Underdiagnosed Paraquat Induced Pneumothorax and Pneumomediastinum, The “Daisley Barton Syndrome”: A Clinical Feature Of Paraquat-Induced Acute Lung Injury
Meyers D1, Rampersad A2*, Christopher M3, Pegus S4, Daisley H5
1 Acting Registrar, Department of Pathology, General Hospital, San Fernando, Trinidad and Tobago, W.I.
2 House Officer, Department of Pathology, General Hospital, San Fernando, Trinidad and Tobago, W.I.
3 House Officer, Department of ENT, General Hospital, San Fernando, Trinidad and Tobago, W.I.
4 Senior House Officer, Department of Internal Medicine, General Hospital, San Fernando, Trinidad and Tobago, W.I.
5 Professor of Pathology, Department of Pathology, General Hospital, San Fernando, Trinidad and Tobago, W.I. and Lecturer in Forensic Medicine, High Wooding Law School, St Augustine Trinidad and Tobago, W.I.
*Corresponding Author
Arlene Rampersad,
House Officer, Department of Pathology,
General Hospital, San Fernando, Trinidad and Tobago, W.I.
E-mail: drarlene19@gmail.com
Received: April 30, 2018; Accepted: July 19, 2018; Published: July 23, 2018
Citation: Meyers D, Rampersad A, Christopher M, Pegus S, Daisley H. Underdiagnosed Paraquat Induced Pneumothorax and Pneumomediastinum, The “Daisley Barton Syndrome”: A Clinical Feature Of Paraquat-Induced Acute Lung Injury. Int J Clin Pharmacol Toxicol. 2018;7(2):296-299. doi: dx.doi.org/10.19070/2167-910X-1800049
Copyright: Rampersad A© 2018. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
The incidence of paraquat ingestion for self-harm, occurs at an alarming rate in Trinidad and Tobago W.I and is virtually synonymous with acute multi-organ failure and death in most settings. Yet recognition of pneumothorax and pneumomediastinum as clinical signs of acute paraquat lung injury remains a challenge amongst medical practitioners. We present a case of paraquat poisoning with these clinical features, which were recognized two days after presentation. The pathophysiological mechanisms for this clinical entity are presented in an effort to alert physicians of this feature as a part of acute paraquat lung injury and to stimulate research in effective therapy.
2.Abbreviations
3.Introduction
4.Case Report
5.Post Mortem Findings
6.Histopathology
7.Discussion
8.Conclusion
9.References
Keywords
Paraquat Poisoning; Pneumothorax and Pneumomediastinum; Acute Lung Injury.
Abbreviations
ROS: Reactive Oxygen Species.
Introduction
The purpose of this manuscript is to highlight the proposed mechanism of paraquat induced acute lung injury, namely pneumothorax and pneumomediastinum. This entity, as a consequence of paraquat ingestion, was first published by Daisley and Barton in 1990 [1], hitherto called the Daisley Barton syndrome. Since then, recognition of this entity has been underdiagnosed and undervalued.
Our case presents the Daisley Barton syndrome as part of paraquat induced acute lung injury which developed in a matter of 2 - 3 days post ingestion.
Case Report
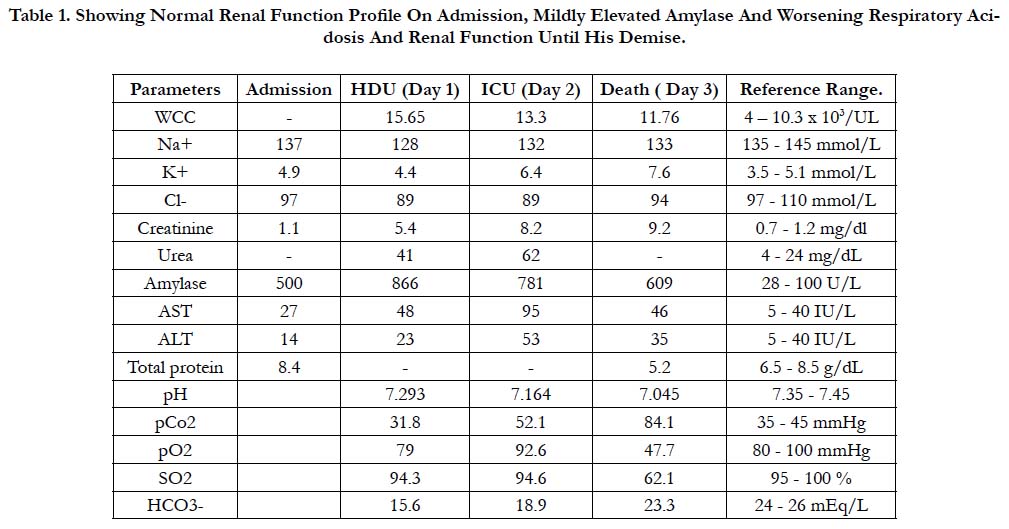
The patient was a 23-year-old male who presented to the district health facility with vomiting, diarrhea and severe chest and abdominal pain after alcohol binge drinking the night prior. He was assessed as having acute alcoholic gastritis with possible acute pancreatitis and was referred to the General Hospital for further management. Initial blood investigations were normal except for a mildly elevated serum amylase, although his renal function declined progressively during his hospital stay (Table 1).
Table 1. Showing Normal Renal Function Profile On Admission, Mildly Elevated Amylase And Worsening Respiratory Acidosis And Renal Function Until His Demise.
His chest x-ray on admission showed a mild increase in respiratory infiltrates. He developed respiratory distress within the first 24 hours of admission, and was noted to have subcutaneous emphysema involving his neck and upper trunk and a left pneumothorax. A left thoracostomy was performed and ' he was' also mechanically ventilated.
The general surgeons attributed the finding of pneumothorax and pneumomediastinum and the copious amount of frank blood emanating from the nostrils and oropharynx to Boerhaave’s syndrome. Respiratory acidosis ensued (Table 1), and Spo2 saturation remained low despite high settings on the ventilator. The patient died on the third day. It was shortly before he died that his relative alluded to the fact that an empty bottle of paraquat was found at his home.
Post Mortem Findings
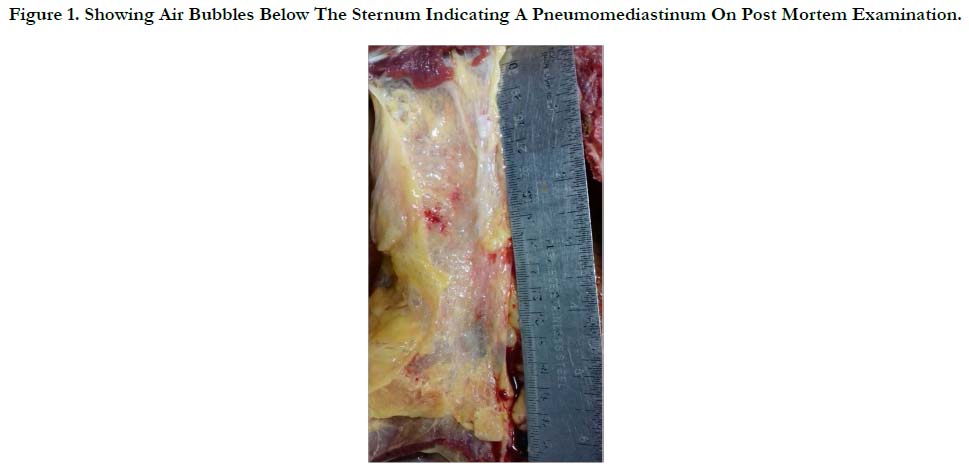
There were erosions to the lower lip and tongue, and significant erosive hemorrhagic gastritis and esophagitis, more so at the gastro esophageal junction. The trachea, bronchi and lower airways were severely hyperemic which was indicative of aspiration pneumonitis. The lungs were rubbery in consistency, markedly overweight, 1000 gms each, and were congested and edematous. There was a left pneumothorax and pneumomediastinum with air churned in the pericardial sac and the mediastinal fat (Figure 1).
Figure 1. Showing Air Bubbles Below The Sternum Indicating A Pneumomediastinum On Post Mortem Examination.
There was also right heart failure with chronic passive venous congestion of the liver and congestive splenomegaly. The brain was edematous and overweight. The kidneys were edematous as seen in acute renal failure. The pancreas was grossly unremarkable.
Histopathology
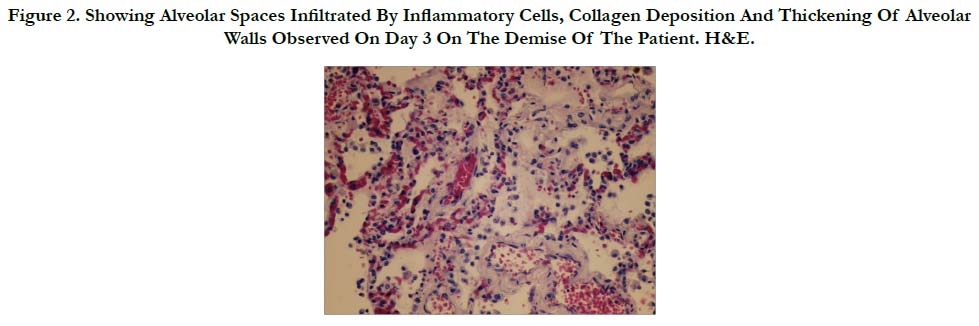
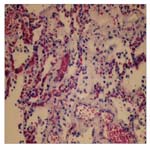
Many histologic sections of the lungs were examined. There were many areas that showed atelectasis and emphysematous changes. The alveolar spaces contained polymorphs, lymphocytes and many macrophages, occasional giant cells, edema and frank blood. There were ballooning of some macrophages with blue collagen like material. These latter macrophages were found within alveolar spaces and alveolar walls (Figure 2).
Figure 2. Showing Alveolar Spaces Infiltrated By Inflammatory Cells, Collagen Deposition And Thickening Of Alveolar Walls Observed On Day 3 On The Demise Of The Patient. H&E.
The alveolar walls showed marked widening with round cells, giant cells and deposits of procollagen. There was alveolar capillary congestion and multiple pulmonary micro-thrombi in small vessels.
The cor-pulmonale was secondary to the pathological repair of the alveoli, producing early pulmonary fibrosis and micro thrombi.
The pancreas did not show acute inflammatory changes nor was there saponification of the omentum and surrounding fatty tissue.
There was evidence of esophagitis demonstrated with denuded mucosa, congested mucosal blood vessels and round cells infiltrates that extended into the submucosal space. There was vascular congestion within the muscle layers. Serial sectioning of the esophagus was done and no inflammatory/bloody sinus tracts breeching the serosa to confirm a ruptured esophagus was observed.
Discussion
Paraquat (1,1-dimethyl-4, 4-bipyridylium dichloride) is a herbicide, which belongs to the bipyridil quaternary ammonium herbicide. Gramoxone is its most common trade name. Absorption by plant leaves inhibits photosynthesis and leads to death of the leaves and ultimately the plant. This is a complex process, which involves cyclic reduction-oxidation reactions, which produces reactive oxygen species (ROS), hydrogen peroxide, and hydroxyl radicals. This hydroxyl radical is extremely reactive and readily destroys unsaturated lipids including membrane fatty acids and chlorophyll.
The hydroxyl radical produces lipid radicals, which react with oxygen to form lipid hydroperoxides; such lipid hydroperoxides (also called Reactive oxygen species) destroy the integrity of cell membranes allowing cytoplasm to leak into intercellular spaces, which leads to rapid leaf wilting and desiccation [2].
Paraquat is very toxic to humans and animals. The LD50 for humans is around 30mg/kg. In Trinidad, it is often used in self-harm/suicide1. It is poorly absorbed in the stomach. Fuller’s earth or activated charcoal can detoxify unabsorbed paraquat in the stomach and should be administered to all patients as a first line of therapy who presents to hospital with a history of paraquat ingestion.
Once paraquat is absorbed, its entry into the blood stream has deleterious effects on all organs.
Hyperamylasemia originating from mild acute pancreatitis has been used as an early predictor of mortality in patients with acute pancreatitis [3]. There was no evidence of pancreatitis in this patient in discussion and his moderately elevated amylase would have had contribution from salivary glands stimulation following alcohol and paraquat consumption and poor renal clearance following his acute renal failure [4, 5].
Both the ingestion of alcohol and paraquat produce esophagitis and gastritis. There was no evidence of esophageal rupture in the patient in discussion thereby negating the phenomenon of Boerhaave’s syndrome. The histological findings of the esophagus are suggestive of Mallory-Weiss syndrome without perforation of the esophagus which would account for the upper gastro intestinal tract hemorrhage in this patients as a result of his binge drinking and or paraquat ingestion [6].
The deleterious effect of paraquat is seen more so in the lung where its concentration is much higher than those in plasma and other organs. This selective accumulation of paraquatin the lung occurs through the polyamine uptake system, and provides an explanation for its selective toxicity to the lung [7].
The pathogenesis of paraquat induced pulmonary toxicity producing Acute Lung Injury is often categorized into an early and late phase, although both phases occur simultaneously (See figure above). The first toxicological effects to the lung correspond to a destructive phase in which type I and type II pneumocytes are destroyed by ROS. Damage of type I epithelial cells causes their rupture, which prevents gas exchange and thereby exposing the basement membrane of the alveolar wall. Type II cells are also destroyed thereby limiting the production of surfactant and the transfer of water and ions and epithelial regeneration [2].
Decrease in surfactant causes increase in surface tension within alveoli, which draws fluid from capillaries to produce edema. It is also postulated that endothelial cells are damaged by reactive oxygen species, causing capillary permeability with resultant edema, and hemorrhage. In the pathogenesis of the paraquat induced acute lung injury, chemokines are released [8, 9], thereby generating an inflammatory response causing an influx of inflammatory cells. These are composed mainly of neutrophils, monocytes and lymphocytes and also endothelial cells and myofibroblasts, which promotes pathological repair with collagen production and deposition within the alveolar wall and alveolar spaces [10, 11].
This production and deposition of collagen in acute paraquat toxicity occurred in the lung as early as day 3 in the patient in discussion (Figure 2).
It is the increase in surface tension within alveoli brought about by the destruction of type II cells which cause secondary atelectasis, rupture and emphysematous changes, thereby allowing alveoli gas to escape, causing pneumothorax and or the escaped gas to dissect along vascular sheaths and connective tissue planes to the mediastinum to cause pneumomediastinum. Peripheral or sub-pleural, pulmonary alveoli rupture would rapidly facilitate pneumothorax and pneumomediastinum. This pathophysiological mechanism of pneumothorax and pneumomediastinum was first described by Macklin [12] in his experimental animal model and has been observed in many clinical settings where there is acute lung injury [13-15].
Yet another mechanism for increase alveolar pressures and the adverse effects of paraquat pulmonary toxicity is the early development of secondary pulmonary hypertension. The initiation of pathological repair at the alveolar level, with widening of the interstitium with inflammatory cells, and early deposits of collagen, together with alveoli capillary congestion and destruction, and microthrombi formation (Figure 2) cause secondary pulmonary hypertension and cor-pulmonale [16]. This inadvertently leads to increase alveolar pressures and the vicious cycle of pneumothorax and pneumomediastinum. This phenomenon of pneumothorax and pneumomediastinum hitherto called the Daisley Barton syndrome [17] together with other pathological features of acute lung injury discussed above leads ultimately to the acute respiratory distress and failure, which was experienced by the patient in the discussion and led to his ultimate death.
Conclusion
Pneumothorax and pneumomediastinum are clinical signs of paraquat induced acute lung injury and its proposed pathophysiology is revised above. Daisley and Barton first described this clinical observation in 1990 [1] . These clinical signs are not uncommon and have a high index of early mortality, and must be looked for in all cases of paraquat poisoning [17, 18].
In regions like Trinidad and Tobago where there is a high incidence of paraquat assisted suicide [19], paraquat poisoning should be included in the differential diagnosis in patients presenting with acute respiratory distress and or evidence of the Daisley Barton syndrome (Daisley Barton 1990 [19] namely pneumothorax and pneumomediastinum in acute paraquat toxicity.
More research needs to be done on therapy to combat paraquat toxicity based on the proposed pathophysiology of acute lung injury [20-22].
References
- Daisley H, Barton EN. Spontaneous pneumothorax in acute paraquat toxicity. West Indian Med J. 1990 Sep;39(3):180-5. PubMed PMID: 2264334.
- Dinis-Oliveira RJ, Duarte JA, Sanchez-Navarro A, Remiao F, Bastos ML, Carvalho F. Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment. Crit Rev Toxicol. 2008;38(1):13-71. PubMed PMID: 18161502.
- Liu S, Wang Q, Zhou R, Li C, Hu D, Xue W, et al. Hyperamylasemia as an early predictor of mortality in patients with acute paraquat poisoning. Med Sci Monit. 2016 Apr 21;22:1342-8. PubMed PMID: 27101346.
- Pieper-Bigelow C, Strocchi A, Levitt MD. Where does serum amylase come from and where does it go?. Gastroenterol Clin North Am. 1990 Dec;19(4):793-810. PubMed PMID: 1702756.
- Barnett JL, Wilson JA. Alcoholic pancreatitis and parotitis: utility of lipase and urinary amylase clearance determinations. South Med J. 1986 Jul;79(7):832-5. PubMed PMID: 2425437.
- Atkinson M, Bottrill MB, Edwards AT, Mitchell WM, Peet BG, Williams RE. Mucosal tears at the oesophagogastric junction (the Mallory-Weiss syndrome). Gut. 1961 Mar;2:1-11. PubMed PMID: 13684977.
- Smith LL, Lewis CP, Wyatt I, Cohen GM. The importance of epithelial uptake systems in lung toxicity. Environ Health Perspect. 1990 Apr;85:25-30. PubMed PMID: 2200666.
- Hu X, Shen H, Wang Y, Zhao M. Liver X receptor agonist TO901317 attenuates paraquat-induced acute lung injury through inhibition of NF-κB and JNK/p38 MAPK signal pathways. Biomed Res Int.. 2017;2017.
- Yan B, Chen F, Xu L, Xing J, Wang X. HMGB1-TLR4-IL23-IL17A axis promotes paraquat-induced acute lung injury by mediating neutrophil infiltration in mice. Sci Rep. 2017 Apr 4;7(1):597. doi: 10.1038/s41598-017-00721-8. PubMed PMID: 28377603.
- Habiel DM, Hogaboam C. Heterogeneity in fibroblast proliferation and survival in idiopathic pulmonary fibrosis. Front Pharmacol. 2014 Jan 23;5:2. doi: 10.3389/fphar.2014.00002. PubMed PMID: 24478703.
- Micallef L, Vedrenne N, Billet F, Coulomb B, Darby IA, Desmoulière A. The myofibroblast, multiple origins for major roles in normal and pathological tissue repair. Fibrogenesis Tissue Repair. 2012 Jun 6;5(Suppl 1):S5. doi: 10.1186/1755-1536-5-S1-S5. PubMed PMID: 23259712.
- Macklin CC. Transport of air along sheaths of pulmonic blood vessels from alveoli to mediastinum: clinical implications. Arch Intern Med. 1939 Nov 1;64(5):913-26.
- Murayama S, Gibo S. Spontaneous pneumomediastinum and Macklin effect: overview and appearance on computed tomography. World J Radiol. 2014 Nov 28;6(11):850-4. PubMed PMID: 25431639.
- Rezvani K, Kurbaan AS, Brenton D. Ecstasy induced pneumomediastinum. Thorax. 1996 Sep;51(9):960-1. PubMed PMID: 8984713.
- Soares DS, Ferdman A, Alli R. Subcutaneous emphysema and pneumomediastinum following cocaine inhalation: a case report. J Med Case Rep. 2015 Sep 13;9:195. doi: 10.1186/s13256-015-0683-8. PubMed PMID: 26364299.
- Moraes D, Loscalzo J. Pulmonary hypertension: newer concepts in diagnosis and management. Clin Cardiol. 1997 Aug;20(8):676-82. PubMed PMID: 9259160.
- Zhou CY, Kang X, Li CB, Li XH, Liu Y, Wang Z, et al. Pneumomediastinum predicts early mortality in acute paraquat poisoning. Clin Toxicol (Phila). 2015 Jul;53(6):551-6. doi: 10.3109/15563650.2015.1046183. PubMed PMID: 26072933.
- Ntshalintshali SD, Manzini TC. Paraquat poisoning: Acute lung injury–a missed diagnosis. S Afr Med J. 2017 Apr 25;107(5):399-401. doi: 10.7196/ SAMJ.2017.v107i5.12306. PubMed PMID: 28492119.
- Daisley H Jr, Simmons V. Forensic analysis of acute fatal poisonings in the southern districts of Trinidad. Vet Hum Toxicol. 1999 Feb;41(1):23-5. PubMed PMID: 9949480.
- Gawarammana IB, Buckley NA. Medical management of paraquat ingestion. Br J Clin Pharmacol. 2011 Nov;72(5):745-57. doi: 10.1111/j.1365-2125.2011.04026.x. PubMed PMID: 21615775.
- Köppel C, Wissmann CV, Barckow D, Rossaint R, Falke K, Stoltenburg- Didinger G, et al. Inhaled nitric oxide in advanced paraquat intoxication. Journal of Toxicology: Clinical Toxicology. 1994 Jan 1;32(2):205-14. PubMed PMID: 8145361.
- Zhu Y, Deng G, Ji A, Yao J, Meng X, Wang J, et al. Porous Se@ SiO2 nanospheres treated paraquat-induced acute lung injury by resisting oxidative stress. Int J Nanomedicine. 2017 Sep 27;12:7143-7152. doi: 10.2147/IJN. S143192. PubMed PMID: 29026307.