A Keratoacanthoma With Squamous Cell Carcinoma Under Immunosuppressive Therapy After Renal Transplantation
Keiji Sugiura1,2, Mariko Sugiura1,2, Kazuharu Uchida3, KunioMorozumi4
1 Department of Environmental Dermatology & Allergology, Daiichi Clinic Nittochi Nagoya Bld., 2F, 1-1 Sakae 2, Nakaku, Nagoya, 460-0008, Japan.
2 Department of Dermatology and Allergy, Masuko Memorial Hospital, 35-28 Takehashicho, Nakamuraku, Nagoya, 453-8566, Japan.
3 Department of Nephrology, Masuko Memorial Hospital, 35-28 Takehashicho, Nakamuraku, Nagoya, 453-8566, Japan.
4 Department of Renal Transplantation, Masuko Memorial Hospital, 35-28 Takehashicho, Nakamuraku, Nagoya, 453-8566, Japan.
*Corresponding Author
Keiji Sugiura M.D., Ph.D,
Department of Environmental Dermatology & Allergology, Daiichi Clinic, Nittochi Nagoya Bld., 2F, 1-1 Sakae 2, Nakaku, Nagoya, 468-0008, Japan.
Tel: +81527602783
Fax: +81-52-204-0835
E-mail: ksugiura@daiichiclinic.jp
Received: January 28, 2022; Accepted: February 22, 2022; Published: March 26, 2022
Citation: Keiji Sugiura, Mariko Sugiura, Kazuharu Uchida, KunioMorozumi. A Keratoacanthoma With Squamous Cell Carcinoma Under Immunosuppressive Therapy After Renal
Transplantation. Int J Clin Dermatol Res. 2022;10(1):275-277. doi: dx.doi.org/10.19070/2332-2977-2200061
Copyright: Keiji Sugiura©2022. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
A 69-year-old Japanese male developed a solitary keratoacanthoma with squamous cell carcinoma (SCC) on his right arm. He had undergonekidney transplantation from his father due to chronic renal failure approx. 45 years earlier and had since been under immunosuppressive treatment (azathioprine and prednisolone). An immunohistopathological examination revealed Ki- 67- and p53-positive cells in the tumor bed of the keratoacanthoma, and our final diagnosis was a solitary keratoacanthoma with SCC. In this case, several factors apparently caused the solitary keratoacanthoma with SCC: the long duration of immunosuppression, the use of azathioprine after renal transplantation, and UV exposure. Immunohistopathological studying is important for the diagnosis of SCC or keratoacanthoma. Keratoacanthomashave malignant potential, and this patient's SCC could have been caused by the keratoacanthoma.
2.Introduction
3.Methods
4.Results
5.Discussion
6.Acknowledgements
7.References
Keywords
Keratoacanthoma; Squamous Cell Carcinoma(SCC); Renal Transplantation; Immunosuppressive Condition; Azathioprine.
Introduction
Three kinds of keratinocyte tumor, squamous cell carcinoma
(SCC), basal cell carcinoma (BCC), and actinic keratosis (AK),
account for 99% of non-melanoma skin cancers, and 20% of
cutaneous malignancies are SCC [1]. The incidence of SCC is
increasing worldwide. A major riskfactor in the development of
SCCis ultra-violet (UV) light exposure; in addition, immunosuppressive
therapy after organ transplantation is closely related to
the carcinogenesis [2]. A 20.5% incidence of non-melanotic skin
cancer in patients who underwent organ transplantation in the
years from 1978 to 2005 was reported [3]. In the USA, >7% of
35,000 renal transplant recipients with 3 years of immunosuppression
treatment developed non-melanotic skin cancer, and this
incidence is 20-fold higher compared to that in immunocompetent
individuals [4].
Kidney transplantrecipients were reported to have a 3- to 12-fold
increased risk of solid organ cancer compared to general populations
[5, 6], and another study indicated that the incidence of
non-melanotic skin cancer in transplant recipients was elevated
by 52.7-fold [7]. Remarkably, the high risk incidence of non-melanotic
skin cancers such as SCC and BCC in kidney transplantrecipients
is60- to 250-fold increasing [8, 9].
Keratoacanthoma, first described by Jonathon Hutchison in 1889
[10], is a benign skin tumor raised from hair follicles [11, 12], and
it often arises on skin exposed to UV light. The characteristics
of keratoacanthoma are single-cause, dome-shaped, and rapid
growth and development in elderly people [11-13], and a keratoacanthoma
possesses malignant potential.The histological findings
of keratoacanthomaare similar to those of well-differentiated
SCC. The removal of a keratoacanthoma is recommended for diagnosis
and treatment.
Case Presentation
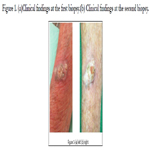
A 69-year-old Japanese male noticed skin swelling on his right
arm beginning in March 2021 (Figure 1-a). The first diagnosis
based on a skin biopsy was irritated seborrheic keratosis, but
the swelling continued and became greaterover the following
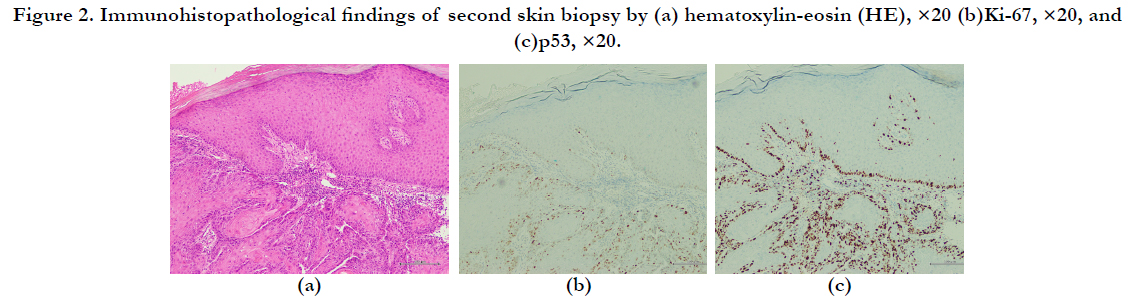
month(Figure 1-b). The results of a second skin biopsy indicated
akeratoacanthoma with Ki-67-positive and p53-positive cells in
the tumor bed (Figure 2a–c).
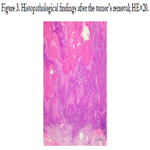
We removed the tumor under local anesthesia with a 1-cm safe
margin, with suspicion of SCC. The final diagnosis based on the
histopathological findings was a solitary keratoacanthoma with
SCC (Figure 3). Forty-five years earlier, due to the patient's chronic
renal failure,hehad received a kidney from his father, and he
had been under immunosuppressive treatment with azathioprine
and prednisolone since then. His several warts had increased in
number after the start of the immunosuppressive treatment, and
liquid nitrogenhad been used to treatmany warts.
Figure 2. Immunohistopathological findings of second skin biopsy by (a) hematoxylin-eosin (HE), ×20 (b)Ki-67, ×20, and (c)p53, ×20.
Discussion
The incidence of non-melanotic skin cancer among tissue-transplant
recipients is higher that of general populations, and SCC is
most common skin cancer.SCC is related to UV exposure. More
than 90% of skin tumors among organ transplant recipients developed
in UV-exposed areas of skin [14]. This feature may be responsible
for the approximately fourfold higher incidence of SCC
compared to that of BCC among transplant recipients; moreover,
the causal factors of invasive SCC are also related to the dose and
duration of immunosuppressive therapy and UV exposure [15].
UV is thus a greater causal factor of skin cancer in immunosuppressed
patients compared to general populations [16]. It is
important that sunblock be used for protecting the skin's DNA
against UV radiation, and in Australia, the use of sunblock is a
standard recommendation for patients undergoing immunosuppressive
therapy [16, 17]. Our patient hadnever used sunblock, as
is true of many elderly males in Japan. The use of sunblock in that
population has been considered to be similar to the use of makeup
by females (and thus"unmanly" behavior).
Skin cancer in patients with immunosuppressive therapy is associated
with the dose, kinds of immunosuppressant medicine used,
and the duration of immunosuppression. A particular immunosuppressive
agent, azathioprine, is related to the development of
multiple SCCs and warts, and it is better to use mammalian target
of rapamycin inhibitors (mTORi)instead of azathioprine for
immunosuppressive protocols in light of the risk of skin cancer
[18]. A common immunosuppressive agent that is catabolized to
6-mercaptopurine,azathioprine affects the synthesis of purines
and can block DNA repair [19, 20], and this agent produces reactive
oxygen species under UV exposure [19, 21]. Azathioprine was
reported to increase the risk of SCC development by fivefold [19,
21]. The use of mTORi has shown significantly lower incidences of both AKand SCC, because these agents have an inhibitory effect
on tumor angiogenesis and an antiproliferative effect on tumor
cells.
A retrospective study showed that risk factors of cancer development
were age and male gender [23]. The cancer risk in kidney
transplant recipients is threefold-increased compared to that of
patients with dialysis [24]. Our patient hadreceived treatment for
many warts, and these warts might be related to him being in
an immunosuppressive condition. His risk factors for skin cancer
were being male, elderly, with a UV-exposed area, not using sunblock,
a long duration of immunosuppression, and immunosuppressive
therapy with azathioprine.
The etiology of keratoacanthoma is uncertain, but keratoacanthoma
can be associated with immunosuppressive conditions
such as UV exposure, drug treatments, and the use of X-rays [25].
Keratoacanthomahas been classified in the group of benign epithelial
tumors with malignant potential, and it sometimes shows
SCC differentiation within the lesion. The histological features
of keratoacanthoma and SCC are similar, and it is often difficult
to differentiate these tumors. Because keratoacanthoma and SCC
are often admixed, there are a few proposed terms such as keratoacanthoma-
like SCC [26] and keratoacanthoma with an SCC
component (KASCC) [27, 28]. The latterkeratoacanthoma has
been classified as SCC keratoacanthoma type [29]. Immunohistochemistry
using p53 and Ki-67 may be helpful for distinguishing
between subungual keratoacanthoma and subungual SCC [30].
Conclusion
We reported a case of SCC in akeratoacanthoma related mainly to
the patient's immunosuppressive condition.This case emphasizes
that performing a skin biopsy or tumor removal and the immunohistopathological
findings are important for the diagnosis and
treatment of a keratoacanthomaand/or SCC in patients who have
undergone organtransplantation.
References
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard.[online] covid19. who. int.
- Azfar NA, Zaman T, Rashid T, Jahangir M. Cutaneous manifestations in patients of hepatitis C. Journal of Pakistan Association of Dermatologists. 2008;18(3):138-43.
- Gulati A, Pomeranz C, Qamar Z, Thomas S, Frisch D, George G, et al. A Comprehensive Review of Manifestations of Novel Coronaviruses in the Context of Deadly COVID-19 Global Pandemic. Am J Med Sci. 2020 Jul;360(1):5-34. Pubmed PMID: 32620220.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020 May;34(5):e212-e213. Pubmed PMID: 32215952.
- Sachdeva M, Gianotti R, Shah M, Bradanini L, Tosi D, Veraldi S, et al. Cutaneous manifestations of COVID-19: Report of three cases and a review of literature. J Dermatol Sci. 2020 May;98(2):75-81. Pubmed PMID: 32381430.
- Gianotti R, Zerbi P, Dodiuk-Gad RP. Clinical and histopathological study of skin dermatoses in patients affected by COVID-19 infection in the Northern part of Italy. J Dermatol Sci. 2020 May;98(2):141-143. Pubmed PMID: 32381428.
- Dalal A, Jakhar D, Agarwal V, Beniwal R. Dermatological findings in SARSCoV- 2 positive patients: An observational study from North India. Dermatol Ther. 2020 Nov;33(6):e13849. Pubmed PMID: 32543757.
- Potekaev NN, Zhukova OV, Protsenko DN, Demina OM, Khlystova EA, Bogin V. Clinical characteristics of dermatologic manifestations of COVID- 19 infection: case series of 15 patients, review of literature, and proposed etiological classification. Int J Dermatol. 2020 Aug;59(8):1000-1009. Pubmed PMID: 32621287.
- Tammaro A, Adebanjo GAR, Parisella FR, Pezzuto A, Rello J. Cutaneous manifestations in COVID-19: the experiences of Barcelona and Rome. J Eur Acad Dermatol Venereol. 2020 Jul;34(7):e306-e307. Pubmed PMID: 32330340.
- Bouaziz JD, Duong TA, Jachiet M, Velter C, Lestang P, Cassius C, et al. Vascular skin symptoms in COVID-19: a French observational study. JEur Acad Dermatol Venereol. 2020 Sep;34(9):e451-e452. Pubmed PMID: 32339344.
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, Aguirre T. Chilblain-like lesions on feet and hands during the COVID-19 Pandemic. Int J Dermatol. 2020 Jun;59(6):739-743. Pubmed PMID: 32329897.
- Alramthan A, Aldaraji W. Two cases of COVID-19 presenting with a clinical picture resembling chilblains: first report from the Middle East. Clin Exp Dermatol. 2020 Aug;45(6):746-748. Pubmed PMID: 32302422.
- Marzano AV, Genovese G, Fabbrocini G, Pigatto P, Monfrecola G, Piraccini BM, et al. Varicella-like exanthem as a specific COVID-19-associated skin manifestation: Multicenter case series of 22 patients. J Am Acad Dermatol. 2020 Jul;83(1):280-285. Pubmed PMID: 32305439.
- Diotallevi F, Campanati A, Bianchelli T, Bobyr I, Luchetti MM, Marconi B, et al. Skin involvement in SARS-CoV-2 infection: Case series. J Med Virol. 2020 Nov;92(11):2332-2334. Pubmed PMID: 32410241.
- De Giorgi V, Recalcati S, Jia Z, Chong W, Ding R, Deng Y, et al. Cutaneous manifestations related to coronavirus disease 2019 (COVID-19): A prospective study from China and Italy. J Am Acad Dermatol. 2020 Aug;83(2):674- 675. Pubmed PMID: 32442696.
- Freeman EE, McMahon DE, Lipoff JB, Rosenbach M, Kovarik C, Takeshita J, et al. Pernio-like skin lesions associated with COVID-19: A case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020 Aug;83(2):486- 492. Pubmed PMID: 32479979.
- Freeman EE, McMahon DE, Lipoff JB, Rosenbach M, Kovarik C, Desai SR, et al. The spectrum of COVID-19-associated dermatologic manifestations: An international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020 Oct;83(4):1118-1129. Pubmed PMID: 32622888.
- Suchonwanit P, Leerunyakul K, Kositkuljorn C. Cutaneous manifestations in COVID-19: Lessons learned from current evidence. J Am Acad Dermatol. 2020 Jul;83(1):e57-e60. Pubmed PMID: 32339706.
- Wollina U, Karadag AS, Rowland-Payne C, Chiriac A, Lotti T. Cutaneous signs in COVID-19 patients: A review. Dermatol Ther. 2020 Sep;33(5):e13549. Pubmed PMID: 32390279.
- Galván Casas C, Català A, Carretero Hernández G, Rodríguez-Jiménez P, Fernández-Nieto D, Rodríguez-Villa Lario A, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020 Jul;183(1):71-77. Pubmed PMID: 32348545.
- Roca-Ginés J, Torres-Navarro I, Sánchez-Arráez J, Abril-Pérez C, Sabalza- Baztán O, Pardo-Granell S, et al. Assessment of Acute Acral Lesions in a Case Series of Children and Adolescents During the COVID-19 Pandemic. JAMA Dermatol. 2020 Sep 1;156(9):992-997. Pubmed PMID: 32584397.
- de Masson A, Bouaziz JD, Sulimovic L, Cassius C, Jachiet M, Ionescu MA, et al. Chilblains is a common cutaneous finding during the COVID-19 pandemic: A retrospective nationwide study from France. J Am Acad Dermatol. 2020 Aug;83(2):667-670. Pubmed PMID: 32380219.
- Piccolo V, Neri I, Filippeschi C, Oranges T, Argenziano G, Battarra VC, et al A. Chilblain-like lesions during COVID-19 epidemic: a preliminary study on 63 patients. J Eur Acad Dermatol Venereol. 2020 Jul;34(7):e291-e293. Pubmed PMID: 32330334.
- Gupta S, Gupta N, Gupta N. Classification and pathophysiology of cutaneus manifestations of COVID-19. Int J Res Dermatol. 2020 Jul;6(4):1-5.
- Zhao Q, Fang X, Pang Z, Zhang B, Liu H, Zhang F. COVID-19 and cutaneous manifestations: a systematic review. J Eur Acad Dermatol Venereol. 2020 Nov;34(11):2505-2510. Pubmed PMID: 32594572.
- Mahieu R, Tillard L, Le Guillou-Guillemette H, Vinatier E, Jeannin P, Croué A, et al. No antibody response in acral cutaneous manifestations associated with COVID-19? J Eur Acad Dermatol Venereol. 2020 Oct;34(10):e546- e548. Pubmed PMID: 32488946.
- Chen J, Wang X, Zhang S, Liu B, Wu X, Wang Y, et al. Findings of acute pulmonary embolism in COVID-19 patients. Available at SSRN 3548771. 2020 Mar 1.
- Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020 May 2;395(10234):1417-1418. Pubmed PMID: 32325026.