Alteration of Peripheral Blood T-cell Subsets in Patients with Cardiovascular Disease; Exposure to Ionizing Radiation (X-rays) and Contrast Medium
Demirhan O1*, Çetinel N1, Çetiner S4, Ça&ğlıyan ÇE2, Cureoglu A2, Uslu IN1, Deveci OS2, Sertdemir Y3, Demirtaş M2
1 Department of Medical Biology and Genetics, Faculty of Medicine, Çukurova University, Adana, Turkey.
2 Department of Cardiology, Faculty of Medicine, Çukurova University, Adana, Turkey.
3 Department of Biostatistics, Faculty of Medicine, Çukurova University, Adana, Turkey.
4 Department of Medical Laboratory Techniques, Faculty of Medicine, Çukurova University, Adana, Turkey.
*Corresponding Author
Dr. Osman Demirhan,
Professor, Department of Medical Biology and Genetics,
Faculty of Medicine, Çukurova University, 01330 Balcalı-Adana, Turkey.
Tel: 90-322-3387140
Fax: 90-322-3386572
E-mail: osdemir@cu.edu.tr
Received: May 08, 2018; Accepted: June 08, 2018; Published: June 11, 2018
Citation: Demirhan O, Çetinel N, Çetiner S, Çağlıyan ÇE, Cureoglu A, Uslu IN, et al., Alteration of Peripheral Blood T-cell Subsets in Patients with Cardiovascular Disease; Exposure to Ionizing Radiation (X-rays) and Contrast Medium. Int J Cardiol Res. 2018;5(2):104-108. doi: http://dx.doi.org/10.19070/2470-4563-1800018
Copyright: Demirhan O© 2018. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Ionizing radiation (IR) induced damage of the immune system, and exposure to IR induces subgroups of T-lymphocytes and different cell groups of immune system give different responses in individuals exposed to long-term IR. The aim of this study was to investigate the effect of exposure to low levels of IR and iodinated contrast media in cellular immunity of patients with cardiovascular disease (CVD) exposed to coronary angiographic imaging. A group of 47 patients with CVD exposed to X-rays (IR) and iodinated contrast media (27 males, 20 females) were subjected to investigating the level of early T-cell marker (CD3), T-helper (CD4) and T suppressor (CD8) of the T-Iymphocyte subgroups. Peripheral blood samples collected before and after angiographic imaging into tubes containing EDTA were investigated for lymphocyte subsets using flow cytometry in Turkey. The age range was 38-75 years (54.31 ± 9.09). The ratio of CD4/CD8 was between 1.525- 1.833. The rates of CD4 and CD8 was significantly difference before and after angiographic imaging (p= 0.000 to 0.001), as the value of CD4 increases, CD8 decreases with it. There was no statistically significant difference in the percentages and absolute value of lymphocyte subsets between the genders (p>0.05). The present date demonstrated that exposure to low dose IR contrast and medium induce switch of the immune system to CD4 and CD8 immune response. Short-term exposure to X-rays is temporarily stimulate cellular immune functions, and have high immune function and the risk, and increase cellular immune function. At the same time, it may possibly cause damage to the vascular endothelium of patients.
2.Abbreviations
3.Introduction
4.Subjects And Methods
4.1 Subjects
4.2 Flow Cytometry Study
4.3 Statistical Analysis
5.Results
6.Discussion
7.Conclusion
8.Acknowledgements
9.References
Keywords
Cardiovascular Disease; Angiographic Imaging; T-Iymphocyte; Flow Cytometry; Ionizing Radiation; Iodinated Contrast Media.
Abbreviations
IR: Ionizing Radiation; CVD: Cardiovascular Disease; EDTA: Ethylene Diamine Tetra Acetate.
Introduction
Cardiac imaging is increasingly used to detect heart diseases and to guide therapy. Along with the increased use of cardiac imaging at clinics there is increased attention to the potential risks related to the methods used. X-rays (IR) and iodinated contrast agents are frequently used for diagnostic applications in the angiography, and these were main risk sources. IR is known to cause harm, and high radiation doses tend to kill cells, while low doses tend to damage or alter the genetic code of irradiated cells. At the time of radiotherapy and radio diagnostic, there is a risk, that it is associated with the irradiation of normal, healthy tissue and the development of the radio induced complications. At the same time, it has also long been known that IR induced damage of the immune system. However, substantial evidence suggests more varied effects of radiation on the immune system, prompting the recharacterization of radiation as immune-modulatory rather than immunosuppressive. The effect of IR on the immune response has become one of the chief research fields in radiation biology and radiation protection [5]. The relationship between IR and the immune system is multifactorial and highly depends on the radiation dose/quality and immune cell types [9]. However it results in changes in morphology and functional activity both at the cellular and system levels causing disturbance of immune reactivity whose final result is modulation of the immune system [11].
Therefore, this study aimed at investigating the effect of exposure to IR and iodinated contrast agent on the immune system, the effect on the balance between early T-cell marker (CD3), T-helper (CD4) and T suppressor (CD8) of the T-Iymphocyte subgroups.
This study was carried out on the venous blood of 47 angiography patients with CVD exposed to coronary angiographic imaging (X-rays and iodinated contrast media, 27 males, 20 females).The patients were subjected to investigating the level of CD3, T-helper (CD4) and T suppressor (CD8) of the T-Iymphocyte subgroups. The patients were referred from Department of Cardiology, Cardiac Imaging, University Çukurova, Adana, Turkey. The age range of the patients ranged from 38 to 75 years (54.31 ± 9.09 average age). Peripheral blood samples collected into tubes containing disodium ethylene diamine tetra acetate (EDTA) before and 24 hours after angiographic imaging. The blood samples were investigated for lymphocyte subsets using flow cytometry in Turkey. This study was approved by the Institutional Ethic Committee and informed consent was obtained from each participant.
The ranges of CD3, CD4, CD8 and the CD4/CD8 rate in the 47 patients with CVD were determined using a flow cytometry instrument (Beckman Coulter, Navios, USA) at the Central Laboratory of the Faculty of Medicine, University of Cukurova, Turkey. 100μl of EDTA blood from each patient received polystyrene tubes. 10μl of CD8 FITC/CD4PE/CD3ECD (Beckman Coulter, USA) was added from the monoclonal antibody mixture and then vortexed. The tubes were placed in Carousel. Carousel TQ-Prep (Beckman Coulter, USA). The device's short-button was pressed. The samples were incubated for 10 minutes at room temperature. Later, 600μl Immunoprep A (erythrocyte lysing agent), 265μl Immunoprep B (leukocyte stabilizer) and 100μl Immunoprep C (cell membrane fixative) solutions were added to the samples in the TQ-Prep Workstation (vortexed after each transfer) (Beckman Coulter, USA). Analysis of each lymphocyte subset was made using an EPICS XLMCL flow cytometer (Beckman Coulter) and the values were determined as a percentage of all the parameters.
All analyses were performed using IBM SPSS Statistics Version 19.0 statistical software package. Continuous variables were summarized as mean and standard deviation and as median and minimum-maximum where appropriate. For comparison of two related (paired) continuous variables, paired samples t-test was used. The statistical level of significance for all tests was considered to be 0.05.
Results
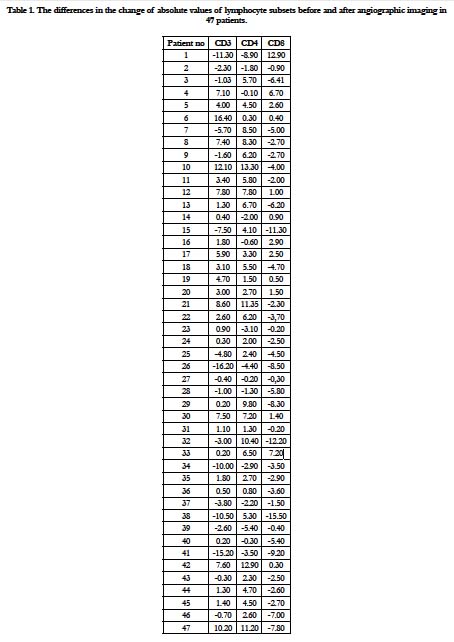
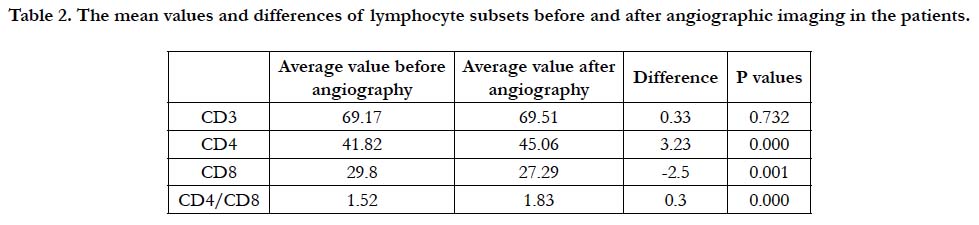
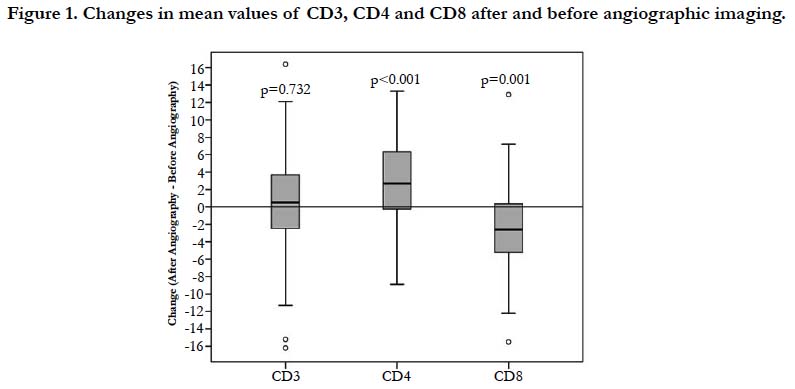
The mean values and difference of 47 patients before and after angiographic imaging of the lymphocyte subsets are given in Table 1 and 2, Figure 1. The absolute values of the lymphocyte subsets before and after angiographic imaging were as follows; CD3: 69.177 and 69.514; CD4: 41.824 and 45.061; CD8: 29.809 and 27.294, respectively. The differences in the change of absolute values CD3, CD4 and CD8 before and after angiographic imaging in every patient (47) were as follows: CD3: increased in 29 patients (% 61.7) and decreased in 18 patients (% 38.3); CD4: increased in 33 patients (% 70.2) and decreased in 14 patients (% 29.8); CD8: increased in 13 patients (% 27.6) and decreased in 34 patients (% 72.4) (Table 1). CD3 ratio is increased, CD4 ratio is increasing while CD8 ratio is decreasing accordingly.
It was found that the absolute value of CD3 was not significant (p=0.732) but, the rates of CD4 and CD8 was statistically significant difference before and after angiographic imaging (p value ranged from 0.000 to 0.001). Although, the ratio of CD4/ CD8 before and after angiographic imaging was between normal values, as the value of CD4 increases, CD8 decreases with it (Table 1 and Figure 1). The rate of CD4/CD8 before and after angiographic imaging was 1.526 and 1.834, respectively. However, there was statistically significant difference in absolute value of CD4/CD8 (p=0.000) (Table 1). Before and after the angiographic imaging, and CD8-FITC/CD4-PE/CD3-ECD values were determined in the patients. Before the angiographic imaging, it was observed that the CD4/CD8 ratio was less than 1.6 in 27 patients, between 1.6-2.0 in 12 patients and above 2.0 in 8 patients. Before the angiographic imaging, 16 of the 27 persons with a CD4 / CD8 ratio below 1.6 were below 1.6 after the angiographic imaging, 4 had values between 1.6 and 2.0, and 7 were above 2.0. Before the angiographic imaging, the value of 8 of 12 persons with a CD4/CD8 ratio of 1.6-2.0 was between 1.6-2.0 after the angiographic imaging, and the value of 4 was above 2.0. Before the angiographic imaging, the value of one of the 8 CD4 / CD8 ratios above 2.0 was reduced to 1.6-2.0 after the value treatment, while the value of 8 remained above 2.0. There was no statistically significant difference in the percentages and absolute value s of lymphocyte subsets between the genders (p>0.05).
Table 1. The differences in the change of absolute values of lymphocyte subsets before and after angiographic imaging in 47 patients.
Table 2. The mean values and differences of lymphocyte subsets before and after angiographic imaging in the patients.
Discussion
Medical radiations(as X-rays) are the largest source of radiation exposure and increase cellular immune function. X-rays is increasingly being used in cardiology to detect heart disease and guide therapy. The immune suppression may make the human body unable to resist the infection of bacteria or virus, whereas immune system overreaction also causes tissue damage and increases the rate of fatality and disability. In studies, increased concentrations of some immunoglobulins and changes in numbers of lymphocytes were observed in blood samples from radar operators and workers at television-transmission stations, but the results were variable and the alterations seemed to be within the normal variation [6].
We analyzed the relation between peripheral immune cell subsets and before-after angiographic imaging. The CD4/CD8 ratio of 1.525-1.833 obtained in this study is within the reported ratio of 1.5 + 0.6 - 2.0 + 0.02 [7, 14, 17]. Nevertheless, after angiography showed a significantly lower percentage of CD8 cells (cytotoxic T-cells) and a higher percentage of CD4 cells (helper T-cells) on day 1 the after angiography in our patients (p=0.000 to 0.001). This result suggest that the patients had temporary immune depression after angiography. At the same time, angiographic processing causes an increase in patient's cellular immunity and may possibly cause damage to the vascular endothelium of patients and increase the release of some inflammatory mediators. In a recent study, CD4% T lymphocytes level was a statistically significant lower among exposed group compared to the control (p<0.001) [3]. However, the observed variations in some cases could not be attributed only to the radiation exposure because of the impact of a number of other exogenous and endogenous factors on the immune system [5]. Several studies assessed the effects of exposure to IR radiation on indicators of immune function in humans. These studies indicate that low dose IR from natural sources or occupational exposure (Wall et al. 2006) [18] may stimulate the immune system and potentiate its effect or function [10]. It has also been reported that low dose radiation warns of immunity [9].
It has been reported that subgroups of T-lymphocytes are affected at different levels and different cell groups of immune system give different responses in individuals exposed to longterm ionizing radiation. Which is in contrast to the previous study showing levels of CD4(+) T lymphocytes was found to be weaker in exposed workers compared with controls, indicating the importance of taking appropriate measures to protect radiology workers from exposure to IR ionizing radiation [4]. Another report on individuals occupationally exposed to IR showing no change for T-cell and B-cell total counts and for the T cell subset percentages of CD4+, CD8+ [12]. These discrepancies might be due to the source and dose of radiation. Because, the interrelationship between ionizing radiation and the immune system is multifactorial and highly depends on the radiation dose/ quality and immune cell types [9]. Ethnic and some differences are factors that may influence the levels of lymphocyte subsets [7, 13, 17]. Environmental factors, and including various infectious agents (Comans-Bitter, Dre Groot, van den Beemd, Neijens, Hop, Groeneveld et al. 1997 [2]; Al Qouzi, Al Salamah, Al Rasheed, Al Musalam, AL Khairy, Kheir et al. 2002) [1] may also influence the number and subsets of lymphocytes. Of equal importance may be the variation introduced by use of different instruments and procedures [7]. In the present study, gender did not affect the percentages and absolute values of lymphocyte subsets. However, same studies reported that only CD4 was significantly higher in female than in male subjects [8, 16]. In addition, it found higher CD8 values for male than female subjects [16]. Santagostino et al., (1999) [15] reported significant differences in CD3, CD4 and NK according to gender, but not in the ratio of CD4/CD8.
All these experimental studies in vivo that aimed to assess effects of short-term and prolonged low level exposure to IR on function and status of the immune system, clearly indicates that various shifts in number and/or activity of immunocompetent cells are possible. Short-term exposure to weak IR fields may temporarily stimulate certain humoral or cellular immune functions, while prolonged irradiation inhibits the same functions. Thus, even though there are indications that changes are occurring, the relevance of these observations in relation to carcinogenicity is unclear. Overall, researchers concluded that there was insufficient evidence to determine that alterations in immune function induced by exposure to IR affect carcinogenesis in humans.
Conclusion
Angiography is generally considered as a safe technology with clinical impact. It is accepted readily as a powerful noninvasive diagnostic tool to investigate the coronary vessels in the body. The present date demonstrated that after angiography, the rate of CD4 in patients is significantly higher than before angiographic imaging, and angiographic processing causes an increase in patient's cellular immunity. Further, an increase in the number of CD4+ T-cells after angiography suggests that this process may possibly cause damage to the vascular endothelium of patients and increase the release of some inflammatory mediators. At the same time, our work may lead to future work in this area with inflammatory cytokines. Thus, there is a clear need to evaluate and establish biologic approaches for determining low-dose radiation effects in patients undergoing diagnostic X-ray procedures. Short-term exposure to X-rays is temporarily stimulate cellular immune functions. Estimation of the risk from IR is difficult. However, IR can be considered as a ‘two-edged sword’ in that it may lead to immune suppression or overreaction, which critically contributes to the patient’s prognosis. Thus, even though there are indications that changes are occurring, the relevance of these observations in relation to carcinogenicity is unclear. However, the results of the researches may vary, but by identifying patients after angiography immune suppression and immune overreaction, we can treat patients in a different, sometimes opposite, way to regulate the immune function in advance. This research may also lead to effective therapeutic strategies eliminating complications after angiography.
Acknowledgements
We would like to express our thanks to the routine cytogenetic laboratory personnel such as technicians for their assistance and contribution. This work was supported by the Çukurova University Research Fund TYL-2015-5066.
References
- Al Qouzi A, Al Salamah A, Al Rasheed R, Al Musalam A, Al Khairy K, Kheir O, et al. Immunophenotyping of peripheral blood lymphocytes in Saudi men. Clin Diagn Lab Immunol. 2002 Mar;9(2):279-81. PubMed PMID: 11874863.
- Comans-Bitter WM, de Groot R, van den Beemd R, Neijens HJ, Hop WC, Groeneveld K, et al. Immunophenotyping of blood lymphocytes in childhood Reference values for lymphocyte subpopulations. J Pediatr. 1997 Mar;130(3):388-93. PubMed PMID: 9063413.
- Farahat S, Mansour N, Shete M, Ramadan M. Immune-modulatory Effect of Ionizing Radiation on Type 1 and Type 2 Immune Responses among Workers in Cardiac Catheterization Units. Br J Med Med Res. 2017;19(2):1-0.
- Godekmerdan A, Ozden M, Ayar A, Gursu MF, Ozan AT, Serhatlioglu S. Diminished cellular and humoral immunity in workers occupationally exposed to low levels of ionizing radiation. Arch Med Res. 2004 Jul-Aug;35(4):324-8. PubMed PMID: 15325507.
- Gyuleva IM, Penkova KI, Rupova IT, Panova DY, Djounova JN. Assessment of some immune parameters in occupationally exposed nuclear power plant workers: Flow cytometry measurements of T lymphocyte subpopulations and immunoglobulin determination. Dose Response. 2015 Nov 17;13(4):1559325815611901. PubMed PMID: 26740807.
- Jauchem JR. Effects of low-level radio-frequency (3 kHz to 300 GHz) energy on human cardiovascular, reproductive, immune, and other systems: a review of the recent literature. Int J Hyg Environ Health. 2008 Mar;211(1-2):1-29. PubMed PMID: 17692567.
- Kaaba SA, Al Fadhli S, Khamis A. Reference values of lymphocyte subsets in the normal healthy adult Kuwaiti Arab population. Immunol Lett. 2002 May 1;81(3):199-203. PubMed PMID: 11947925.
- Kam KM, Leung WL, Kwok MY, Hung MY, Lee SS, Mak WP. Lymphocyte subpopulation reference ranges for monitoring human immunodeficiency virus-infected Chinese adults. Clin Diagn Lab Immunol. 1996 May;3(3):326-30. PubMed PMID: 8705678.
- Liu SZ. Cancer control related to stimulation of immunity by low-dose radiation. Dose Response. 2007 Aug 28;5(1):39-47. doi: 10.2203/doseresponse. 06-108.Liu. PubMed PMID: 18648611.
- Luckey TD. Radiation hormesis overview. Radiation Protection Management. 1999 Jul;16:22-34.
- McBride WH, Chiang CS, Olson JL, Wang CC, Hong JH, Pajonk F, et al. A sense of danger from radiation. Radiat Res. 2004 Jul;162(1):1-19. PubMed PMID: 15222781.
- Rees GS, Daniel CP, Morris SD, Whitehouse CA, Binks K, et al. Occupational exposure to ionizing radiation has no effect on T‐and B‐cell total counts or percentages of helper, cytotoxic and activated T‐cell subsets in the peripheral circulation of male radiation workers. Int J Radiat Biol. 2004 Jul;80(7):493-8. PubMed PMID: 15360087.
- Reichert T, DeBruyère M, Deneys V, Tötterman T, Lydyard P, Yuksel F, et al. Lymphocyte subset reference ranges in adult Caucasians. Clin Immunol Immunopathol. 1991 Aug;60(2):190-208. PubMed PMID: 1712687.
- Roman S, Moldovan I, Călugăru A, Regalia T, Sulică A. Lymphocyte subset reference ranges in Romanian adult Caucasians. Rom J Intern Med. 1995 Jan-Jun;33(1-2):27-36. PubMed PMID: 8535349.
- Santagostino A, Garbaccio G, Pistorio A, Bolis V, Camisasca G, et al. An Italian national multicenter study for the definition of reference ranges for normal values of peripheral blood lymphocyte subsets in healthy adults. Haematologica. 1999 Jun;84(6):499-504. PubMed PMID: 10366792.
- Uppal SS, Verma S, Dhot PS. Normal values of CD4 and CD8 lymphocyte subsets in healthy indian adults and the effects of sex, age, ethnicity, and smoking. Cytometry B Clin Cytom. 2003 Mar;52(1):32-6. PubMed PMID: 12599179.
- Vithayasai V, Sirisanthana T, Sakonwasun C, Suvanpiyasiri C. Flow cytometric analysis of T-lymphocytes subsets in adult Thais. Asian Pac J Allergy Immunol. 1997 Sep;15(3):141-6. PubMed PMID: 9438546.
- Wall BF, Kendall GM, Edwards AA, Bouffler S, Muirhead CR, Meara JR. What are the risks from medical X-rays and other low dose radiation?. Br J Radiol. 2006 Apr;79(940):285-94. PubMed PMID: 16585719.