Safety, Tolerability and Cost Effectiveness of Commercially Available vs. Freshly Prepared Salicylic Scid Peel in Treatment of Acne: An Indian Perspective
Pandya I1, Pandya P2*, Shah R3, Padhiar B4
1 Senior Resident, Department of Dermatology, AMC MET Medical College and LG hospital, Ahmedabad, Gujarat, India.
2 Assistant Professor, Department of Dermatology, GMERS Medical College, Gandhinagar, Gujarat, India.
3 Assistant Professor, Department of Pharmacology, GMERS Medical College, Gandhinagar,Gujarat, India.
4 Professor and Head, Department of Dermatology, GMERS Medical College, Gandhinagar, Gujarat, India.
*Corresponding Author
Dr. Purna Pandya,
Assistant Professor, Department of Dermatology, GMERS Medical College, Gandhinagar, Gujarat-382012, India.
Tel: +919428405724
E-mail: dr.purnapandya2017@gmail.com
Received: May 02, 2019; Accepted : February 10, 2020; Published: February 13, 2020
Citation: Pandya I, Pandya P, Shah R, Padhiar B. Safety, Tolerability and Cost Effectiveness of Commercially Available vs. Freshly Prepared Salicylic Scid Peel in Treatment of Acne: An Indian Perspective. Int J Clin Dermatol Res. 2020;8(2):253-258. doi: dx.doi.org/10.19070/2332-2977-2000055
Copyright: Pandya P© 2020. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Aim: To evaluate safety profile, tolerability and cost-effectiveness of superficial chemical peeling agent salicylic acid (SA) either commercially available or freshly prepared in treatment of acne.
Methodology: Total 123 patients with acne who gave consent for salicylic acid (SA) peeling were randomized into two groups A (commercially available SA) and B (freshly prepared SA). All adverse drug reactions (ADRs) either spontaneously reported or identified by physician were recorded and analyzed for causality, severity and its preventability. Cost effectiveness was assessed by comparing cost for one session in each group while tolerability was assessed using subjective scale and analyzed.
Results: Out of total 123 patients, 32 (26.01%) developed ADR. Total 47 adverse events (38.21%) were recorded in these 123 patients. Most common ADR was burning sensation (16,34.04%) followed by erythema (9,19.15%) and dry skin (6,12.77%). Adverse events were reported significantly higher (p<0.05) in freshly prepared SA peel group (29, 62.96%) as compare to commercially available SA peel group (18, 37.03%). Majority of ADRs (28, 59.57%) were of “possible” category of WHOUMC criteria. out of 47 adverse drug reactions, 41 (87.23%) were mild and 6 (12.77%) were moderate. 34.05% of ADRs were definitely preventable. 57.14% patients in freshly prepared group and 68.33% in commercially available group rated the treatment as acceptable without any discomfort at all. Cost of freshly prepared SA peel was significantly lower (33.33 INR vs. 0.7INR; p<0.05).
Conclusion: Freshly prepared salicylic acid peel can serve as cheaper alternative in developing countries like India but close monitoring for adverse drug reactions required.
2.Introduction
3.Methodology
4.Results
5.Discussion
6.Conclusion
7.References
Keywords
Acne Vulgaris; Salicylic Acid Peel; Safety and Tolerability; Adverse Effects of Salicylic Acid; Causality Assessment of ADRs; Severity and Preventability of ADRs; Cost Effectiveness of SA Peel.
Introduction
Chemical peels are methods to cause a chemical ablation of defined skin layers to induce an even and tight skin as a result of the regeneration process [1, 2]. The actual peeling procedure involves the application of a caustic chemical substance to destroy layers of the skin such that they are then spontaneously eliminated over several days and repair mechanisms of the epidermis and dermis are induced. The mechanical action of peeling, even when limited to the epidermis, is able to stimulate regeneration via pathways in the dermis that are not well understood. The depth of destruction depends on the substance used and its concentration. The use of chemical peels has been reported since antiquity, but a standardized and scientifically based technique has emerged only over the past decades [3]. The chemical face peel is among the 5 most common cosmetic procedures performed for individuals in the 35- to 50-year age group in USA [4].
Various chemical peeling agents and strategies are used to repair the effects of photodamage and acne such as acne wrinkles, dyschromia, and actinic keratosis [5]. Salicylic acid peel has been established as an effective treatment modality of treatment of acne and it is used widely be different dermatologists [6]. Although generally safe and predictable, this peel has a significant period of 5 to 7 days during which visible peeling of the skin occurs with erythema and other side effects. This morbidity is a deterrent to many prospective patients because it can interfere with work and social interactions [7]. Majority of patients perceive acne itself as a cause of social, psychological and emotional distress and adverse effects caused by drugs can enhance this disease burden to the patient and adversely affects quality of life [8]. Adverse effect of salicylic acid ranges from dryness, mild erthyma and itching to severe S J syndrome and salicylism [9].
Moreover, cost of the cosmetic treatment is also very high in developing countries like India. Various formulations are available for salicylic acid peels in market with huge prize differences. Cost of the treatment is also one of the selection criteria for any treatment for the given patient especially when cost is bared by the patient himself which enhances the out of the pocket health budget [10]. In India, health care costs are more impoverishing than ever before and almost all hospitalizations, even in public hospitals leads to Catastrophic Healthcare Expenditure (CHE) and over 63 million people are facing poverty every year due to health care costs alone [10]. Healthcare access in India is affected with 70:70 paradox; 70 per cent of healthcare expenses are incurred by people from their pockets, of which 70 per cent is spent on medicines alone, leading to impoverishment and indebtedness [11]. For decades, economic planners of India regarded health expenditure as financially nonproductive social spending and public financing levels were low and total spending on healthcare was about 4.1% of GDP [12]. At the prescriber level, if we can give the patient a more cost-effective alternative, it can help in reducing the health expenditure of the patients.
Although salicylic acid appears to be the miracle cosmetic ingredient of the 1990s, there are genuine safety concerns associated with its extended use [13]. According to principles of rational therapy, any drug is selected on the basis of efficacy, safety, suitability and cost of the treatment for the patient. Therefore, this study was aimed to evaluate the tolerance, safety profile and cost effectiveness of superficial chemical peeling agent salicylic acid either commercially available or freshly prepared in treatment of acne vulgaris.
This was a randomized open label study carried out in dermatology department of a tertiary care teaching hospital in western India over the period of one year May 2016 to April 2017.
Inclusion criteria: Patients attending to dermatology outpatient department were screened and those diagnosed with acne vulgaris (n = 123) were included. Diagnosis of acne was mainly based on clinical examination by the qualified dermatologist. Patients of age 12 years and more and both gender with mild to moderate acne with facial lesions only were included in the study. Only newly diagnosed and those who did not take any treatment for last 15 days were included for the study.
Exclusion criteria: Pregnant and lactating mothers, patients with known history of hypersensitivity reaction to salicylates or aspirin, patients with history of herpes simplex, patients with drug induced acne and patients with history of keloid formation were excluded from the study. Patients not willing to participate in the study and not willing to give written informed consent were also excluded.
The study protocol was approved by Human Research Ethics Committee of the institute prior to commencement of study. Permission from the hospital superintendent and head of the dermatology department was also obtained before conducting the study. All the patients participating in the study were explained clearly about the purpose and nature of the study in the language they understood. Written informed consent was obtained before including them in the study. In case of minor, written informed consent from the parent/legal guardian was obtained in addition to assent from the adolescent.
All outdoor patients, new as well as old, meeting the inclusion criteria attending to dermatology department were interviewed for the first time on the day of enrollment and their case sheets were reviewed to gather necessary information -as on that day- to fill up case record forms. Detailed history and examination was carried out by treating dermatologist. Details of the symptoms, duration, site, and type of lesions, any keloidal tendencies in the patient or in the family, presence of viral infection, local tumors and evolving dermatoses were noted. Counting of lesions was done in good nature light with the help of a hand lens. Acne grading was done using lesion count: grade 1 (total number of lesions < 10/100 cm2), grade 2 (10 – 20/100 cm2), grade 3 (20 - 30/100 cm2) and grade 4 (>30/100 cm2) [14]. After the examination and checking the suitability of using SA superficial chemical peel patients were randomly assigned to two groups.
All patients were randomly assigned into group A and B using random number table.
Group A: Patients with acne vulgaris treated with commercially available 30% salicylic acid peeling.
Group B: Patients with acne vulgaris treated with freshly prepared 30% salicylic acid peeling (Which was prepared by adding 3gms of salicylic acid powder into 10 ml of denatured spirit).
All the patients were applied with superficial peeling either commercially available or freshly prepared as standard method of application [15].
Before peeling, the face was washed with soap and water to remove any makeup, dust and debris and was scrubbed with spirit gauze. Peeling was done with cotton wool applicator dipped in required solution with smooth strokes to the affected areas with patient lying supine at an angle of 45° with closed eyes and plugged ears. Application was completed within 30 seconds and termination was done by cleaning the face with cold water but avoiding rubbing.
Patients were made to sit in front of a fan, if required, immediately after peeling. The contact time ranged from 5-7 minutes for each session. Avoidance of the use of soaps and sun exposure at least for one following day was strongly advised. Patients were prescribed daily use of physical block sunscreens during daytime with SPF >30. They were cautioned not to apply any cream or face wash containing salicylic acid, or retinoids. All the patients were followed up every 15 days till 3 months and tolerance to therapy and development of any side effects were recorded at each follow up visit.
All the ADRs either spontaneously reported and those identified by physician were reported and analyzed. The primary researcher was trained in identification and reporting and analysis of the adverse drug events. In case of conflict in analysis of the reports, the opinion of the treating physician was also obtained. All ADRs were analyzed for its causality assessment using WHO-UMC criteria [16]. Severity of ADEs were analyzed using scale of Hartwig and Siegle [17] and preventability of ADEs was analyzed using criteria of Schumock and Thornton modified by Lau et al., 2003 [18].
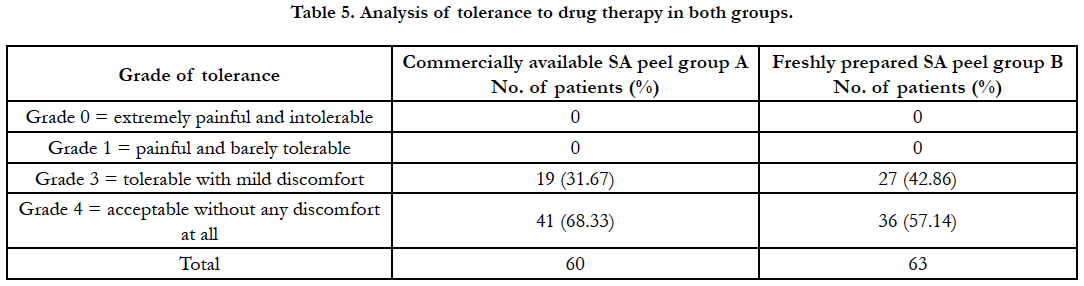
Tolerance of the treatment was assessed subjectively from Grade 0 to 4: (Grade 0 = extremely painful and intolerable, Grade 1 = painful and barely tolerable, Grade 2 = tolerable with significant discomfort, Grade 3 = tolerable with mild discomfort, Grade 4 = acceptable without any discomfort at all).
Calculation of cost of freshly prepared SA peel and commercially available SA peel per sitting was done.
All data were analyzed with the help of Microsoft excel 2010. Data were represented as actual frequency, mean, percentage, standard deviation as appropriate. Chi-square test was used for analysis and association of qualitative data. Unpaired t test was used for comparison between the groups, and paired t test was used for within group comparisons. P values < 0.05 were considered significant.
Results
Total 123 patients enrolled for the study of which 60 were assigned to group A (commercially available SA peel group) and 63 were assigned to group B (freshly prepared SA peel group). The mean age of the patients was 23.47 ± 5.62 and 22.99 ± 6.35 years respectively in commercially and freshly prepared SA groups. Majority (55.28%) patients belong to 21-30 years of age with female preponderance. Highest patients (52.03%) had Fitzpatrick skin type IV, followed by type III in 37.40% patients and type V in 6.5% of patients. Most common presentation of acne is with papules in all (100%) patients, followed by comedones in 93.4%, pustules in 22% and nodules or cyst in 10% of patients. Onset of acne was in between the ages of 12-15 years in more than half (56.7%) of the patients. There was no significant difference among the baseline characteristics of acne patients in both the groups (p>0.05).
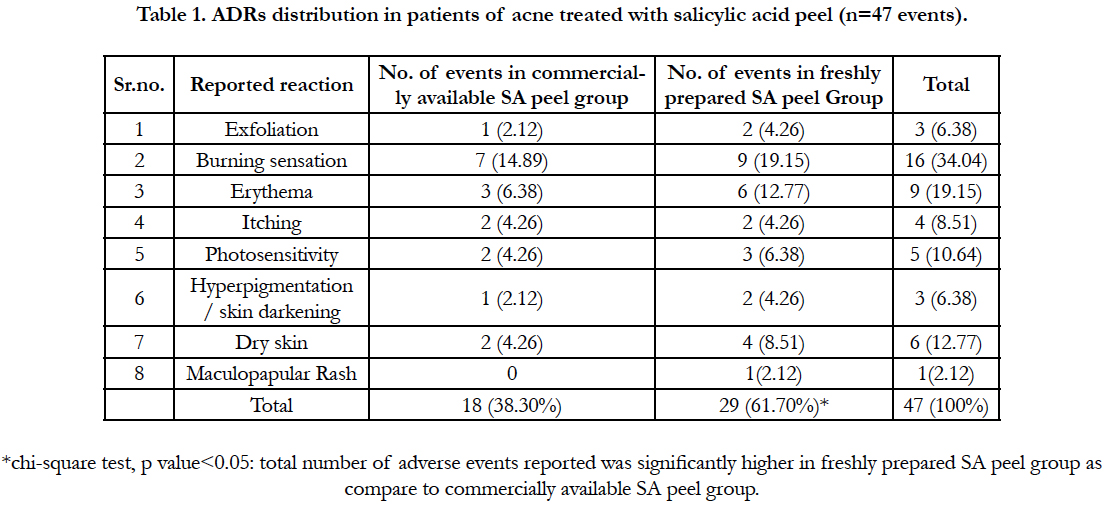
Out of total 123 patients enrolled for the study, 32 (26.01%) developed some or other ADR. Total 47 adverse events (38.21%) were recorded in these 123 patients. Most common ADR was burning sensation followed by erythema and dry skin. Comparison of ADRs in both the groups has been shown in Table 1. Adverse events were reported significantly higher (p<0.05) in freshly prepared SA peel group (29, 62.96%) as compare to commercially available SA peel group (18, 37.03%).
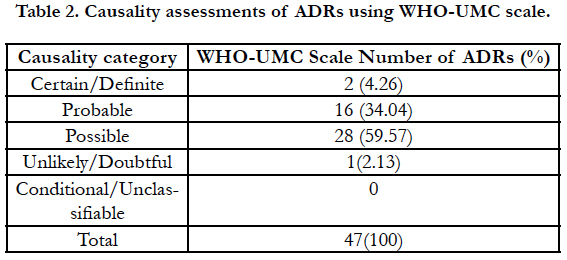
It was carried out using WHO-UMC criteria as shown in Table 2. Majority of ADRs (28, 59.57%) were of “possible” category followed by “probable” category (16, 34.04%), “certain” category (2, 4.26%) and only one case (2.13%) fell in category of unlikely/ doubtful.
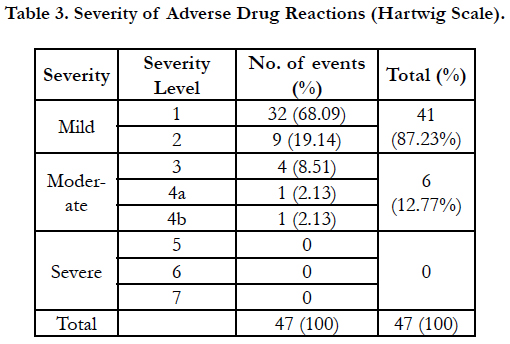
On evaluating severity assessment by Hartwig scale, out of 47 adverse drug reactions, 41 (87.23%) were mild and 6 (12.77%) were moderate. None of the patient developed serious ADR (Table 3).
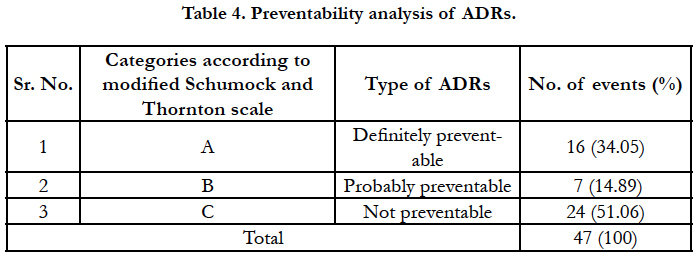
The preventability assessment of ADRs was carried out using modified Schumock and Thornton scale. As shown in Table 4, majority of ADRs were not preventable (24, 51.06%) followed by definitely preventable 16 (34.05%) and 7 (14.89%) probably preventable.
Both the peels were well tolerated by almost all the patients (Table 5). 57.14% patients in freshly prepared group and 68.33% in commercially available group rated the treatment as acceptable without any discomfort at all.
Commercially available peel was costing 1000 INR for 30ml. considering usage of 1 ml per session, cost of peeling was counted as 33.33 INR. For freshly prepared peel, cost of salicylic acid powder cost was 804rs for 500 gram and denatured spirit 1000 ml costs INR 70. One ml of freshly prepared peel will cost 0.7 INR. There was significant difference in the cost of treatment among both groups (p<0.05).
Discussion
Salicylic acid has been used widely as a superficial chemical peeling agent in the treatment of acne vulgaris since past one decade. Chemical peeling is a skin-wounding procedure that can have some potentially undesirable side-effects and tolerance to this procedure may vary from person to person. This study was aimed at comparative evaluation of adverse drug reactions, tolerability of therapy and cost-effectiveness of therapy in patients of acne prescribed with either commercially available or freshly prepared salicylic acid peel.
This superficial peeling procedure is usually well tolerated in skin photo types III to VI [19, 20]. Majority of our patients belonged to skin type III and above and tolerated the procedure very well. None of the patients found it unacceptable or painful. Very few experienced it as an uncomfortable procedure but the vast majority graded it as a well-tolerated and acceptable experience. These results were comparable to earlier studies with superficial peeling agents [19-21]. Complications related to salicylic acid chemical peeling that have been reported in the literature include infections (herpes, bacterial), milia, premature peeling, persistent erythema, allergic reactions, post-inflammatory hyper or hypopigmentation, lines of demarcation, lines created by tears dripping on to the face and neck, and scarring (hypertrophic, atrophic or keloidal) [22]. None of the patient developed any complications in both the group in this study.
Out of total 123 patients enrolled for the study, 32 (26.01%) developed some or other ADR. Total number of events reported was 47 as few patients had developed more than one adverse drug reactions. The findings are falling in the broad range of ADRs occurring with topical drugs according to different literature [23, 24]. Patients in the freshly prepared salicylic acid group developed significant more ADRs (p<0.05) than patients in commercially available SA peel group. Most of the adverse reactions (exfoliation, burning, irritation, stinging, erythema, dryness etc) that occurred in our study were already expected of such treatment. These were easily manageable and did not affect the compliance of the patients. None of the patients developed post-inflammatory hyper or hypopigmentation of the affected or surrounding unaffected skin. This was quite encouraging because post-inflammatory dyspigmentation (hyper or hypopigmentation) was initially considered as a risk factor in the dark-skinned population [22]. None of the patient reported with activation of herpes virus infection of Rey’s syndrome or allergic reaction with salicylic acid peel.Few patients reported that they could not follow the advice given by doctors like avoiding sunlight, application of sunscreen and calamine lotions at the day time for ADRs which may be possible reason for development of some of the ADRs. Further, we also experienced that due to insufficient data on deachallenge or rechallenge, it became very difficult to assign the category of ‘certain’ of ‘definite’ to any ADR which is responsible of only 2 ADRs reported as ‘certain’.
Severity of ADRs is also one of the important parameters for evaluation. For this purpose the most commonly and best used scale is Hartwig’s scale. We observed that 87.23% of adverse events were of mild severity suggesting no discontinuation of the offending drug required or withholding the causative drug without any other intervention was sufficient to treat the ADR. Only 6 (12.77%) adverse events were at level 3 or 4 meaning that they required admission to the hospital for management of ADR, or prolongation of hospital stay by at least one day in case of already hospitalized patients and required either an antidote or interventional treatment. None of the patient developed severe ADRs. The carry home massage would be that salicylic acid can cause frequent mild reactions which need monitoring and prompt treatment of them.
Preventability analysis of ADRs in our study showed that nearly half of the ADRs (23, 48.94% %) were ‘definitely’ or ‘probably’ preventable, which is consistent with the broad range of figures (30-70%) suggested in literature [25, 26]. It is not possible to prevent all the ADRs but some ADRs (type A - Augmented – dose related) can be predicted considering the pharmacological actions of a drug. Considering the burden of ADR, related morbidity and cost involved in its treatment, it is desirable to take measures for prevention of ADRs. Though all the preventive measures are difficult to execute, but simple measures like previous history of allergy, avoiding sunlight after drug application, application of sunscreen lotions and moisturizers can easily be practiced. Enhance education of patient about prescribing can also help in reducing medication errors and ADRs [27].
It was found in the study that cost of treatment was significantly lower in freshly prepared SA peel group (p<0.05) which can be a cost effective alternative for SA peel. Although the study has highlighted the tolerance, safety and cost effectiveness of freshly prepared SA peel as compared to commercially available peel, few limitation of the study was single centre for data collection and shorter duration of study follow up. Also because of small number of ADRs reported during study, it was not possible to analyze causality, severity and preventability group wise and compare them. Larger studies focusing on this aspect are warranted in future for better understanding of wide spread use of SA as a superficial peeling agent.
Conclusion
Acne is a common dermatological condition and widely treated with superficial peeling agents like salicylic acid. Rate of occurrence of ADRs was reported as 26.01% with salicylic acid peel in this study. Significantly more number of ADRs was reported in the freshly prepared SA peel group as compared to commercially available SA peel group. Though all the ADRs were mild and moderate in severity, nearly half of them were preventable. Both types of preparations of SA peel were well tolerated by most of the patients but cost of therapy was significantly less with freshly prepared SA peel. Selection of the type of SA peel preparation should consider safety, tolerability and cost of the therapy also. It is very prudent to timely identify and diagnosis any ADRs and take appropriate steps for treatment and prevention of them.
References
- Brody HJ. Chemical peeling. In: Patterson AN, editor. Year book St. Louis: Mosby; 1992. p. 7-22.
- Monheit GD, Chastain MA. Chemical peels. Facial Plast Surg Clin North Am. 2001 May;9(2):239-55.Pubmed PMID: 11457690.
- Mendelsohn JE. Update on chemical peels. Otolaryngol Clin North Am. 2002 Feb;35(1):55-72. Pubmed PMID: 11781207.
- Fulton JE, Porumb S. Chemical peels: their place within the range of resurfacing techniques. Am J Clin Dermatol 2004; 5(3):179-87. Pubmed PMID: 15186197.
- Griffiths CE, Finkel LJ, Ditre CM, Hamilton TA, Ellis CN, Voorhees JJ. Topical tretinoin (retinoic acid) improves melasma. A vehicle-controlled, clinical trial. Br J Dermatol. 1993 Oct;129(4):415-21. Pubmed PMID: 8217756.
- Kim RH, Armstrong AW. Current state of acne treatment: Highlighting lasers, photodynamic therapy, and chemical peels. Dermatol Online J. 2011 Mar 15;17(3):2. Pubmed PMID: 21426868.
- Niemeier V, Kupfer J, Demmelbauer-Ebner M, Stangier U, Effendy I, Gieler U. Coping with acne vulgaris. Evaluation of the chronic skin disorder questionnaire in patients with acne. Dermatology. 1998;196(1):108-15. Pubmed PMID: 9557243.
- Al Robaee AA. Assessment of general health and quality of life in patients with acne using a validated generic questionnaire. Acta Dermatovenerol Alp Pannonica Adriat. 2009 Dec;18(4):157-64. Pubmed PMID: 20043053.
- Bari AU, Iqbal Z, Rahman SB. Tolerance and safety of superficial chemical peeling with salicylic acid in various facial dermatoses. Indian J Dermatol Venereol Leprol 2005 Mar-Apr;71(2):87-90. Pubmed PMID: 16394379.
- Golechha M. Healthcare agenda for the Indian government. Indian J Med Res 2015 Feb ;141(2): 151-3. Pubmed PMID: 25900948.
- Jayakrishnan T, Jeeja MC, Kuniyil V, Paramasivam S. Increasing out-ofpocket health care expenditure in India-due to supply or demand. Pharmacoeconomics. 2016;1(105):1-6.
- Dror DM, Putten-Rademaker V, Koren R. Cost of illness: evidence from a study in five resource-poor locations in India. Indian J med Res. 2008 Apr;127(4):347-61. Pubmed PMID: 18577789.
- Food and Drug Administration[Internet]. US: Beta Hydroxy Acids in Cosmetics. Center for Food Safety and Applied Nutrition, Office of Cosmetics and Colors Fact Sheet ; 2000 [Cited 2000 March 7].Available from: https://www.fda.gov/cosmetics/cosmetic-ingredients/beta-hydroxy-acids
- Tutakne MA, Chari KV. Acne, rosacea and perioral dermatitis. IADVL Textbook and atlas of dermatology. 2003;2:689-710.
- Garg VK, Sinha S, Sarkar R. Glycolic Acid Peels Versus Salicylic–Mandelic Acid Peels in Active Acne Vulgaris and Post-Acne Scarring and Hyperpigmentation: A Comparative Study. Dermatol Surg. 2009 Jan;35(1):59-65. Pubmed PMID: 19076192.
- World Health Organization. The Importance on Pharmacovigilance. Safety Monitoring on Medicinal Products. Office of Publications, Geneva , Switzerland; 2002.
- Hartwing SC, Siegel J, Schnelder PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm. 1992 Sep;49(9):2229-32. Pubmed PMID: 1524068.
- Lau PM, Stewart K, Dooley MJ. Comment: hospital admissions resulting from preventable adverse drug reactions. Ann Pharmacother. 2003 Feb;37(2):303-4.
- Grimes PE. The safety and efficacy of salicylic acid chemical peels in darker racial-ethnic groups. J Dermatol Surg. 1999 Jan;25(1):18-22. Pubmed PMID: 9935087.
- Lee HS, Kim IH. Salicylic acid peels for the treatment of acne vulgaris in Asian patients. Dermatol Surg . 2003 Dec;29(12):1196-9. Pubmed PMID: 14725662.
- Kligman D, KligmanAM. Salicylic acid as a peeling agent for the treatment of photoaging. Dermatol Surg.1998 Mar;24(3):325-8. Pubmed PMID: 9537006.
- Nordlund JJ. Postinflammatory hyperpigmentation. Dermatol clin. 1988 Apr 1;6(2):185-92.
- Dipiro JT, Talbert RL, Yee GC. Pharmacotherapy: a pathophysiologic approach. 6th ed. South Carolina: The McGraw-Hill Companies; 2008.
- Rathi SK. Acne vulgaris treatment: the current scenario. Indian J Dermatol. 2011 Jan;56(1):7-13. Pubmed PMID: 21572783.
- Bates DW, Cullen DJ, Laird N, Peterson LA, Small AD, Servi D, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE prevention study group. JAMA. 1995 jul;274(1):29-34. Pubmed PMID: 7791255.
- Ducharme MM, Boothby LA. Analysis of adverse drug reactions for preventability. Int J Clin Pract. 2007 Jan;61(1):157-61. Pubmed PMID: 17229189.
- Dean B, Schachter M, Vincent C, Barber N. Causes of prescribing errors in hospital inpatients: a prospective study. Lancet. 2002 Apr 20;359(9315):1373-8. Pubmed PMID: 11978334.