Deterioration of Brain Cell Function Induced by Dapoxetine Administration in Male Rats
Marwa AM1*, Sayed O2, El-Hanbuli HM3, Abo-sief AF4
1 Lecturer of Forensic Medicine and Clinical Toxicology, Department of Forensic Medicine and Clinical Toxicology, Faculty of Medicine, Fayoum University, Fayoum, KimanFaris Str., Egypt.
2 Lecturer of Chemistry, Department of Chemistry, BioChemistry Division, Faculty of Science, Fayoum University, KimanFaris Str., Egypt.
3 Assistant Professor of Pathology, Department of Pathology, Faculty of Medicine, Fayoum University, KimanFaris Str., Egypt.
4 Lecturer of Andrology, Department of Andrology, Sexology & STDs, Faculty of Medicine, Beni-Suef University, Salah Salem Str., Egypt.
*Corresponding Author
Marwa A Mwaheb,
Lecturer of Forensic Medicine and Clinical Toxicology,
Department of Forensic Medicine and Clinical Toxicology,
Faculty of Medicine, Fayoum University, Fayoum, KimanFaris Str., Egypt.
E-mail: marwa.mwaheb@yahoo.com
Received: March 02, 2018; Accepted: April 11, 2018; Published: April 13, 2018
Citation: Marwa AM, Sayed O, El-Hanbuli HM, Abo-sief AF. Deterioration of Brain Cell Function Induced by Dapoxetine Administration in Male Rats. Int J Clin Pharmacol Toxicol. 2018;7(1):284-289. doi: dx.doi.org/10.19070/2167-910X-1800047
Copyright: Marwa A Mwaheb© 2018. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Background and Aim of Work: Dapoxetine is a Selective Serotonin Reuptake Inhibitor (SSRI) decided for the remedy of premature ejaculation (PE) in adult men. The objective of this research was to determine the influence of dapoxetine on the brain of male rats.
Methods: The rats were divided into three empirical groups and one control group of 10 rats each. The empirical groups daily took dapoxetine hydrochloride at doses of 2.0, 4.0 or 8.0 mg/kg (each dose for each group). 1 in 0.5 ml saline for 60 days.
Results: There was a significant elevation in lipid peroxidation level in all treated groups in respect to control group but there was a decrease in total antioxidant level, and elevation in Acetylcholinesterase activity and Vascular Endothelial Growth Factor level in all treated groups compared to control group. The histological brain damage score was zero for all rats in the control group only, it showed increased mean from group II till group IV. The study demonstrated that Apoptotic Index (determined by the immunohistochemistry) was lowest in the control group and increased significantly from group II through IV.
Conclusion: Dapoxetine administration in rats in large doses for a temporal period affected the brain cells and must be carefully used.
2.Introduction
3.Material and Method
3.1 Experimental Animal Design
3.2 Biochemical Assays
3.3 Histopathology and Immunohistochemistry Studies
3.4 Statistical Analysis
4.Results
4.1 Biochemical Results
4.2 Histopathological and Immunohistochemistry Results
4.3 Relation between Biochemical and Immunohistochemical Results
5.Discussion
6.Conclusion
7.References
Keywords
Dapoxetine; Brain Damage; Oxidative Stress; Anti-Inflammatory; Lipid Peroxidation.
Introduction
Premature ejaculation (PE) is the most common sexual problem worldwide. Human ejaculation is thought to be controlled by many different physical pathways. In addition to the contribution of the psychological factors to the biological ones which make the pathophysiology of PE even more complex. In the last years, the treatment modalities have consisted of different psychotherapy options. However, discoveries on the central regulation of ejaculation and the therapeutic role of serotonergic agents in PE revolutionised the treatment of this condition [1]. Well-established evidences from the studies have expounded the efficacy and safety of selective serotonin reuptake inhibitors (SSRIs) in lateness of ejaculation, confirming their role for the therapy of lifelong and gained premature ejaculation (PE) [2].
A modernistic serotonin transporter inhibitor, dapoxetine, has been in proceeding specifically for the curative of PE. Results from placebo-controlled, randomized, multicenter phase III trials have pretended that men with PE extraditing dapoxetine 30 or 60mg long-practiced increased intravaginal ejaculatory latency and higher levels of observation through ejaculation and gratification with sexual intercourse [3, 4]. Dapoxetine was effective when taken unwanted, 1-3 h before intercourse. In dissimilarity, large scale studies of efficacy and safety in men with PE have not been behaved with traditional SSRI antidepressants [5, 6].
Neurotoxicity testing of new complex with the desirable pharmacological effects represents one of the major bottlenecks in drug development since it was time consumed and required of many animal experiments. The neurotoxicity is likely to be the result of a collection of many mechanisms, including oxidative brain injury, prompting of apoptosis and neuronal harm, neuroglia inflammatory reaction, and lessening neurotransmitter biosynthesis [7]. Therefore, the existent research is the first undertaken to repress neuron inflammation and neuronal apoptosis by means of dapoxetine toxicity in the empirical animal model.
Forty male albino rats weighting average of 150 g were preserved in the laboratory under steady conditions of temperature (24 ± 2°C) at minimum one week before and through the empirical work, being kept on a standard diet and water, were ready adlibitum. The animals were preserved in harmony with the rules described by the faculty of Science and study was suitable by Animal Ethics Committee of Fayoum University, Egypt. The rats were divided at random into three empirical study groups (GII, GIII and GIV) and one control group (GI) of 10 rats each. The study groups were daily took dapoxetine hydrochloride (Inspire Pharmaceutical Company, IPC Pharma, Cairo, Egypt) at doses of 2.0, 4.0 or 8.0 mg per kg body weight [8] (every dose for each group) 1 in 0.5 ml saline, while the controls were on 0.5 ml saline. All were kept for 60 days. At the end of the experimental period, animals were killed and the whole brain of each animal was rapidly removed, thoroughly washed with isotonic saline, dried on filter paper and then weighed. Each brain was sagitally split into two halves. One half of each brain was instantly homogenized to give 10% (w/v) homogenate in ice-cold medium containing 50 mM Tris-HCl (pH 7.4) and 300 mM sucrose [9]. The homogenate was centrifuged at 1800 xg for 10 min at 4°C. The supernatant (10%) was detached and stored at -70°C for biochemical analyses. The second half of each brain was preserved in 10% buffered formalin for histopathological and immunohistochemical examination.
Brain lipid peroxidation was estimated by malondialdehyde (MDA) via use of thiobarbituric acid (Sigma-Aldrich Chemie Gmbh, Steinheim, Germany) [10]. Total antioxidant status (TAS) was predestined by a colorimetric method rely on the capability of brain antioxidants to inhibit the production of thiobarbituric acid reactive substances(TBARS). TBARS are output following benzoate disintegration by means of hydroxyl radicals that form in a Fenton-type reaction [11].
Brain acetylcholinesterase activity (AChE) was decided colorimetrically using a kit provided by Sigma-Aldrich (St Louis, MO, USA) [12]. Brain tumour necrosis factor alpha (TNF-α) [13] concentricity was evaluated in the brain homogenate using a rat TNF-α ELISA technique using human TNF-αELISA kit from Orgenium Co., Vantaa FINLAND. Brain derived neurotrophic factor (BDNF) was measured by ELISA technicality using [14] kit from RayBiotech Co., USA. Brain vascular endothelial growth factor (VEGF) was evaluated by ELISA steps using [15] a kit purchased from In vitro gen Co., CAMARILLO, and VENTURA, California, USA. Brain (IL1β) was measured by ELISA steps [16] using a kit from Assay Pro-USA.
Paraffin section (5μm in thickness) was prepared from fixed brain halves for processing the histological and immunohistochemical studies.
For general histology and evaluation of the brains' status; sections were stained with haematoxylin and Eosin (H&E). Estimate of tissue alterations in 20 various fields for each section was conducted by the pathologist who was insensible of the treatment. Histopathological brain damage was scored by grading neuoronal necrosis, neurophagia, neuronal edema, vascular congestion, focal hemorrhage and perivascular edema with a maximum score of 18. Each modulation was scored as: 0, absent or very rare; 1, mild; 2, moderate; or 3, severe [17].
For immunohistochemistry using BAX antibodies deparaffinized, rehydrated sections were heated in a microwave oven in 10 mmol/L citrate buffer (PH6) for antigen retrieval. Sections were in glorioused in 0.3% H2O2 in methanol to block endogenous peroxidase and were pre-incubated with normal mouse or rabbit serum to block nonspecific binding of antibodies. The sections were then incubated with primary antibody. The avidin-biotinperoxidase-complex manner was used for discovery, with diaminobenzidine tetrachloride as the chromogen. The primary antibody used was rabbit monoclonal BAX antibody (ab32503) (1:250; abcam, USA). As a negative control for immunostaining, the second antibody was used without the first antibody.
Scoring of IHC stained cortical neurocytes was done under light microscopy. Brown immunostaining of cytoplasm for Bax was considered as positive apoptotic neuronal cells. Semi-quantification analysis of the apoptotic index (AI) was decided by counting a total of at least 1000 cells per slide subdivided in 10 fields chosen at random x 400 magnification. AI% = [number of positive cells/total number of calculated cells] x 100 is the percentage of positive cells in 1000 cells [18].
Equal variance between the groups was first checked using the Levene’s test for homogeneity of variances and statistical analysis of the difference between the mean values was carried out using one-way analysis of variance (ANOVA) followed by the least significant difference (LSD) test for multiple comparisons. SPSS statistical software 22.0 for Windows (SPSS, Inc., Chicago, IL, USA) was used for all analyses. A value of p < 0.05 was considered statistically significant for all tests.
The records in Table (1) showed a significant increase (p ≤ 0.001) in lipid peroxidation level (MDA) in all treated groups compared to the control group. Also, the study illustrated a significant decrease (p ≤ 0.001) in total antioxidant level in all treated groups in respect to control group but no significant change between group III and group IV (p = 0.211).
Table (2) showed a significant increase (p ≤ 0.001) in TNF-α level in all treated groups compared to control group. There was a significant elevation (p ≤ 0.001) in IL-1β level in all treated groups compared to control group but no significant change between group III and group IV (p = 0.521). Acetylcholinesterase activity notarized a significant elevation (p ≤ 0.001) in all treated groups compared to control group, but there was a significant decrease (p ≤ 0.001) in the brain-derived neurotrophic factor (BDNF) level in all treated groups compared to control group yet no significant change between group III and group IV (p=0.117). There was an increase (p≤ 0.001) in vascular endothelial growth factor (VEGF) level in all treated groups when compared to control group, while Acetylcholine level was lower (p ≤ 0.001) in all treated groups compared to control group with no significant change between group III and group IV (p = 0.275).
Table 2. Mean ± S.D. of Plasma TNF-α, BDNF, VEGF and Acetylcholinesterase Activity in all Different Groups.
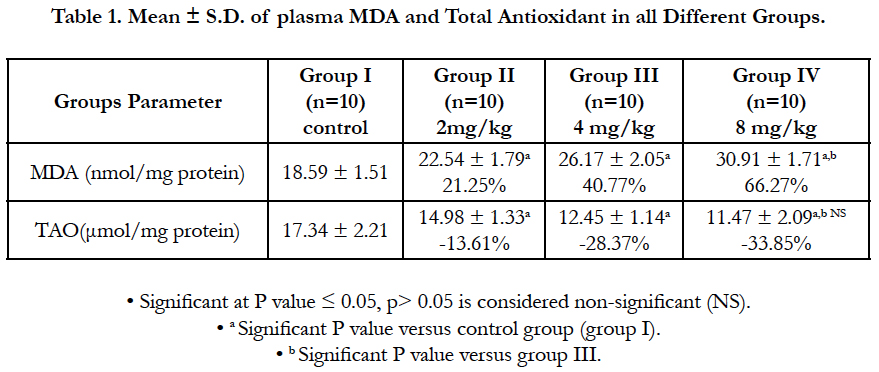
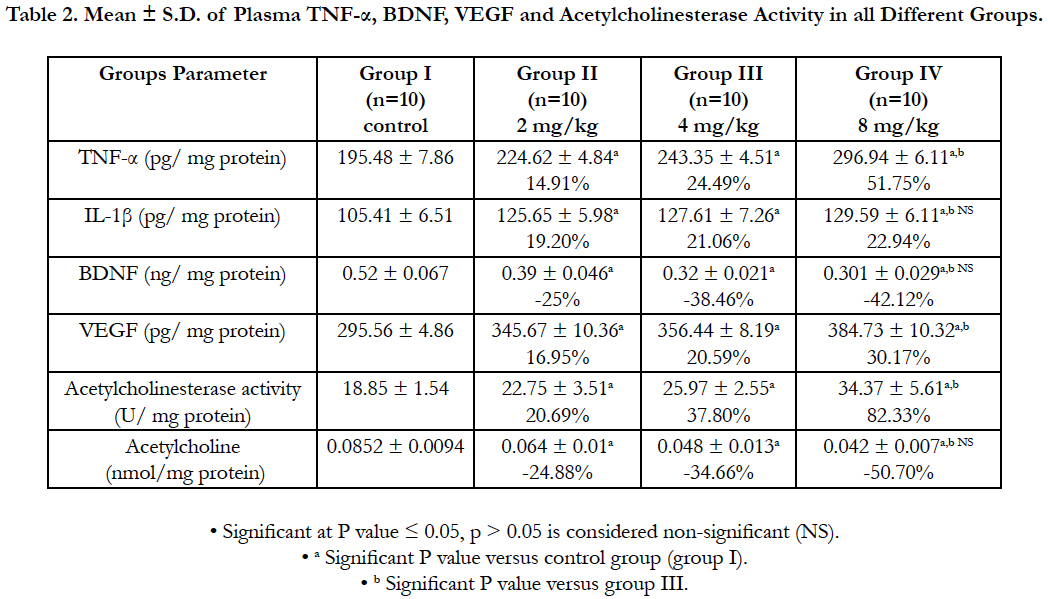
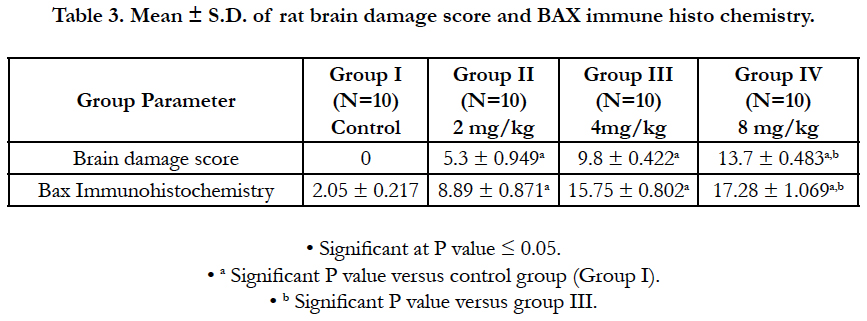
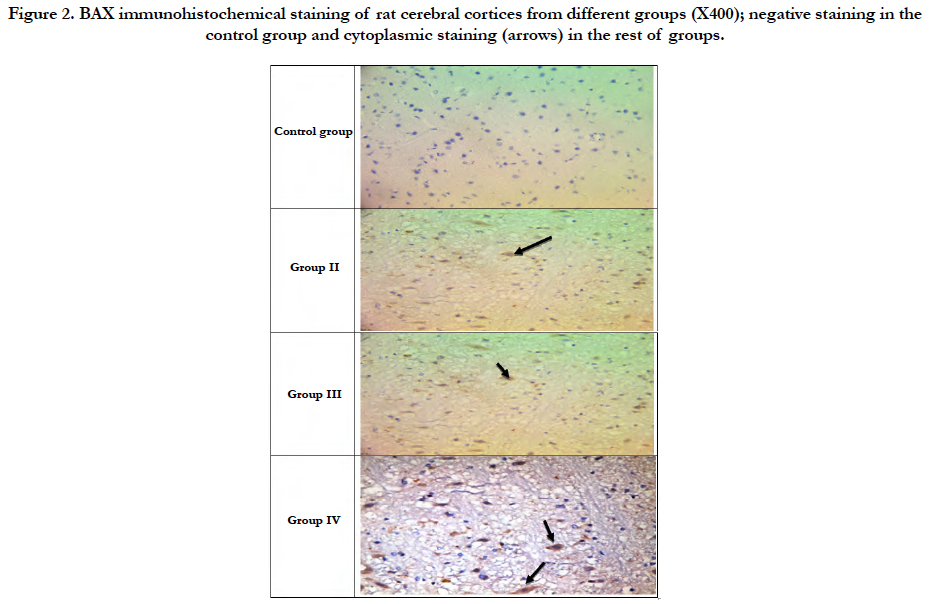
Examination of H&E stained slides of rat brains (Figure 1) revealed normal brain histology in the control group (Group I), neuronal necrosis started to appear in cerebral cortices in group II (Mean ± SD = 2.8 ± 0.422) and scored 3 in all rats in groups III and IV. Also, neurophagia appeared in group II (Mean ± SD = 1.8 ± 0.422) and scored 3 in all rats in groups III and IV. Neuronal edema was noticed only in group IV (Mean ± SD = 1.9 ± 0.316). Vascular congestion was noticed in all treated groups; (Mean ± SD = 0.9 ± 0.316 and 2.8 ± 0.422 for groups II and III respectively), while the score was 3 for all rats in group IV. Focal hemorrhage was present in groups III and IV only and all rats in both groups scored 1. Perivascular edema was noticed in group IV only (Mean ± SD = 1.8 ± 0.422). The brain damage score was zero for all rats in the control group only, it showed increased mean from group II till group IV (Table 3). The existent study demonstrated that AI (determined by the immunohistochemistry of BAX) was lowest in the control group and increased significantly from group II through IV (Table 3 and Figure 2).
Figure 1. Brain (Cerebral cortex) of rat from control group (A) showing no histopathological changes, from group II (B) showing necrosis of neurons and neuronophagia (arrow), from group III (C) showing congestion of cerebral blood vessel (small arrow) and necrosis of neurons (large arrow) and from group IV (D) showing necrosis of neurons and neuronophagia (arrow), neuronal oedema (E) (arrow) (H & E X 400), congestion of cerebral blood vessel (small arrow) and focal haemorrhage (large arrow) (F) (H & E X 400).
Figure 2. BAX immunohistochemical staining of rat cerebral cortices from different groups (X400); negative staining in the control group and cytoplasmic staining (arrows) in the rest of groups.
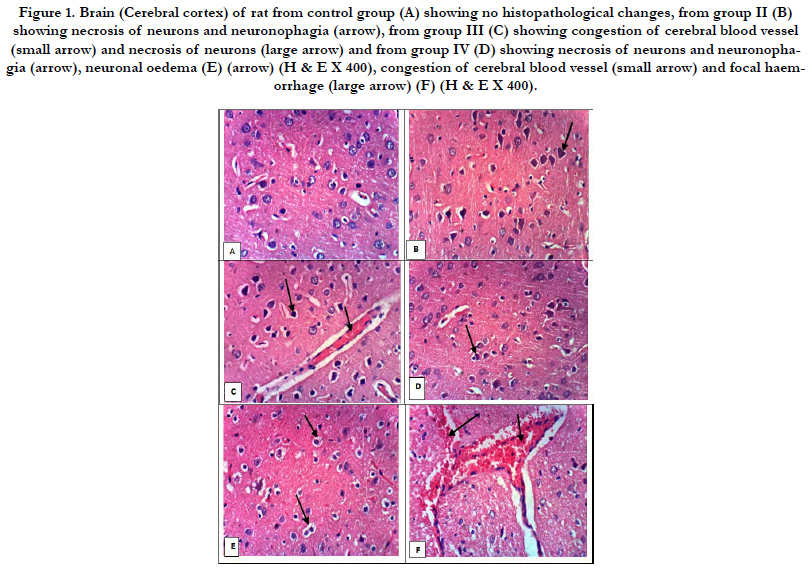
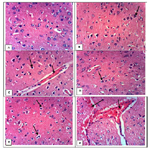
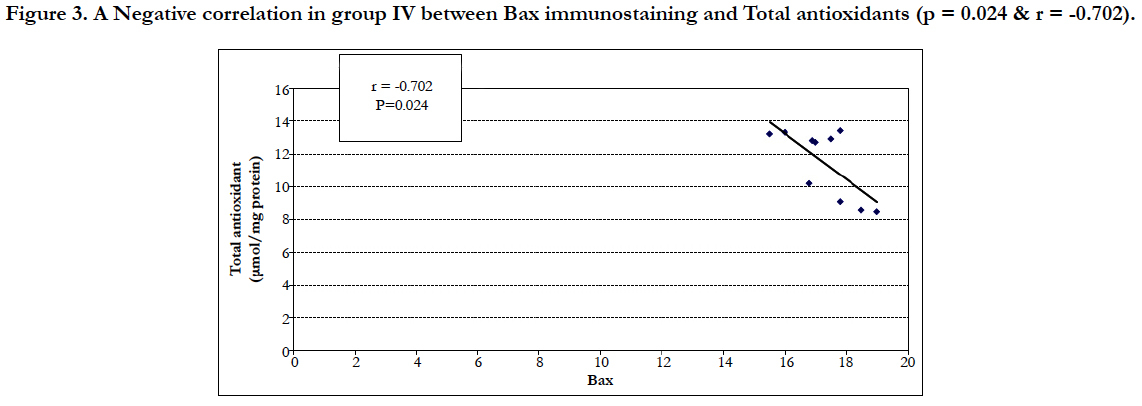
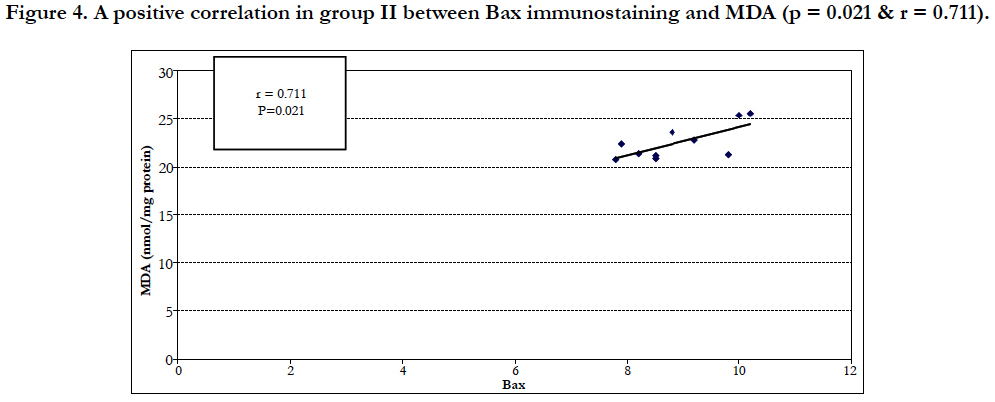
In group IV there was a negative correlation between the apoptotic index (BAX immunostaining) and the total antioxidants (Figure 3), and there was a positive correlation between BAX immunostaining and MDA in Group II (Figure 4).
Figure 3. A Negative correlation in group IV between Bax immunostaining and Total antioxidants (p = 0.024 & r = -0.702).
Figure 4. A positive correlation in group II between Bax immunostaining and MDA (p = 0.021 & r = 0.711).
Discussion
Dapoxetine is a fast-acting SSRI that is used to treat PE and it is often considered as the first line drug therapy [19]. It is not approved for use in the USA and paroxetine has been used as it has a relatively greater efficacy in delaying ejaculation [20]. The existent study recorded for the first time that dapoxetine motivated oxidative brain cell injury. The brain is more exposed to oxidative stress than any other organ due to its large oxygen consumption, high lipid content, and low mitotic rate and antioxidant levels [21]. Different proteins are involved in the regulation of programmed cell death. However, the importance of apoptosis in toxicology has been underestimated given the difficulty of identifying apoptotic cells [22]. Some members of the Bcl family, such as Bcl-2 are known anti-apoptotic proteins. Whereas, the cytoplasmic expression of Bax is pro-apoptotic that enhance apoptosis [23].
The results showed that a significant rise in TNF-α level in all treated groups compared to control group, this is agreed by other research studied aluminum intoxication in rats and reported an elevation in brain Caspase-3 and TNF-α levels, and this was reflected histologically, as increased brain apoptosis, severe degenerations of cerebral cortex neurons and small pyramidal cells of the hippocampus [24] which is also similar to the existent histologic and immunohistochemical results. Also, another research reported an increase in the brain level of pro inflammatory molecules, such as TNF-α, IL-1b in rat group treated with arsenic in respect to the normal control group. This happened because arsenic induced the production of free radicals that cause destruction to lipid, protein, and DNA of the body [25].
The brain-derived neurotrophic factor (BDNF) is the basic regulate element for the existence and distinction of eclectic populations of neurons during development. It is synthesised preponderantly in the CNS by neurons, and is highly be expressed in the hippocampus and cortex, two brain area which are recognized to be important for education and memory [26]. The existent study showed a significant decrease (BDNF) level in all dapoxetine treated groups in comparison to control group but no significant change between group III and group IV, the results that go with a research that has been recorded a reduction in expression of BDNF in aluminium intoxication, that keeps normal neuronal circuits in the brain, and declared that it played a significant role in the development of plentiful neurodegenerative disorders [24]. The current research also coincided with another research of arsenic toxicity that causes brain deterioration and significant reduction in the brain level of BDNF as compared to the normal control group. This inhibition of BDNF was supposed to be a result of the spoilage of the nerve and glial cells in the brain due to the output of reactive oxygen species [25].
The neurotransmitter acetylcholine (Ach) is fundamental for the central and peripheral control of movement, autonomic nervous system function, arranged of sleep, and numerous cognitive procedure including timing, attention, education, and memory [27, 28]. The results of the study also clarified that Acetylcholinesterase activity showed a significant elevation (p ≤ 0.001) in all dapoxitine treated groups as compared to control group, this is similar to what reported by other study which showed an increase in the brain Acetylcholinesterase activity in Alzheimer disease group induced by oral administration of Aluminum chloride (AlCl3) [29].
Conclusion
Our data suggested that dapoxetine administration in rats lead to focal cerebral inflammation in low dose but cause necrosis in increased dose. However, further clinical trial studies are still required.
References
- Patel K, Hellstrom WJ. Central regulation of ejaculation and the therapeutic role of serotonergic agents in premature ejaculation. Curr Opin Investig Drugs. 2009 Jul;10(7):681-90. PubMed PMID: 19579174.
- Hatzimouratidis K, Amar E, et al. Guidelines on male sexual dysfunction: erectile dysfunction and premature ejaculation. Eur Urol. 2010 May;57(5):804-14. PubMed PMID: 20189712.
- Pryor JL, Althof SE, et al. Efficacy and tolerability of dapoxetine in treatment of premature ejaculation: an integrated analysis of two double-blind, randomised controlled trials. The lancet. 2006 Sep 9;368(9539):929-37. PubMed PMID: 16962882.
- Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999 Feb 10;281(6):537-44. PubMed PMID: 10022110.
- Metz ME, Pryor JL, Nesvacil LJ, Abuzzahab Sr F, Koznar J. Premature ejaculation: a psychophysiological review. J Sex Marital Ther. 1997 Mar 1;23(1):3-23. PubMed PMID: 9094032.
- Chia SJ. Management of premature ejaculation-a comparison of treatment outcome in patients with and without erectile dysfunction. Int J Androl. 2002 Oct;25(5):301-5. PubMed PMID: 12270028.
- Karam A, Nadia A, Abd EF, Nemat A, Siham MA. Protective effect of ginger (zingiber officinale) on Alzheimer's disease induced in rats. J Neuroinfect Dis. 2014;5(159):2.
- Guideline TT, Guideline O. OECD Guidelines for the Testing of Chemicals.
- Tsakiris S, Schulpis KH, Marinou K, Behrakis P. Protective effect of Lcysteine and glutathione on the modulated suckling rat brain Na+, K+- ATPase and Mg2+-ATPase activities induced by the in vitro galactosaemia. Pharmacol Res. 2004 May;49(5):475-9. PubMed PMID: 14998558.
- Wills ED. Lipid peroxide formation in microsomes. General considerations. Biochem J. 1969 Jun;113(2):315-24. PubMed PMID: 4390101.
- Koracevic D, Koracevic G, et al. Method for the measurement of antioxidant activity in human fluids. J Clin Pathol. 2001 May;54(5):356-61. PubMed PMID: 11328833.
- Ellman GL, Courtney KD, Andres Jr V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88-95. PubMed PMID: 13726518.
- Seriolo B, Paolino S, et al. Effects of anti‐TNF‐α treatment on lipid profile in patients with active rheumatoid arthritis. Ann N Y Acad Sci. 2006 Jun;1069:414-9. PubMed PMID: 16855168.
- Thoenen H. The changing scene of neurotrophic factors. Trends Neurosci. 1991 May;14(5):165-70. PubMed PMID: 1713715.
- He H, Venema VJ, et al. Vascular endothelial growth factor signals endothelial cell production of nitric oxide and prostacyclin through flk-1/KDR activation of c-Src. J Biol Chem. 1999 Aug 27;274(35):25130-5. PubMed PMID: 10455194.
- Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005 Aug;5(8):629.
- Esrefoglu M, Bak AA, et al. Melatonin is effective in reducing stress-induced organ damage in Wistar albino rats. Turk J Biol. 2014 Jul 1;38(4):493-501.
- Xu C, Shu WQ, et al. Protective effects of green tea polyphenols against subacute hepatotoxicity induced by microcystin-LR in mice. Environ Toxicol Pharmacol. 2007 Sep;24(2):140-8. PubMed PMID: 21783802.
- Giuliano F, Hellstrom WJ. The pharmacological treatment of premature ejaculation. BJU Int. 2008 Sep;102(6):668-75. PubMed PMID: 18485043.
- Rivera P, González R, et al. Use of paroxetine on-demand in premature ejaculation. Actas Urol Esp. 2005 Apr;29(4):387-91. PubMed PMID: 15981427.
- Stevanović ID, Jovanović MD, Jelenković A, et al. The effect of inhibition of nitric oxide synthase on aluminium-induced toxicity in the rat brain. Gen Physiol Biophys. 2009;28 Spec No:235-42. PubMed PMID: 19893106.
- Gomez-Lechon MJ, O'Connor JE, et al. Identification of apoptotic drugs: multiparametric evaluation in cultured hepatocytes. Curr Med Chem. 2008;15(20):2071-85. PubMed PMID: 18691057.
- Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000 Oct 12;407(6805):802-9. PubMed PMID: 11048732.
- Said MM, Abd Rabo MM. Neuroprotective effects of eugenol against aluminiuminduced toxicity in the rat brain. Arh Hig Rada Toksikol. 2017 Mar 1;68(1):27-37. PubMed PMID: 28365674.
- Lin AM, Fang SF, Chao PL, Yang CH. Melatonin attenuates arsenite‐induced apoptosis in rat brain: involvement of mitochondrial and endoplasmic reticulum pathways and aggregation of α‐synuclein. J Pineal Res. 2007 Sep;43(2):163-71. PubMed PMID: 17645694.
- Ghoneim FM, Khalaf HA, et al. Protective effect of chronic caffeine intake on gene expression of brain derived neurotrophic factor signaling and the immunoreactivity of glial fibrillary acidic protein and Ki-67 in Alzheimer’s disease. Int J Clin Exp Pathol. 2015 Jul 1;8(7):7710-28. PubMed PMID: 26339337.
- Kivipelto M, Helkala EL, Laakso MP, et al. Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. BMJ. 2001 Jun 16;322(7300):1447-51. PubMed PMID: 11408299.
- Kaizer RR, Correa MC, Gris LR, et al. Effect of long-term exposure to aluminum on the acetylcholinesterase activity in the central nervous system and erythrocytes. Neurochem Res. 2008 Nov;33(11):2294-301. PubMed PMID: 18470612.
- Hanaa HA, Asmaa MZ, Bosy A. Zingiber officinale and Alzheimer’s Disease: Evidences and Mechanisms. Int. J Pharm Sci Rev Res. 2014 Aug;27(2):142- 152.