brain Mitochondrial Oxidase, Deoxyhemoglobin, Ceramide and Sphingomyelin Levels and Their Potential Application to Human Substance Abuse
Altura BM1-7*, Gebrewold A1, Carella A1, Barbour RL8, Wu F1, Altura BT1, 3-7
1 Department of Physiology and Pharmacology, The State University of New York Downstate Medical Center, Brooklyn, New York, USA.
2 Department of Medicine, The State University of New York Downstate Medical Center, Brooklyn, New York, USA.
3 The Center for Cardiovascular and Muscle Research, The State University of New York Downstate Medical Center, Brooklyn, New York, USA.
4 The School for Graduate Molecular and Cellular Science, The State University of New York Downstate Medical Center, Brooklyn, New York, USA.
5 Bio-Defense Systems, Inc, Rockville Centre, New York, USA.
6 Orient Biomedica, Estero, Florida, USA.
7 Magnesium for Health Foundation, Patterson, California, USA.
8 Department of Pathology, State University of New York, Brooklyn, New York, USA.
*Corresponding Author
B. M. Altura,
Professor, Box 31, SUNY Downstate Medical Center,
450 Clarkson Ave., Brooklyn, NY 11203, USA.
Tel: 718-270-2194
E-mail: burton.altura@downstate.edu
Received: September 27, 2019; Accepted: October 14, 2019; Published: October 16, 2019
Citation: Altura BM, Gebrewold A, Carella A, Barbour RL, Wu F, Altura BT. Optical Spectroscopy and Prevention of Deleterious Brain- damaging Cerebral Vascular Effects of Cocaine by Magnesium Ions: Effects on Brain Mitochondrial Oxidase, Deoxyhemoglobin, Ceramide and Sphingomyelin Levels and Their Potential Application to Human Substance Abuse. Int J Cardiol Res. 2019;6(1):137-143. doi: http://dx.doi.org/10.19070/2470-4563-1900023
Copyright: B. M. Altura© 2019. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Previously, four of us suggested that acute cocaine HCl administration can result in concentration-dependent vasoconstriction, vasospasm and decreased cerebral blood flow. Here, we present new in vivo results using rapid (240 nm/min) optical backscatter measurements, with an intact cranial preparation in the rat, indicating that acute infusion of cocaine HCl directly (via branch of internal carotid) into the rat brain rapidly produces dose-dependent vasoconstriction of the cerebral microcirculation associated with a pronounced reduction in tissue blood content, pronounced elevation in deoxyhemoglobin, significantly increased levels of reduced cytochrome oxidase aa3 and microvascular damage as the dose of cocaine was increased. Furthermore, we present in vivo experiments demonstrating the capability of magnesium ions (Mg2+) to attenuate and prevent these deleterious responses of cocaine HCl. Optical backscatter spectra (500-800 nm) were obtained by directing a single sending and receiving fiber to a portion of the left parietal cranium (in anesthetized rats), shaved to a translucent appearance to facilitate optical penetration. In the absence of added Mg2+, infusion of a solution of cocaine HCl at 0.34 ml/ min produced prompt vasoconstriction as evidenced by a greater than 90% loss of oxyhemoglobin from the field of view and increases in levels of reduced cytochrome oxidase to between 50% and >90%. These effects were partially, to nearly completely attenuated by the addition of MgCl2 to the infusion containing added cocaine HCl. Of particular interest was the observation that attenuation of the vasoconstrictive effects of cocaine by Mg persisted despite a subsequent cocaine challenge without added Mg2+. In other experiments, based on previous studies, we noted rapid increases in production and cellular release of ceramides concomitant with reductions in brain sphingomyelin in response to cocaine administration which were either attenuated or inhibited by prior administration of either Mg2+ or a blocker of ceramide synthesis, namely, myriocin. Our new results indicate that, depending upon dose, cocaine HCl can produce prompt and severe vasoconstriction and that infusion of Mg2+ can largely attenuate and prevent this response. In addition, we demonstrate that infusion of cocaine directly into the brain results in rapid synthesis and release of ceramides which can be attenuated/inhibited by pretreatment of animals with either Mg2+ or myriocin.
2.Materials and Methods
2.1 Animal model, optical spectroscopy and protocol
2.2 Optical Measurements
2.3 Interpretation of optical spectra
2.4 Level of deoxyhemoglobin
2.5 Levels of reduced cytochrome oxidase aa
2.6 Measurement of brain ceramides and sphingomyelin and use of myriocin or Mg
2.7 Statistics
3.Results
4.Discussion
5.Acknowledgements
6.References
Introduction
Clinical and experimental studies have now, unequivocally, established that ingestion, intravenously administered, or snorting of cocaine or abuse of cocaine can produce a variety of dangerous effects in different areas of the brain, including profound reductions in blood flows and strokes [1-7]. These actions include atrophy of cortical, subcortical, prefrontal cortical, hippocampal, medullary and cerebral areas of the brain associated with headaches, blackouts, functional neuronal deficits and pyschoses. Clinically, it is known that cocaine abuse can result in hemorrhagic strokes and cerebral infarctions [1-7]. The first report of cocaine-induced stroke was reported in 1977, more than 40 years ago by Brust and Richter [8].
Previous studies from our laboratories, using image-splitting in vivo television microscopy, have shown that acute infusion of cocaine HCl, produced graded concentration-dependent spasms of cerebral and medullary arterioles and arteries in the intact rat brain causing rupture of venular postcapillaries, thus resembling stroke-like events [9]. Moreover, examination of isolated canine, rat, monkey and baboon cerebral and basilar arteries contracted in a dose-dependent manner upon the addition of cocaine HCl in isolated organ baths maintained under physiologic conditions [10, 11]. In addition, we have shown in intact, unopened rat brains, using 31P-nuclear magnetic resonance (NMR) spectroscopy, that administration of cocaine HCl can produce concentrationdependent brain ischemia, preceded by rapid falls in brain intracellular free Mg2+ ([Mg2+]i) [12]. Increasing doses of cocaine HCl induced hemorrhagic strokes in these rat models preceded by falls in phosphocreatine and ATP and rises in intracellular phosphate levels [12]. Several additional studies on the intact rat brain, using in situ 31P-NMR spectroscopy and direct invivo observations of the cerebral microcirculation suggest that administration of Mg2+ can prevent these hemorrhagic strokes [9, 12]. Whether or not these effects of Mg2+ result in diminution or a complete loss of mitochondrial ischemic events in the brain caused by cocaine is not known.
There is a growing body of both clinical and experimental literature to suggest that central nervous system (CNS) injury usually results in early and pronounced alterations in blood and brain levels of Mg2+ [13-20]. Mg2+ deficiency prior to induction of experimental brain injury with cocaine and percussion injury is associated with higher mortality and worsened neurological outcomes [21].
Several years ago, optical near-infrared spectroscopy had been used as a noninvasive technique to determine and measure cerebral oxygen availability in the intact rat brain [22, 23]. In the study, herein, using rapid optical backscatter measurements, and visible near-infrared optical spectroscopy, we have tested the hypothesis that administration of magnesium chloride will largely block the ischemic effects of cocaine HCl in the brain as determined by noninvasive measurements of mitochondrial cytochrome oxidase aa3, deoxyhemoglobin and microvascular damage.
Recently, our laboratories have provided evidence to indicate that low levels of [Mg2+]0 results in rapid synthesis and release of ceramides in canine and rat cerebral arterial smooth muscle cells and in all four chambers of rat heart cardiomyocytes [24-26]. We have reported that ceramides induce potent concentrationdependent contractions of canine and subhuman primate cerebral arteries [26-28]. In addition, using in situ high-resolution television-optics of the pial and medullary microvasculatures, we have found that many ceramides induce vasoconstriction-spasms of venules leading to adherence of leukocytes, macrophages and monocytes on the postcapillary venular walls [28]. In view of these new experiments, we hypothesized that if cocaine HCl resulted in rapid reductions in brain [Mg2+]I, like we have reported previously [12, 21], then infusion of cocaine into the brain should cause alterations in the levels of brain ceramides (i.e., rises) which might be inhibited or attenuated by a specific antagonist of ceramide synthesis like myriocin.
A description of the methods used for the surgical preparation of the animals has been described previously [29]. Briefly, male Wistar rats (175-230 g) were anesthetized with sodium pentobarbital (35-45 mg/kg, intramuscularly, Nembutal) and cannulae were placed into a branch of the internal carotid artery (PE10 tubing) and a femoral vein (PE20 tubing). To improve optical penetration, the skull was exposed and shaved, very carefully), to a translucent appearance [29]. The animal was then placed in a prone position and the head stabilized by use of a three-point stereotaxic positioner [29]. The underlying tissue was resected and the calvarium thinned by careful scaping with a scalpel until the outline of the cerebral vessels was evident [29]. Repeated infusions of small doses of pentobarbital (3-5 mg) were made as needed to maintain a light plane of surgical anesthesia [29].
Fiber optic bundles were used to intercept light entering the sample and reference cells of a Perkin-Elmer Lambda 5 spectrophotometer as described previously [30]. The bundle from the sample port was directed to a 20X Newport microscopic objective lens that focused the light onto the end of a 1-mm diameter single glass fiber [30]. The fiber was used to illuminate the tissue. A second, receiving fiber, positioned 2.5-3.0 mm from the illuminating fiber, served to capture the backscattered signals [30]. The fiber optic bundle from the reference port and the receiving fiber were directed to a homemade “black-box” containing a Hammamatsu model 463 end-on photomultiplier tube. The intensity of the reference signal could be adjusted by varying the aperture of an adjustable iris [30].
The system was calibrated against the 656.1 mm emission line from a deuterium lamp [30]. On each day of use, the intensity of the optical signals from the sample and reference fibers was balanced by performing a background correction against a barium sulfate planchet. Once calibrated, the fibers were oriented normally to the surface and placed in light contact with the thinned calvarium. A drop of microscope oil was placed at the point of contact to improve optical couplingand stabilize the signal. Optical measurements were made by scanning at 240 mm/ min between 800 and 500 nm in steps of 1 nm resolution with the instrument set in the transmittance mode [30]. Results were displayed in the absorbance mode.
Relative blood content: Variations in the relative blood content in the viewing field were estimated by comparing the intensity of the signal about the isobestic point in the range of 586- 591 nm from control spectra (no infusion) to spectra obtained during infusion of Ringers solution at high flow rates (0.8 ml/ min). Under these conditions, global blanching of the brain was apparent, with no evidence of the hemoglobin signal being present and only the background-reduced cytochrome spectrum could be seen [30]. At wavelengths below 600 nm, the amplitude of the latter was reduced by ~ 40% when compared with control spectra. The wavelength internal for the apparent “isobestic point” was derived by examining multiple sets of control spectra and spectra from KCl-arrested animals (n=8). This interval is not indicative of instability in the measurements, but rather likely, results from differences among the preparations in coupling efficiencies and thickness of the underlying cranium [30]. This is supported by two lines of evidence: 1. Replicate measurements in control animals were reproducible; and 2. The observed signal-tonoise level is typically greater than 50:1.
An estimate of the level of deoxyhemoglobin in the animal spectra was madeby comparing differences in signal intensity at 576 and 587 nm to measurements performed using the same setup on mixtures containing whole rat blood with added 5% v/v microscopic beads (2.02 um, 10% solids, Seragen Diagnostics) that had been equilibrated to different O2 tensions (n-6) [30]. The latter was accomplished by varying a mixture of gases containing 95% O2/5% CO2 and 95% N2/5% CO2. The corresponding oxygen saturation of the mixtures (without added latex beads) was independently determined using a Radiometer OSM3 oximeter operated in the animal mode for a rat. The mean value of hemoglobin O2 saturation calculated from 15 animal control spectra by this method was 91+/-4%, which is similar to that found in vivo.
Levels of reduced cytochrome oxidase were estimated by comparing the difference in signal intensity at 605 and 620 nm [30]. The differences seen in control spectra and KCl-arrested animals was defined as fully oxidized and fully reduced, respectively. Intermediate values were calculated by linear interpolation.
Difference spectra were computed by subtracting the data obtained, at the indicated infusion rate, in the presence of added cocaine HCl and cocaine HCl plus added MgCl2, similar to methods we published previously [30].
A total of 78 male Wistar rats were used in these studies including animals used in the Mg2+ coadministration experiments. Continuous infusion was performed using a Harvard infusion pump at low settings of 0.07, 0.14 and 0.34 ml/min. The infusate was Ringers solution with and without added cocaine HCl. In some experiments, the latter was supplemented with added MgCl2 at concentrations between 5 and 20 mM. Control studies involved infusing Ringers solution at the above flow rates and optical scans were started within 30 s after initiating the infusion. Following this, solutions containing cocaine HCl were infused at the different flow rates using a similar protocol. Subsequently, a solution containing added MgCl2 (5-20 mM) and cocaine HCl was infused at the indicated flow rates. In six of eight preparations, the initial concentration of MgCl2 tested was 5 and 10 mM for the remaining two. For the former, in two preparations, minimal or only moderate attenuation of the cocaine HCl-induced vasoconstrictive response was observed. In these cases, following a10-20 min period of no infusion, a subsequent challenge at the same flow rate (i.e.,0.34 ml/min) was given but with a higher concentration of added MgCl2.
Using different groups of animals (six each), which were sacrificed by an overdose of pentobarbital sodium (100 mg/kg), several different experiments were performed: 1. infusion of cocaine HCl with or without MgCl2; 2. Infusion of cocaine HCl with or without Ringers solution; and 3. Infusion of cocaine HCl after animals received either 10 mg/kg of myriocin or MgCl2 given intravenously via tail vein injection.
Ceramides and sphingomyelin were measured in extirpated cerebral hemispheres after the foregoing groups of study protocols according to methods we previously published [26].
Where appropriate, means +/- S.E.M. were calculated and compared using paired and unpaired t-tests as well as analysis of variance (ANOVA) with Scheffe’s contrast test. A p-value of less than 0.05 was considered significant.
Results
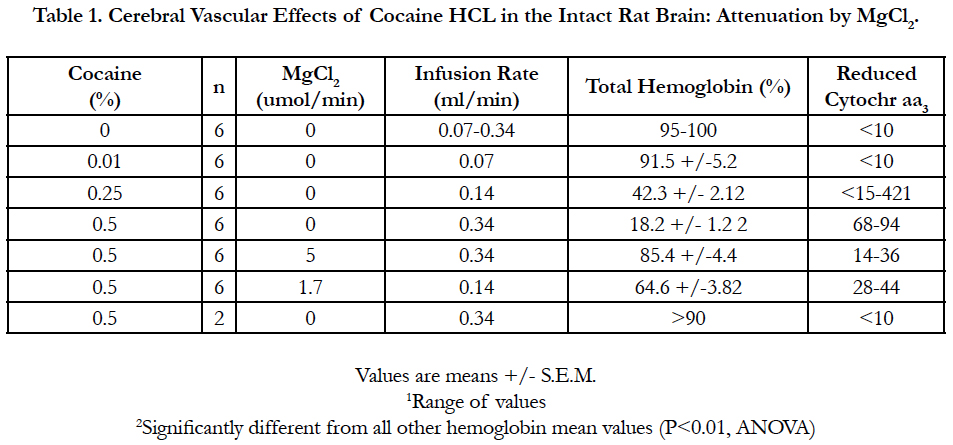
The data are summarized in Tables 1 and 2. Infusion of cocaine HCl showed that it produced a dose-dependent vasoconstrictive response resulting, at the highest flow rate (0.34 ml/min), near complete exclusion of the hemoglobin signal from the field of view of the receiving fiber. The resultant spectra revealed that the background tissue cytochromes in their reduced state were essentially indistinguishable from that observed 60 min following death of the animal or by infusing Ringers solution at low flow rates high enough to exclude blood from the brain (>1.36 ml/ min). The peaks of the spectra at approximately 550 and 605 nm were consistent with the known absorption maximum of reduced cytochrome c + c1 and aa3, respectively [31].
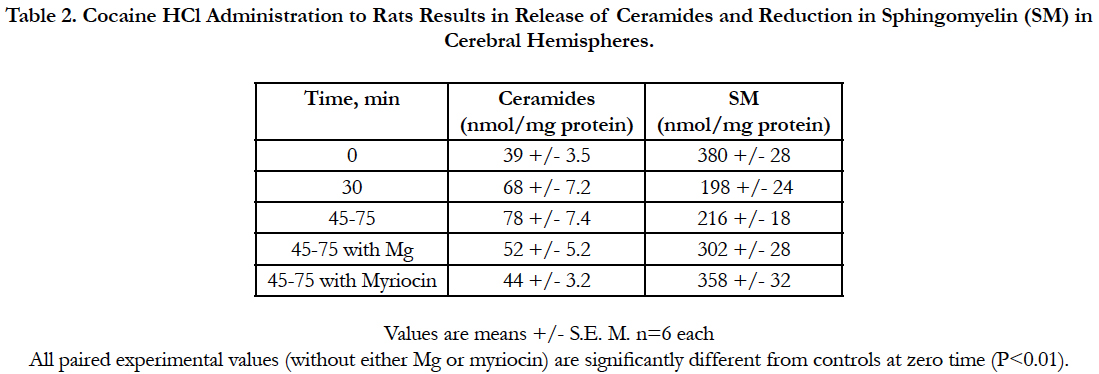
Table 2. Cocaine HCl Administration to Rats Results in Release of Ceramides and Reduction in Sphingomyelin (SM) in Cerebral Hemispheres.
At the highest flow rates, the cocaine-induced vasoconstrictive response was even observable by the unaided eye in room light, and was seen as a global blanching of the brain tissue. Significantly, when 5 mM MgCl2 was included in the infusate containing 0.5 % cocaine HCl, the hemoglobin signal recovered to approximately 85% of the control level. A similar finding was observed in two other animal preparations. While estimates of the level of deoxyhemoglobin in these spectra were difficult to quantify, due to the change in signal intensity, at the reported isobestic point (i.e., approximately 587 nm), the observation that the absorption maximal at approximately 545 and 576 nm have nearly equivalent amplitudes, and the lack of any distinctive peak at 560 nm would suggest it is quite low. This finding, together with the observed increase in the total hemoglobin signal, clearly indicates that the coadministration of MgCl2 at a dose of 1.7 umol/min significantly attenuated the profound vasoconstrictive effect caused by infusion of cocaine HCl.
Coadministration of 10 mM MgCl2 to the 0.5% cocaine HCl infusate nearly completely attenuated the cocaine vasoconstrictive effects. Although not shown, it should be noted, here, that the primary spectrum for this infusion protocol overlapped nearly completely with the spectrum obtained for infusion of 0.5% cocaine HCl at 0.07 ml/min. Of particular interest in this set of experiments, however, was the observation that a subsequent cocaine challenge in the absence of added MgCl2, performed 10 min following the previous infusion, failed to produce the expected vasoconstrictive response, thus suggesting either tachyphylaxis, a threshold effect or a hysteresis in the vascular response.
Analysis of the concentrations of ceramides in the cerebral hemispheres 30 min after intravenously administered cocaine HCl, as predicted, indicated progressive rises in the synthesis of total ceramides (increasing with time elapsed), which was attenuated by MgCl2, also as predicted, and completely inhibited by prior treatment with myriocin (see Table 2). Sphingomyelin levels were reduced with elapsed time and prevented from deficit reductions in the presence of either MgCl2 or treatment with myriocin (Table 2).
Discussion
As of this writing, the available techniques used for diagnostic brain imaging can be classified into structural and functional imaging methods. Structural imaging of the brain is utilized to acquire anatomical information (e.g., X-ray computed tomography [CT], magnetic resonance imaging [MRI], and ultrasound imaging) while the goal of functional imaging of the brain is to acquire information on the physiological state of cerebral and other brain tissues ( e.g., blood flows, oxygen consumption, metabolic activity, neuronal activity, etc.). These methods include functional MRIs (fMRIs), electroencephalography (EEG), magnetoencephalography (MEG), positron emission tomography (PET), and single photon emission computed tomography (SPECT). Near-infrared spectroscopy NIRS) was designed to measure concentration changes in hemoglobin and mitochondrial cytochromes in the brain, noninvasively [32,33]. NIRS, although primarily utilized to assess brain tissue oxygenation, has also demonstrated considerable potential for neuroimaging (e.g., functional NIRS) [32, 34-35]. Approximately 20 years ago, Villringer and Chance used noninvasive approaches employing near-infrared light to interrogate the human cortex through the intact scalp and skull [23]. It is now thus possible, as utilized herein, to employ visible light to illuminate the brain.
Our present results, using optical reflectance spectroscopy, confirm and extend our previous work and that of others that have utilized only invasive techniques (e.g., isotopes; in vivo microcirculatory studies) and 31P-NMR spectroscopy to assess dynamics of cerebral blood flow changes in the intact brain in response to administration or ingestion of various doses of cocaine [2, 3, 5, 9-12, 21]. The increased, observed incidence of strokes seen in human subjects after cocaine abuse, and the controversy concerning the mechanism (s) of cocaine-induced strokes, makes the noninvasive approach taken herein of special importance. Although the optical approach taken, herein, does not allow one an examination of discrete, microscopic localized areas of the brain microvasculature (i.e., arterioles, metarterioles, precapillary sphincters, and venules or capillaries per se), it can discern noninvasively tissue oxygenation, blood volume, the mitochondrial state, and the degree of tissue ischemia in a closed cranium, thus allowing rapid, repeat or continuous assessment of blood flow distribution prior to, during or post-ischemic (or stroke-like) syndromes. In our opinion, the technology used, herein, is a major advancement, particularly in the substance abuse field.
Due to the great dependence of cerebral function on oxidative metabolism, occurrence of ischemic events in the brain could significantly interfere with the oxidative capacity of the brain cerebral tissues leading to the desaturation of oxyhemoglobin in the capillary bed, as found in the present study, and the resultant reduction of mitochondrial function as indicated by the precipitous concentration-dependent rise in reduced cytochrome oxidase. With respect to the latter, the results in the present study suggest that oxidative function would be severely compromised due to the high levels (i.e., 50-90%) of reduced cytochrome oxidase. These results remind us that Mg deficiency in rats yielded a very similar result, i.e., a precipitous rise in reduced cytochrome oxidase in cardiac myocytes, cerebral vascular smooth muscle cells, and cerebral-medullary tissue cells [26, 36-42, unpublished findings]. Whether the reduction in cerebral vascular smooth muscle cell, glial cell, and brain reduction in free [Mg2+]I followed by membrane entry and release of intracellular Ca2+, which we have reported on previously [41, 43-46], is in large measure responsible for the cocaine-induced reduction in mitochondrial metabolism, and is the major mechanism for mitochondrial dysfunction, remains to be determined. However, when the present findings are taken together with the cocaine-induced rises in brain ceramides , reported herein, we believe the latter is the most likely mechanism along with direct vasoconstrictive actions on cerebral arterial, arteriolar and precapillary vessels we have reported on previously [21, 24, 26-28].
Since more than 90% of the O2 consumed by mammals and humans involves cytochrome aa3 or complex IV (cytochromec- O2 oxidoreductase) [47], the present study would suggest that cocaine-induced decreases in brain O2 content (indicated by >75-90% increases in deoxygenated hemoglobin) must perforce reduce the cerebral cytochrome aa3 redox state. Others have reported previously that when oxygen saturation of arterial blood reaches 88% in humans, significant reduction of cytochrome aa3 is observed [22]. Most importantly, the present study also demonstrates that coadministration of Mg2+ with cocaine HCL results in a reversibility of the dramatic increase in reduced cytochrome oxidase. The fact that administration of myriocin, by itself, markedly reduces the increase in reduced cytochrome aa3, as well as resulting in an attenuation of the cocaine-induced increase in deoxyhemoglobin, clearly supports a role for synthesis and release of ceramides in the observed biochemical and biophysical cocaine-induced disturbances in mitochondrial functions. Our finding of a concomitant decrease in cerebral sphingomyelin (SM) (with time after cocaine) suggests that some of the rise in cerebral concentration of ceramides must be due to the breakdown of some SM. This probability is currently being explored in our labs.
Important roles for Mg2+ in the pathophysiology of brain injury and trauma have been suggested previously by numerous experimental and human studies which demonstrate that brain injury, including that induced by numerous substances of abuse (i.e., alcohol, PCP, marijuana-cannabis products, amphetamines, psilocybin, heroin, mescaline, etc.) is accompanied early by decreases in brain total and ionized Mg2+ [12, 13, 15, 21, 39, 48-60] concomitant with marked depression in blood free Mg2+ levels; in these cases, ionized levels of Mg2+ are clearly more affected than total Mg levels. Ingestion or injection of cocaine HCl prior to brain trauma clearly intensifies the depression in circulating levels of Mg2+ in human subjects [6]. We have found that dietary deficiency of Mg intake for short periods of time (i.e., 21 days) is associated with significantly higher mortality to subsequent administration of cocaine and higher stroke mortality [21]. In this context, our laboratory has reported that dietary deficiency in Mg intake can rapidly (even over a few days) lower brain free Mg levels but not necessarily brain total Mg levels [61].
Experimentally, using different forms of brain trauma, it has been demonstrated that administration of Mg will either prevent or reduce the losses in brain free Mg levels, reduce neuromotor deficits, and reduce memory loss in both anesthetized and freely moving animals [13, 17, 20, 21, 26, 62-65]. In addition, Mg2+ has been shown to be neuroprotective in hippocampal brain slice preparations subjected to neurotoxic or anoxic degeneration caused by excitatory amino acids or global ischemia [66, 67].
Our present data, as well as our data acquired in alcohol-, marijuana-cannabis-, amphetamines-, heroin-, fentanyl-, and psychedelic drug-induced strokes [9, 12, 17, 21, 51-60], are consistent with the latter findings. The present findings indicating that Mg2+ infusion prevents or ameliorates cocaine-induced mitochondrial dysfunction (as observed by precipitous rises in reduced cytochrome oxidase) are consistent with findings that decreased extracellular Mg2 results in increased cortical cell death due to oxidative injury [68]. Since cocaine HCl administration to rats results in, initially and rapidly (less than 3 min), in marked losses of brain [Mg2+]I [52], Mg2+ infusions could be expected to ameliorate cocaine-induced brain injury.
Previously, we have shown that decreased extracellular Mg2+ results in significantly increased intracellular free [Ca2+]I in dissociated hippocampal neurons as well as in primary cultured type-2 rat astrocytes [17, 69, 70] and rat, canine and baboon primary cultured cerebral vascular smooth muscle cells [17]. It is, thus, likely that the cocaine-induced loss of brain [Mg2+]I by inducing Ca2+-dependent cerebral, cortical, and medullary vasoconstriction followed by a proinflammatory response (viz., increased levels of reactive oxygen species, cytokines such as IL-2 and TNF-alpha coupled to adherence of leukocytes, monocytes and macrophages to the brain venular postcapillary walls [21, 25, 26], clearly induces vascular smooth muscle, endothelial and neuronal cell damage [9, 12, 14, 15, 17, 25, 30, 38]. An early biomarker of these events, from the work described herein, appears to be concentration dependent increases in reduced cytochrome oxidase and rises in ceramides (Tables 1 and 2).
Since mitochondria may regulate cell death( i.e., apoptosis) [71, 72], and an early marker of this event is a release of mitochondrial cytochrome c into the cytoplasm [71, 72], it is possible that the early and rapid appearance of increased brain reduced cytochrome oxidase levels, which are seen in the present study, could be indicative of such early apoptotic events with increasing doses of cocaine HCl. In this context, we have shown that cocaine HCl appears to induce apoptosis in a variety of tissues (e.g., heart, peripheral vascular muscle, coronary arterial muscle cells, liver cells, among other) [21, 73, 74 unpublished findings], including the brain [73, unpublished findings]. Very recently, we have identified two additional pathways of programmed cell death in Mg-deficient cardiac, vascular muscle and brain tissues, viz., necroptosis and ferroptosis [for reviews, see 75, 76], both of which we have found in cocaine-treated animals [unpublished findings]. Both of these new cell death pathways activated by cocaine HCl were attenuated markedly by pretreatment of the animals with myriocin [unpublished findings]. If our hypothesis is borneout, then coadministration of Mg2+ with blockers of ceramide synthesis, may turn out to be: 1. an inhibitor of several pathways involved in programmed cell death; and 2. useful in the treatment of cocaine-induced toxicity, tolerance, and brain damage, including strokes. Lastly, we believe our new findings could make it possible to rapidly monitor, noninvasively, the therapeutic benefits and actions of Mg2+ and ceramide antagonists in the intact human brain by use of optical reflectance spectroscopy.
Acknowledgements
Much of our studies were supported, in part, by research grants from The National Institutes of Health (National Heart, Lung and Blood Institute; The National Mental Health Institute; The National Institute on Drug Abuse; and The National Institute on Alcoholism and Alcohol Abuse) and unrestricted research grants from several pharmaceutical companies (Sandoz Pharmaceuticals; CIBA-GEIGY Corp.; Bayer Corp.). It is important to state that some of studies done and mentioned, herein, dither were partially initiated or carried out, in part, while some of us were on the Faculty of The Albert Einstein College of Medicine).
References
- Kaku DA, Lowenstein DH. Emergence of recreational drug abuse as a major risk factor for stroke in young adults. Annals of internal medicine. 1990: 113(11):821-7.
- Levine SR, Brust JC, Futrell N, Ho KL, Blake D, et al. Cerebrovascular complications of the use of the crack form of alkaloidal cocaine. New England Journal of Medicine. 1990; 323(11):699-704.
- Levine SR, Brust JC, Futrell N, Brass LM, Blake D, et al. A comparative study of the cerebrovascular complications of cocaine: alkaloidal versus hydrochloride-a review. Neurology. 1991; 41(8):1173.
- Sloan MA, Kittner SJ, Rigamonti D, Price TR. Occurrence of stroke associated with use/abuse of drugs. Neurology. 1991; 41(9):1358.
- Brust JC. Neurological aspects of substance abuse. Butterworth-Heinemann; 2004.
- Howington JU, Kutz SC, Wilding GE, Awasthi D. Cocaine use as a predictor of outcome in aneurysmal subarachnoid hemorrhage. Journal of neurosurgery. 2003 Aug 1;99(2):271-5.
- Westover AN, McBride S, Haley RW. Stroke in young adults who abuse amphetamines or cocaine: a population-based study of hospitalized patients. Archives of general psychiatry. 2007; 64(4):495-502.
- Brust JCM, Richter RW. Stroke associated with cocaine abuse. NY State J Med. 1977; 77: 1471.
- Huang QF, Gebrewold A, Altura BT, Altura BM. Cocaine-induced cerebral vascular damage can be ameliorated by Mg2+ in rat brain. Neuroscience letters. 1990; 109(1-2):113-6.
- He GQ, Zhang A, Altura BT, Altura BM. COCAINE-INDUCED CEREBRAL ARTERIAL VASOSPASM-POSSIBLE RELATION TO STROKE AND SUDDEN-DEATH. InFASEB JOURNAL. 1992 Feb 26; 6(4): A986-A986.
- He GQ, Zhang A, Altura BT, Altura BM. Cocaine-induced cerebral vasospasm and its mechanism of action. J Pharmacol Exp Ther. 1994 Mar; 268(3): 1532-1539.
- Altura BM, Gebrewold A, Altura BT, Gupta RK. MAGNESIUM PROTECTS AGAINST COCAINE-INDUCED HEMORRHAGIC 31PNMR IN-VIVO STUDY. Frontiers in Bioscience. 1997 May 15; 2:a9-12.
- Altura BT. The role of magnesium in etiology of strokes and cerebrovasospasm. Magnesium: Exp Clin Res. 1982; 1:277-91.
- Altura BM, Altura BT, Gebrewold A. Alcohol-induced spasms of cerebral blood vessels: relation to cerebrovascular accidents and sudden death. Science. 1983 Apr 15; 220(4594):331-3.
- Altura BM, Altura BT. Alcohol, the cerebral circulation and strokes. Alcohol. 1984 Jul 1; 1(4):325-31.
- Vink R, McIntosh TK, Demediuk P, Faden AI. Decrease in total and free magnesium concentration following traumatic brain injury in rats. Biochemical and biophysical research communications. 1987 Dec 16;149(2):594-9.
- Altura BM, Altura BT. Role of magnesium and calcium in alcohol‐induced hypertension and strokes as probed by in vivo television microscopy, digital image microscopy, optical spectroscopy, 31P‐NMR, spectroscopy and a unique magnesium ion‐selective electrode. Alcoholism: Clinical and Experimental Research. 1994 Oct; 18(5):1057-68.
- Memon ZI, Altura BT, Benjamin JL, Cracco RQ, Altura BM. Predictive value of serum ionized magnesium levels in head injuries. Scand J Clin Lab Invest. 1995 Dec; 55(8):671-7.
- Heath DL, Vink R. Blood-free magnesium concentration declines following graded experimental traumatic brain injury. Scand J Clin Lab Invest. 1998 Apr;58(2):161-6.
- Bareyre FM, Saatman KE, Hetfaer MA, Sinson G, Weisser JD, et al. Alterations in ionized and total blood magnesium after experimental traumatic brain injury: relationship to neurobehavioral outcome and neuroprotective efficacy of magnesium chloride. J Neurochem. 1999 Jul; 73(1):271-80.
- Altura BM, Gebrewold A, Carella A, Halevy S, Li W, Altura BT. Short-term Mg deficiency prior to induction of experimental cocaine-induced brain injury and percussion injury is associated with higher mortality and worsened neurological complications in rats. 2019. Submitted.
- Hampson NB, Caatporesi EM, Stolp BW, Moon RE, Shook JE, Griebel JA, et al. Cerebral oxygen availability by NIR spectroscopy during transient hypoxia in humans. J Appl Pjysiol. 1990 Sep; 69(3):907-13.
- Villringer A, Chance B. Non-invasive optical spectroscopy and imaging of human brain function. Trends in neurosciences. 1997 Oct 1;20(10):435-42.
- Morrill GA, Gupta RK, Kostellow AB, Ma GY, Zhang A, Altura BT, et al. Mg2+ modulates membrane lipids in vascular smooth muscle: a link to atherogenesis. FEBS Lett. 1997 May 19; 408(2):191-4.
- Altura BM, Shah NC, Shah GJ, Zhang A, Li W, Zheng T, et al. Shortterm magnesium deficiency upregulates ceramide synthase in cardiovascular tissues and cells; cross-talk among cytokines, Mg2+, NF-kB, and de novo ceramide. Am J Physiol Heart Circ Physiol. 2011 Oct 7; 302(1):H319-32.
- Altura BM, Shah NC, Shah GJ, Altura BT. Magnesium Deficiency, Sphingolipids, and Telomerase: Relevance to Atherogenesis, Cardiovascular Diseases, and Aging. Handbook of Famine, Starvation, and Nutrient Deprivation: From Biology to Policy. 2018:1-23.
- Zheng T, Li W, Wang J, Altura BT, Altura BM. Sphingomyelinase and ceramide analogs induce contraction and rises in [Ca2+] i in canine cerebral vascular muscle. American Journal of Physiology-Heart and Circulatory Physiology. 2000 May 1; 278(5):H1421-8.
- Altura BM, Gebrewold A, Zheng T, Altura BT. Sphingomyelinase and ceramide analogs induce vasoconstriction and leukocyte-endothelial interactions in cerebral venules in the intact rat brain: insight into mechanisms and possible relation to brain injury and stroke. Brain Res Bull. 2002 Jul;58(3):271-8.
- Lassoff S, Gebrewold A, Altura BM. A new method of craniotomy in the rat: in-vivo investigation of the pial microcirculation. Microcirculation. 1982 Jan 1; 2(3):345-53.
- Barbour RL, Gebrewold A, Altura BM. Optical spectroscopy and cerebral vascular effects of alcohol in the intact brain: Effects on tissue deoxyhemoglobin, blood content, and reduced cytochrome oxidase. Alcoholism: Clin Exp Res. 1993 Nov; 17(6):1319-24.
- Tamura M, Oshino N, Chance B, Silver IA. Optical measurement of intracellular oxygen concentration of rat heart in-vitro. Arch BiochemBiophys 1978 Nov 1;191(1):8-22.
- Hoshi YO, Tamura MA. Dynamic multichannel near-infrared optical imaging of human brain activity. Journal of Applied Physiology. 1993 Oct 1; 75(4):1842-6.
- Jobsis FF. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science. 1977 Dec 23;198(4323):1264-7.
- Kato T, Kamei A, Takashima S, Ozaki T. Human visual cortical function during photic stimulation monitoring by means of near-infrared spectroscopy. Journal of Cerebral Blood Flow & Metabolism. 1993 May; 13(3):516-20.
- Hirth C, Obrig H, Villringer K, Thiel A, Bernarding J, Mühlnickel W, Flor H, Dirnagl U, Villringer A. Non-invasive functional mapping of the human motor cortex using near-infrared spectroscopy. Neuroreport. 1996 Aug;7(12):1977-81.
- Barbour RL, Altura BM, Reiner SD, Dowd TL, Gupta RK, Wu F, et al. Influence of Mg2+ on cardiac performance, intracellular free Mg2+ and pH in perfused rat hearts as assessed with 31P-NMR spectroscopy. Magnesium & Trace Elem 1991-1992;10(2-4):99-116.
- Altura BM, Barbour RL, Reiner SD, Zhang A, Cheng TP, Dowd TL, Gupta RK, Wu F, Altura BT. Influence of Mg2+ on distribution of ionized Ca2+ in vascular muscle and on cellular bioenergetics and intracellular free Mg2+ and pH in perfused hearts probed by digital imaging microscopy, 31 PNMR and reflectance spectroscopy. Imaging in Alcohol Research, NIAAA Monograph. 1992; 21:235-71.M
- Wu F, Altura BT, Gao J, Barbour RL, Altura BM. Ferrylmyoglobin formation induced by acute magnesium deficiency in perfused rat heart causes cardiac failure. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 1994 Jan 11;1225(2):158-64.
- Altura BM, Altura BT. Magnesium in cardiovascular biology. Sci. Am. Sci. Med. 1995 Jun;2(3):28-37.
- Altura BM, Zhang A, Altura BT. Alcohol, myocardial bioenergetics, phospholipids, and ionic balance. Alcohol and the Cardiovascular System. Bethesda, National Institutes of Health. 1996:279-315.
- Altura BM, Gebrewold A, Altura BT, Brautbar N. Magnesium depletion impairs myocardial carbohydrate and lipid metabolism and cardiac bioenergetics and raises myocardial calcium content in‐vivo: Relationship to etiology of cardiac diseases. IUBMB Life. 1996 Dec; 40(6):1183-90.
- Altura BM, Shah NC, Li Z, Jiang XC, Perez-Albela JL, Altura BT. Magnesium deficiency upregulates serine palmitoyl transferase (SPT 1 and SPT 2) in cardiovascular tissues: relationship to serum ionized Mg and cytochrome c. American Journal of Physiology-Heart and Circulatory Physiology. 2010 Jun 25; 299(3):H932-8.
- Altura BM, Altura BT. Influence of magnesium on drug-induced contractions and ion content in rabbit aorta. American Journal of Physiology-Legacy Content. 1971 Apr 1; 220(4):938-44.
- Turlapaty PD, Altura BM. Extracellular magnesium ions control calcium exchange and content of vascular smooth muscle. European journal of pharmacology. 1978 Dec 1;52(3-4):421-3.
- Zhang A, Cheng TP, Altura BM. Magnesium regulates intracellular free ionized calcium concentration and cell geometry in vascular smooth muscle cells. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 1992 Feb 19;1134(1):25-9.
- Altura BM, Altura BT. Magnesium: forgotten mineral in cardiovascular biology and atherogenesis. InNew Perspectives in Magnesium Research. Springer, London. 2007; 239-260.
- Capaldi RA. Structure and function of cytochrome c oxidase .Annu Rev Biochem. 1990; 59: 569-596.
- Vink R, McIntosh TK, Demediuk P, Faden AI. Decrease in total and free magnesium concentration following traumatic brain injury in rats. Biochemical and biophysical research communications. 1987 Dec 16; 149(2):594-9.
- Heath DL, Vink R. Blood-free magnesium concentration declines following graded experimental traumatic brain injury. Scandinavian journal of clinical and laboratory investigation. 1998 Jan 1; 58(2):161-6.
- Helpern JA, Van de Linde AM, Welch KM, Levine SR, Schultz LR, Ordidge RJ, et al. Acute elevation and recovery of intracellular [Mg2+] following human focal cerebral ischemia. Neurology. 1993 Aug 1;43(8):1577-1581.
- Altura BT, Altura BM. Cerebrovasospasms induced by phencyclidine are prevented by calcium antagonists and magnesium ions. Magnesium. 1983; 2:52-6.
- Altura BM, Gupta RK. Cocaine induces intracellular free Mg deficits, ischemia and stroke as observed by in-vivo 31P-NMR of the brain. Biochimica et Biophysica Acta (BBA)-Biomembranes. 1992 Nov 9;1111(2):271-4.
- Altura BM, Altura BT, Gupta RK. Alcohol intoxication results in rapid loss in free magnesium in brain and disturbances in brain bioenergetics: relation to cerebrovasospasm, alcohol-induced strokes, and barbiturate anesthesia induced deaths. Magnesium and trace elements. 1991;10(2-4):122-35.
- Altura BM, Gebrewold A, Altura BT, Gupta RK. Role of brain [Mg2+] i in alcohol-induced hemorrhagic stroke in a rat model: a 31P-NMR in vivo study. Alcohol. 1995 Mar 1;12(2):131-6.
- Altura BM, Altura BT. Role of magnesium in patho-physiological processes and the clinical utility of magnesium ion selective electrodes. Scandinavian Journal of Clinical and Laboratory Investigation. 1996 Jan 1; 56(sup224):211-34.
- Altura BM, Altura BT. Association of Alcohol in Brain Injury, Headaches, and Stroke with Brain–Tissue and Serum Levels of Ionized Magnesium: A Review of Recent Findings and Mechanisms of Action. Alcohol. 1999 Oct 1; 19(2):119-30.
- Altura BM, Zhang A, Shah NC, Shah GJ, Gebrewold A, Altura BT. Euphoria from drinking alcoholic beverages may be due to reversible constriction of cerebral blood vessels: potential roles of unrecognized ionized hypomagnesemia, and release of ceramides and platelet-activating factor. Clin Res and trials. 2016; 2(6): 242-245.
- Altura BM, Gebrewold A, Carella A, Shah NC, Altura BT. Increased Risk of Stroke using Marijuana-Cannabis Products: Evidence for Dangerous Effects on Brain Circulation and the Unrecognized Roles of Magnesium. Drug and Alcohol Addiction. 2018;1(1):1.
- Altura BM, Gebrewold A, Carella A, Shah NC, Altura BT. Stroke: A real danger of the therapeutic use of psychedelic drugs and the role of magnesium depletion. EC Pharmacol and Toxicol. 2018; S1(1): 18-23.
- Altura BM, Gebrewold A, Carella A, Shah NC, Shah GJ, Altura BT. Stroke, headaches and hallucinations: Real dangers of the recreational use of amphetamines and ecstasy-like drugs; unrecognized role of hypomagnesemia. EC Pharmacology and Toxicol. 2019; 7(7): 646-652.
- Altura BM, Gebrewold A, Zhang A, Altura BT, Gupta RK. Short-term reduction in dietary intake of magnesium causes deficits in brain intracellular free Mg2+ and [H+] i but not high-energy phosphates as observed by in vivo 31P-NMR. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 1997 Aug 21; 1358(1):1-5.
- BAREYRE FM, SAATMAN KE, RAGHUPATHI R, MCINTOSH TK. Postinjury treatment with magnesium chloride attenuates cortical damage after traumatic brain injury in rats. Journal of neurotrauma. 2000 Nov;17(11):1029-39.
- Feldman Z, Gurevitch B, Artru AA, Oppenheim A, Shohami E, Reichenthal E, Shapira Y. Effect of magnesium given 1 hour after head trauma on brain edema and neurological outcome. Journal of neurosurgery. 1996 Jul 1; 85(1):131-7.
- Heath DL, Vink R. Neuroprotective effects of MgSO4 and MgCl2 in closed head injury: a comparative phosphorus NMR study. Journal of neurotrauma. 1998 Mar;15(3):183-9.
- McIntosh TK, Vink R, Yamakami I, Faden AI. Magnesium protects against neurological deficit after brain injury. Brain research. 1989 Mar 20;482(2):252-60.
- Clark GD, Rothman SM. Blockade of excitatory amino acid receptors protects anoxic hippocampal slices. Neuroscience. 1987 Jun 1;21(3):665-71.
- Kass I, Cottrell J, Chambers G. Magnesium and cobalt , not nimodipine, protect against ischemic damage in the rat hippocampal slice . Anesthesiol. 1988; 69: 710-721.
- Regan RF, Jasper E, Guo Y, Panter SS. The effect of magnesium on oxidative neuronal injury in vitro. Journal of neurochemistry. 1998 Jan;70(1):77-85.
- Zhang A, Fan SH, Cheng TP, Altura BT, Wong RK, Altura BM. Extracellular Mg2+ modulates intracellular Ca2+ in acutely isolated hippocampal CAI pyramidal cells of the guinea-pig. Brain research. 1996 Jul 29;728(2):204-8.
- Li W, Zheng T, Babu AN, Altura BT, Gupta RK, Altura BM. Importance of magnesium ions in development of tolerance to ethanol: studies on cultured cerebral vascular smooth muscle cells, type-2 astrocytes and intact rat brain. Brain research bulletin. 2001 Sep 15; 56(2):153-8.
- Green DR. Apoptosis- pathwayswraps stone blunts scissors. Cell. 2000 Jul 7;102(1):1-4.
- Lockshin RA, Zakeri Z. Programmed cell death and apoptosis: origins of the theory. Nature reviews Molecular cell biology. 2001 Jul; 2(7):545-550.
- Su J, Li J, Li W, Altura BT, Altura B. Cocaine induces apoptosis in cerebral vascular muscle cells: potential roles in strokes and brain damage. European journal of pharmacology. 2003 Dec 15; 482(1-3):61-6.
- Su J, Li J, Li W, Altura BT, Altura BM. Cocaine induces apoptosis in primary cultured rat aortic vascular smooth muscle cells: possible relationship to aortic dissection, atherosclerosis, and hypertension. Int J Toxicol. 2004; 23 (4): 233-237.
- Altura BM, Shah NC, Shah GJ, Altura BT. Regulated RIPK3 necroptosis is produced in cardiovascular tissues and cells in dietary magnesium deficiency; roles of cytokines and their potential importance in inflammation and atherogenesis. J Med Surg Pathol. 2018;2:104.
- Altura BM, Gebrewold A, Carella A, Zhang A, Shah NC, Shah GJ, et al. Regulated ferroptosis cell death is produced in cardiovascular tissues and cells in dietary magnesium deficiency: initiation of roles of glutathione, mitochondrial alterations and lipid peroxidation in inflammation and atherogenesis. EC Pharmacol and Toxicol. 2018; 6(7): 535-541.