Efficacy of Montelukast and Ranitidine as an Add on Therapy for Chronic Urticaria
S Sweetlin1, Dinesh S2*
1 Assistant Professor of Pharmacology, Government Villupuram Medical College, Tamil Nadu, India.
2 Associate Professor of Pharmacology, Government Stanley Medical College, Chennai, Tamil Nadu, India.
*Corresponding Author
Dr. Sivagami Dinesh,
Associate Professor of Pharmacology,
Government Stanley Medical College, Chennai, Tamil Nadu, India.
E-mail: sivagami_dinesh@yahoo.in
Received: January 10, 2018; Accepted: April 12, 2018; Published: April 18, 2018
Citation: Marwa AM, Sayed O, El-Hanbuli HM, Abo-sief AF. Deterioration of Brain Cell Function Induced by Dapoxetine Administration in Male Rats. Int J Clin Pharmacol Toxicol. 2018;7(1):284-289. doi: dx.doi.org/10.19070/2167-910X-1800048
Copyright: Dinesh S© 2018. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Chronic urticaria is a highly distressing disease affecting a person’s quality of life. In most cases, monotherapy fails. Hence, combinations of antihistamines with montelukast, H2 blockers, ciclosporin, dapsone, omalizumab are used with varying results. This study was done to assess the efficacy and safety of the combination therapy of Montelukast and Cetirizine with Ranitidine and Cetirizine in chronic urticaria patients.
Methodology: In this randomized controlled study, hundred patients were recruited, randomized and medications were given to group A (Cetirizine + Montelukast) and group B (Cetirizine + Ranitidine). Complete history, clinical examination and laboratory investigations were done at the beginning of the study. Patients were educated to keep a daily record of Urticaria Activity Score (UAS7) over seven consecutive days in a descriptive chart. Review of patient’s UAS7 record and clinical examination were done at every weekend. Sum of score at the end of every week for 4 weeks were calculated and recorded.
Results: The mean weekly UAS in group A were 18.67, 10.07, 4.65, and 1.74 and in group B were 27.77, 19.38, 13.68 and 8.04 respectively. Significant difference in symptom reduction between group A and group B was found to be favouring group A. The mean total UAS in group A was 35.13, group B is 68.87 (p < 0.001).
Conclusion: Montelukast seems to be a promising medication as add-on therapy to Cetirizine both in the aspect of efficacy and safety in patients affected by chronic urticaria.
2.Introduction
3.Objectives
4.Methodology
4.1 Study Setting
4.2 Study Participants
4.3 Inclusion Criteria
4.4 Exclusion Criteria
4.5 Study Procedure
4.6 Assessment of Efficacy
4.7 Assessment of Safety
4.8 Statistical Analysis
5.Results
6.Discussion
7.Conclusion
8.Limitations
9.References
Keywords
Chronic Urticaria; Cetirizine; Montelukast; Ranitidine.
Introduction
Urticaria is a circumscribed, elevated, erythematous, generally itchy swelling (edema) involving the upper layer of the dermal skin. It is a clinical manifestation of either immunologic inflammatory response to external triggers, or, in some cases, they may be idiopathic. Urticaria is said to be acute if it lasts less than 6 weeks. Most acute episodes are due to adverse allergic reactions to foods in children or to viral illnesses. Episodes of urticaria lasing beyond 6 weeks are said to be chronic urticaria. Most of the patients with chronic urticaria have no underlying disorders or causes that can be discerned.
Approximately urticaria occurs in 15 to 20% of the general population at least once in their lifetime [1]. Chronic urticaria in addition to reducing a person’s quality of life, affects outcome at workplace, school [2]. Although the global incidence and prevalence of chronic urticaria are not known exactly, it is approximately estimated to occur in at least 0.1% and possibly up to 3% of the general population [3]. Chronic urticaria is a relatively common condition in India. But exact disease burden in Indian scenario is unknown.
The urticaria occurs most frequently after adolescence, with the highest incidence in young adults, though persons of any age may experience urticaria and/or angioedema. Incidence of Chronic urticaria is two times higher in women than men. An Indian study showed that out of 500 cases of urticaria. 37% were suffering from physical urticaria [4]. HLA-DRBI*04, HLA-DQBI*0302, HLA-DRBI*15 and HLA-DQBI*06 are present with higher frequency in patients with chronic urticaria as compared with a control population [5].
Urticaria is known to be due to a number of pathophysiological mechanisms. Urticaria may develop after IgE- or IgE receptor - mediated reactions; due to abnormalities of the complement system and other plasma effectors system; after direct mast cell degranulation; or is association with activation of the arachidonic acid metabolic pathways of the cells. The major effectors cell in most forms of urticaria is mast cells, though other cell types may be involved. Urticaria results in a local increase in the permeability of capillaries and venules. Vascular permeability in skin is produced by the interaction of both H1 and H2 histamine receptors. Activation of H1 receptors in the skin induces itching, flare, erythema, whealing and contraction of smooth muscle in respiratory and gastro-intestinal tract. Stimulation of H2 receptors leads to erythema and whealing in the skin and increased gastric acid secretion.
Diagnosing urticaria continues to be a challenge, as its etiology is often unknown. Diagnostic studies should be based on findings elicited by the history and physical examination. There is little role for routine prick skin testing or the radio allegro sorbent test (RAST) in the diagnosis of specific IgE-mediated antigen sensitivity in chronic urticaria/angioedema. In chronic urticaria, disease activity assessment in scientific researches as well as in routine clinical practice could be done using Urticaria Activity Score (UAS7), which is an unified scoring method which was suggested in the EAACI/GA2LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management of urticaria and has been validated. In this score, the signs and symptoms of chronic urticaria assessment is done by the patient themselves thus improving the score’s validity [2].
The combination of chlorpheniramine (H1 antagonist) is found to be more successful in inhibiting a histamine skin reaction when compared with an H1 antagonist alone, and it is recommended for the treatment of chronic idiopathic Urticaria [6]. Other studies with Cetirizine and ranitidine, diphenhydramine and ranitidine, terfenadine and ranitidine showed similar results [7, 8]. It has been observed that the H1 antagonist-H2 antagonist combination inhibits the release of allergic mediators, whether IgE dependent or otherwise [9-11]. However, antihistamines are only partially effective in inhibiting wheal formation in some chronic Urticaria patients; hence it is very probable that other mediators apart from histamine may play a role in wheal formation in chronic Urticaria [12, 13]. Injected leukotriene D4 has been found to be more potent than histamine in causing a wheal and flare [14]. Montelukast blocks the action of leukotriene D4 on the cysteniny1 leukotriene receptor CysLT1 in the lungs. Leukotriene receptor antagonists like montelukast have been tried in chronic urticaria with variable results. Since leukotriene-mediated urtication is not blocked by other agents, leukotriene antagonists can be helpful [15].
There are many clinical trials and isolated observations with multiple treatments either as monotherapy or in combination. Most of the trials have assessed the efficacy of add on therapy of Montelukast with other antihistamines like Hydroxyzine, Desloratidine, Fexofenadine, Obastine etc, but with Cetirizine, the trials are less, especially in Indian population. Similarly trials on role of add on therapy of H2 blocker in chronic Urticaria among Indian population are also very less.
Objectives
1. To compare the efficacy of the combination therapy of Montelukast and Cetirizine with Ranitidine and Cetirizine in patients with chronic Urticaria.
2. To assess the safety of the combination therapy of Montelukast and Cetirizine with Ranitidine and Cetirizine in patients with chronic Urticaria.
This study was carried out as a prospective, randomized, open label comparative study by the Department of Dermatology in our medical college hospital between March 2015 and March 2016.
All patients who were attending the outpatient facility of our department with history and clinical features of urticaria were included in the study. A total of 100 patients were enrolled during the study period, selected with inclusion and exclusion criteria. However, there were four drop outs in Group A and three drop outs in Group B.
• Chronic urticaria patients not responding to two weeks of treatment with Cetirizine 10mg once daily dosage.
• Age: 18-60 years.
• Patients who are willing to give informed consent.
• Urticaria of less than 6 weeks duration.
• Age: <18yrs & >60yrs.
• Pregnant and lactating women.
• Chronic urticaria patients who were treated with steroids & other immunosuppressants.
• Patients with any focal sepsis.
• Drug induced urticaria.
• Urticaria associated with other skin disorders like eczema, etc.,
• Patients with chronic bronchial asthma who were taking steroids/Montelukast.
• Patients with cholestatic jaundice.
Approval was obtained from the Institutional Ethics Committee prior to the commencement of the study. Each participant was explained in detail about the study procedure and a written informed consent was obtained prior to randomization. In cases where the participant was illiterate, left thumb impression was obtained.
Participants who fulfilled the selection criteria were recruited for the study from the outpatient department. A total of 144 participants were screened, of which 100 were recruited for the study. Based on the first come first served criteria, the odd numbered participants were assigned into Group A (Cetirizine + Montelukast) and even numbered participants were assigned to group B (Cetirizine + Ranitidine). Complete history including demographic particulars, clinical examination and baseline laboratory investigations were recorded at the beginning of the study.
GROUP A: Participants were asked to ingest tablet Cetirizine 10 mg and tablet montelukast 10 mg once daily at night after food intake.
GROUP B: Participants were instructed to take tablet Ranitidine 150 mg twice daily 1 hour before food in the morning and night along with tablet Cetirizine 10 mg once daily at night after food intake.
The duration of the study was for 6 weeks, 4 weeks of therapy with 2 weeks of follow up. Participants were educated to keep a daily record of Urticaria activity score over 7 consecutive days in a descriptive chart provided to them. In that chart, the participants were asked to circle the score that corresponded to the number of wheals that occurred and the score that represented the intensity of their pruritus (itching) on a daily basis. Daily, two times (morning and evening) participants scored pruritus, number of hives, over the preceding 12 hours (reflective) and soon at the time of assessment (instantaneous). These assessments were made on awakening (before dosing) and 12 hours after dosing.
Review of the participant’s completed record and clinical examinations of patients according to the Urticaria Activity Score7 (UAS7) were done at the end of every week. Sum of scores were calculated at the end of every week for 4 weeks and the data recorded. Baseline laboratory investigations were repeated at the end of 4th week. Patients were followed up for 2 weeks after completion of the study.
The efficacy was assessed by the decrease in the weekly Urticarial Activity Score which in turn showed the improvement in the participant’s symptomatology. Vital signs were monitored at all visits, whereas electrocardiography and laboratory tests were performed at screening and at the end of 4th week and 6th week.
Participants were advised to report any occurrences of adverse during treatment and follow up period and the same were recorded. Causality assessment of adverse drug reactions was done using WHO scale. Severity assessment was done by Modified Hartwig Seigel Severity assessment scale. Safety evaluations included were any incidence of treatment-induced or any emergency adverse events discontinuations due to adverse events, and changes from baseline in vital signs, laboratory parameters, and electrocardiographic intervals.
The details of the data collected were analyzed statistically using SPSS software (version 20) according to per protocol analysis. Hence, 93 patients who completed the study were included in the statistical analysis. Mean scores were computed for the UAS 7 scores. Association between demographic characteristics and the improvement in the scores were analyzed using Chi square test and student independent t-test. The difference in mean Urticaria activity score (UAS) every week within the same group for 4 weeks was analyzed using analysis of variance (ANOVA) whereas the difference in Urticaria activity score (UAS) between group A and B assessed by student independent t-test. The variations in biochemical investigations between group A and group B were analyzed by student independent t-test. Percentage incidence of adverse effects among the study groups were analyzed using Chisquare test. A probability < 0.05 was considered to be statistically significant.
Results
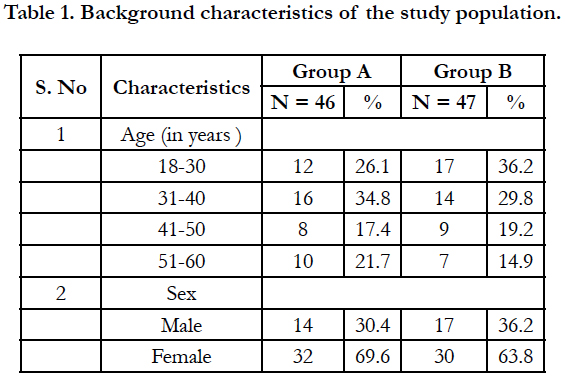
This study was carried out among 93 participants with chronic urticaria, of which 46 participants belonged to Group A and 47 participants belonged to Group B. The mean age of the participants in Group A was 37.9 ± 11.02 years while the mean age in Group B was 36.4 ± 12.1 years. The background characteristics of the study participants are given in table 1.
Majority of the participants in group A belonged to 31-40 years (34.8%) while in group B, the majority of the participants belonged to 18-30 years (36.2%). In both the groups, females were more than males.
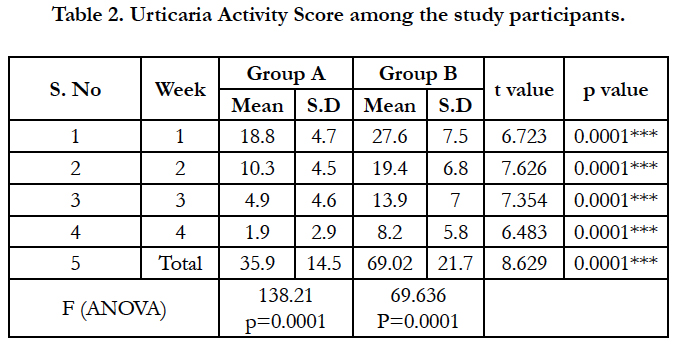
The particulars related to chronic urticaria are given in Table 2. The mean duration of urticaria in Group A was found to be 8.35 ± 5.3 months while in group B, the mean duration of urticaria was 8.23 ± 4.3 months. The weekly urticaria activity score was found to be reducing in both the groups, over the period of 4 weeks, however, the reduction in the scores were rapid in Group A compared to Group B at the end of 4th week (t=6.483; p<0.0005).
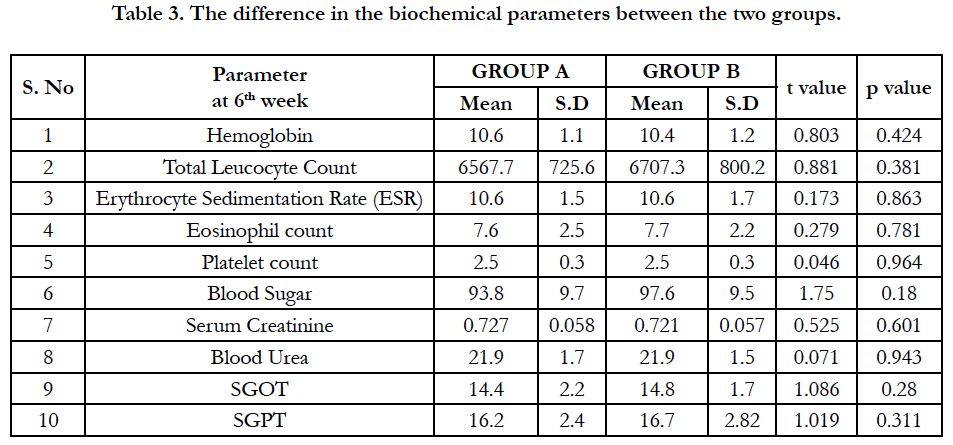
The difference in the biochemical parameters between the two groups is given in table 3. There was no significant difference found between the two groups at baseline or 4th week or 6th week.
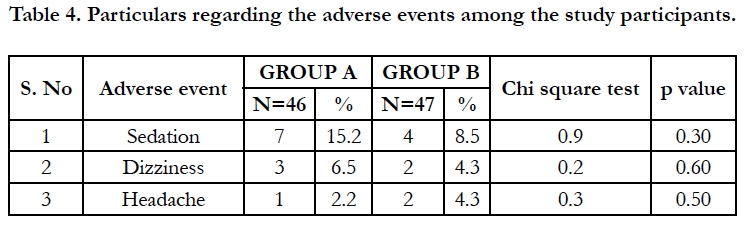
The particulars regarding the adverse events are given in table 4. The adverse events were mild and no serious adverse effects were reported. Among the adverse events, it was found that sedation was the most common followed by dizziness and headache.
Discussion
Chronic urticaria known since ancient times, is a highly distressing disease that can invariably disturb a person’s personal, social and occupational life altogether. This chronic disease manifests as pruritic, raised wheals of reddish colour all over the body of varying sizes with serpiginous margins with blanched centers which may sometimes coalesce [16]. It may appear daily or on most days of a week for a duration of greater than 6 week. The current recommendation by the EAACI/GA2LEN/EDF/WAO guideline is to aim for complete control of symptom in urticaria.
The treatment of chronic urticaria remains a challenging task for physicians. A step-wise approach is currently advocated by the 2009 treatment guidelines [17]. First line therapy comprises a nonsedating H1-antihistamine at standard doses. After two weeks, if no response is obtained, the dose has to be increased up to four times the standard or licensed dose. Third line of therapy includes the addition of a leukotriene receptor antagonist (LTRA). For severe or resistant cases, immunosuppressant’s such as ciclosporin, dapsone, H2-antihistamines and omalizumab are also used [18]. Short-course systemic steroids are recommended for exacerbations.
From India, there are no published studies regarding the use of montelukast in urticaria. Though it is known that monotherapy with montelukast is probably not advisable, there is a need for validation in the Indian population, regarding the outcome of addition of montelukast to an antihistamine in patients with chronic urticaria.
Similarly, data about the efficacy of H2 blockers as an additional therapy to antihistamines in treating chronic urticaria are limited. The combined effect of H1-H2 antihistamines is more due to interactions at the CYP3A4 level or other isoenzyme families resulting in mutual increase in the area under the plasma concentration- time curve (AUC) - rather than due to any genuine “synergic effect”.
In a study by Watson et al., Famotidine combined with Diphenhydramine had shown better symptom improvement in chronic urticaria than prescribing Diphenhydramine alone [19]. There are not enough confirmatory data from clinical trials to recommend combination of 2nd generation antihistamines with leukotriene antagonists or H2 blockers; the role of these drugs in chronic urticaria remains to be established.
In our study among the 93 patients with chronic urticaria, the mean duration of urticaria among the study subjects in group A and group B were 7.93 and 8.11 months respectively. The efficacy outcome measured by the mean weekly urticaria activity score (UAS) at the end of 1st, 2nd, 3rd and 4th week was significantly better among group A participants compared to group B participants. Every week, mean urticaria activity score was found to be decreasing than previous week in both the groups but comparatively high values were seen in group B than group A in the same week, hence showing significant benefit of Montelukast add on therapy.
The mean total urticaria activity score among montelukast group is 35.13 whereas that among ranitidine group is 68.87 (p<0.001) showing significant reduction in wheals and pruritus among group A compared to group B. This is consistent with the findings of a double blind cross over study conducted by M. Kosnik & T. Subic who showed that response to add-on treatment with montelukast was seen among patients with particularly long standing disease [20]. In a study by Wan et al., lesser response rate with Montelukast add-on to Loratadine vs. Loratadine along therapy was reported, but this study in contrast to our study was conducted in newly diagnosed chronic urticaria patients [14].
Hence, most patients are antihistamine responsive and their major pathological mediator being histamine and not leukotriene which might have skewed the results of this study towards relative poor response with add on montelukast. Pronounced favourable responses to montelukast, especially in aspirin intolerant chronic patients were reported by Pacor et al and Erbagci Z et al., [21, 22]. Our safety outcome measures like haematological and biochemical parameters were analyzed by student t-test showing no statistical difference among the study groups implies that both the drugs doesn’t have any untoward effects on these parameters.
A lower incidence of adverse events was encountered in the study. All adverse events were rated as mild. Mild adverse effects such as sedation, dizziness and headache occurred among study groups which does not shown any statistical significant difference among the groups and all the adverse effects subsided without any medications. Moreover, the relapse rates after 2 weeks of follow up were significantly lower in the Montelukast group compared to the Ranitidine group.
The results of this study demonstrate that montelukast administered 10mg once daily as an add-on therapy to Cetirizine 10 mg once daily is more effective than ranitidine 150 mg twice daily add-on therapy for the treatment of Urticarial symptoms in patients with chronic urticaria. Thus, montelukast can be safely used in combination with antihistamines for chronic urticaria patients whose response is poor to antihistamines alone.
Conclusion
From our study, we conclude that combination therapy of Montelukast and Cetirizine is found to be more efficacious than Ranitidine and Cetirizine in the treatment of chronic urticaria patients not responding to Cetirizine along. This is evidenced by statistically significant difference in UAS (p<0.05). Therefore, we conclude that Montelukast is an effective adjuvant to Cetirizine in chronic urticaria. In view of safety, Montelukast was well tolerated with lesser side effect profiles. Relapse of symptoms was also found to be lesser in the Montelukast group. Thus, Montelukast seems to be a promising medication both in the aspect of efficacy as well as safety in patients with chronic urticaria.
Limitations
Our study did not observe the effect on quality of life of chronic urticaria. It is worth to given a trial of montelukast as add on medication in chronic urticaria patients. However, a trial including a larger group of Indian population is recommended.
References
- Bajaj AK, Yadav S, Upadhyay A. Chronic urticaria: An overview. Indian J Dermatol. 2006 Jul 1;51(3):171.
- Zuberbier T, Aberer W, Asero R, et al. The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management of urticaria: the 2013 revision and update. Allergy. 2014 Jul 1;69(7):868-87. PubMed PMID: 24785199.
- Greaves MW. Chronic urticaria. N Engl J Med. 1995 Jun 29;332(26):1767-72. PubMed PMID: 7760895.
- Singh M, Kaur S, Kanwar AJ. Evaluation of the causes of physical urticarias. Indian J Dermatol Venereol Leorol. 1990 Mar 1;56(2):109.
- Yosipovitch G, Patel TS. Pathophysiology and clinical aspects of pruritus. Fitzpatrick’s Dermatology in General Medicine, 8th edition. The McGraw- Hill Companies. 2012:1147-57.
- Bleehen SS, Thomas SE, Greaves MW, Newton J, Kennedy CT, et al. Cimetidine and chlorpheniramine in the treatment of chronic idiopathic urticaria: a multi‐centre randomized double‐blind study. Br J Dermatol. 1987 Jul;117(1):81-8. PubMed PMID: 3307890.
- Dhanya NB, Rai R, Srinivas CR. Histamine 2 blocker potentiates the effects of histamine 1 blocker in suppressing histamine-induced wheal. Indian J Dermatol Venereol Leprol. 2008 Sep-Oct;74(5):475-7. PubMed PMID: 19052407.
- Paul E, Pfeffer M, Bödeker RH. Effect of terfenadine and ranitidine on histamine and suxamethonium wheals. Eur J Clin Pharmacol. 1988;34(6):591-4. PubMed PMID: 3139429.
- Dorsch W, Reimann HJ, Neuhauser J. Histamine1-histamine2 antagonism: Effect of combined clemastine and cimetidine pretreatment on allergen and histamine-induced reactions of the guinea pig lungin vivo andin vitro. Agents Actions. 1982 Apr;12(1-2):113-8. PubMed PMID: 6177209.
- Philbin DM, Moss J, Akins CW, Rosow CE, et al. The use of H1 and H2 histamine antagonists with morphine anesthesia: a double-blind study. Anesthesiology. 1981 Sep;55(3):292-6. PubMed PMID: 6115596.
- Irwin RB, Lieberman P, Friedman MM, Kaliner M, Kaplan R, et al. Mediator release in local heat urticaria: protection with combined H1 and H2 antagonists. J Allergy Clin Immunol. 1985 Jul;76(1):35-9. PubMed PMID: 2861221.
- Ferrer M, Luquin E, Sanchez-Ibarrola A, Moreno C, Sanz ML, Kaplan AP. Secretion of cytokines, histamine and leukotrienes in chronic urticaria. Int Arch Allergy Immunol. 2002 Nov;129(3):254-60. PubMed PMID: 12444324.
- Wedi B, Novacovic V, Koerner M, Kapp A. Chronic urticaria serum induces histamine release, leukotriene production, and basophil CD63 surface expression-inhibitory effects of anti-inflammatory drugs. J Allergy Clin Immunol. 2000 Mar;105(3):552-60. PubMed PMID: 10719307.
- Wan KS. Efficacy of leukotriene receptor antagonist with an anti-H1 receptor antagonist for treatment of chronic idiopathic urticaria. J Dermatolog Treat. 2009;20(4):194-7. PubMed PMID: 19085267.
- Nettis E, Dambra P, D'oronzio L, Loria MP, Ferrannini A, Tursi A. Comparison of montelukast and fexofenadine for chronic idiopathic urticaria. Arch Dermatol. 2001 Jan;137(1):99-100. PubMed PMID: 11176678.
- Venarske D, deShazo RD. Molecular mechanisms of allergic disease. South Med J. 2003 Nov;96(11):1049-54. PubMed PMID: 14632350.
- Zuberbier T, Asero R, Bindslev-Jenson C, et al. EAACI/GA(2)LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy. 2009 Oct;64(10):1417-26. PubMed PMID: 19772512.
- Maurer M, Rosén K, Hsieh HJ, Saini S, Grattan C, et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med. 2013 Mar 7;368(10):924-35.
- Watson NT, Weiss EL, Harter PM. Famotidine in the treatment of acute urticaria. Clin Exp Dermatol. 2000 May;25(3):186-9. PubMed PMID: 10844490.
- Kosnik M, Subic T. Add-on montelukast in antihistamine-resistant chronic idiopathic urticaria. Respir Med. 2011 Oct;105 Suppl 1:S84-8. PubMed PMID: 22015095.
- Pacor ML, Di Lorenzo G, Corrocher R. Efficacy of leukotriene receptor antagonist in chronic urticaria. A double‐blind, placebo‐controlled comparison of treatment with montelukast and cetirizine in patients with chronic urticaria with intolerance to food additive and/or acetylsalicylic acid. Clin Exp Allergy. 2001 Oct;31(10):1607-14. PubMed PMID: 11678862.
- Erbagci Z. The leukotriene receptor antagonist montelukast in the treatment of chronic idiopathic urticaria: a single-blind, placebo-controlled, crossover clinical study. J Allergy Clin Immunol. 2002 Sep;110(3):484-8. PubMed PMID: 12209099.