Evaluation of Efficacy of Advanced Sign Anti Aging Cream in Imparting Anti Aging Attributes: An Open Label Three Arms, Monocentric Comparative Study
Chavan M*, Velaskar S, Arora K, Mehendaley S
Kaya Limited, Andheri East, Mumbai, MH, India.
*Corresponding Author
Mohan Chavan,
Kaya Limited, Andheri East, Mumbai, MH, India.
E-mail: mohanc@kayaindia.net
Received: March 24, 2018; Accepted : May 17, 2018; Published: May 21, 2018
Citation: Chavan M, Velaskar S, Arora K, Mehendaley S. Evaluation of Efficacy of Advanced Sign Anti Aging Cream in Imparting Anti Aging Attributes: An Open Label Three Arms, Monocentric Comparative Study. Int J Clin Dermatol Res. 2018;6(2):165-171. doi: dx.doi.org/10.19070/2332-2977-1800038
Copyright: Chavan M© 2018. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Skin aging is a complex biological process influenced by a combination of intrinsic and extrinsic factors. These factors lead together to physiological alterations and changes in skin appearance. Anti aging creams help to restore and retain not only beauty but also health of skin reversing the years.
An open label, three arms, monocentric, comparative study aimed to evaluate the efficacy of advanced signs anti aging cream in imparting anti aging attributes in healthy female subjects in comparison to baseline. A total of 40 healthy female subjects with Fitzpatrick skin types III to IV, aged 40-45 years with signs of aging and willing to give informed consent were included in the study. Subjects with a known history or present condition of allergic response, having active skin diseases, taking oral medications, pregnant, lactating or nursing women and who were not willing to give informed consent were excluded from the study. All the included subjects were advised to apply advanced signs anti aging cream, twice-a-day, for a period of 8 weeks. The assessments were made at Day 14, Day 28 and Day 56. To compare the difference in skin attributes due to age, the data obtained from the study population was compared with the data of 80 subjects of age group 30-35 years.
Significant reduction in fine lines, wrinkles, spots and pigmentation and improvement in skin firmness, skin texture, evenness of skin tone and hydration effect were observed in subjects. The benchmarking data concluded the efficacy of test product in reversing the years.
2.Introduction
3.Materials and Methods
3.1 Study Design
3.2 Primary and Secondary Objectives
3.3 Inclusion and Exclusion Criteria
3.4 Study Procedure
3.5 Primary and Secondary end points
3.6 TP Application and Compliance
3.7 Assessment for Efficacy
3.8 Assessment for Safety
3.9 Statistical Analysis
4.Results and Discussion
4.1 Efficacy Evaluation by Dermatological Assessment
4.2 Subject Self Assessment Questionnaire
4.3 Instrumental Measurements
4.4 Safety Evaluation by Dermatological Assessment for Application Site Reaction
4.5 Subject Assessment for Application Site Reaction
4.6 Comparative Visual Assessment
4.7 Comparison with Benchmark Data
5.Conclusion
6.References
Keywords
Skin Aging; Anti Aging Cream; Fitzpatrick Skin Types; Anti Aging Attributes; Benchmarking Data.
Introduction
Skin aging is a result of series of biochemical activities in the body which becomes evident and follows different trajectories in different organs, tissues and cells with time [1-2]. Aging signs of internal organs are invisible but the marks of passing time gets easily reflected on the outer skin. Skin appearance is an important aspect which may make all the differences in how old an individual looks. Wrinkled skin can make you look a decade older than you really are; in contrast to this smooth and supple skin can take years off your appearance right away [1, 3].
Skin aging is a complex biological process influenced by combination of endogenous or intrinsic and exogenous or extrinsic factors. These factors synergistically affect the aged look of the skin. Aging due to intrinsic factors is called true or chronological aging. It inevitably occurs as a natural consequence of physiological changes over time. Genetics, anatomical variations, hormonal changes, metabolic process are some of the intrinsic factors. Aging due to extrinsic factors is called photoaging. It is due to controllable factors and occurs at different degrees of intensity, due to solar exposure, smoking, and gravity, as well as other general lifestyle factors, such as diet, sleep, and overall health [4-11].
Anti aging creams help to restore and retain not only beauty but also health of skin reversing the years. They act on skin to look younger by reducing visible wrinkles, expression lines, blemishes, pigmentation changes, discolorations and other environmentally (especially from the sun) related conditions of the skin [3, 12].
A study was planned to evaluate the efficacy of anti aging cream in reducing the signs of ageing, such as fine lines and wrinkles, at a defined time point, in comparison to baseline. This evaluation helped to understand if the product application for 8 weeks could change the skin attributes of subjects in the age group 40-45 years to bring it closer to the skin attributes seen in 10 years younger age group.
Materials and Methods
The study aimed at evaluating the efficacy of the test product in imparting anti ageing attributes such as reduction in fine lines, wrinkles, spots and pigmentation and improvement in skin firmness, skin texture, evenness of skin tone and hydration effect in healthy female subjects of age group 40-45 years with Fitzpatrick skin type III-V; after test product usage for a period of 8 weeks. The test product (TP) is advanced signs anti aging cream.
An open label, three arms, monocentric, comparative study was conducted to evaluate the efficacy of the test products in imparting anti ageing attributes in healthy female subjects in comparison to baseline. The study was conducted in compliance with the ICH Harmonized Tripartite Guidelines for Good Clinical Practice (GCP), and the provisions of Declaration of Helsinki. The protocol and any amendments, the study specific informed consent documents, were submitted to the Ethics Committee for formal approval for the conduct of the study.
Primary objectives were to evaluate the reduction in signs of ageing such as fine lines and wrinkles and tracking the improvement in signs of ageing pertaining to pigmentation such as age spots and other pigmentation, at a defined time point, in comparison to baseline. While the secondary objectives were to evaluate the improvement in skin firmness, skin texture and visible pores, evenness of skin tone and skin hydration, at a defined time point, in comparison to baseline and to assess the safety and acceptance of the test product.
Female adult subjects in general good health as determined from a recent medical history, subjects in the age group of 40-45 years (both ages inclusive), subject with Fitzpatrick skin types III to V, subjects with signs of ageing such as fine lines, wrinkles, spots, pigmentations, uneven skin tone, etc, subjects with mild to moderate hyperpigmentation spots (ephilides, lentigines, PIH) were included in the study. Subjects willing to give a voluntary written informed consent, photography release and agree to come for regular follow up, subjects willing to abide by and comply with the study protocol, subjects who have not participated in a similar investigation in the past four weeks, subjects who are willing not to participate in any other clinical study during participation in the current study were also included in the study. While subjects with a known history or present condition of allergic response to any cosmetic products, having active skin diseases (e.g. moderate to severe acne vulgaris face or nodulocystic acne, psoriasis, active atopic dermatitis, melasma, lichen planus pigmentosus/ Ashy dermatosis, pigmented contact dermatitis or other cutaneous manifestations), with severe to chronic hyperpigmentation spots (ephilides, lentigines, PIH) were excluded from the study. Subjects on oral medications (e.g. steroids, anti-oxidant), systemic treatment which may modify the cutaneous state on the day of inclusion or in the previous 30 days, including retinoid therapy, not willing to discontinue other topical facial products, subjects who are pregnant, lactating or nursing were also excluded from the study. Intense sun exposure/photo allergenicity/toxicity, chronic illness which may influence the cutaneous state, participating in any other cosmetic or therapeutic trial, any underlying uncontrolled medical illness including diabetes mellitus, hypertension, liver disease or history of alcoholism, HIV, hepatitis, or any other serious medical illness were the other exclusion criteria.
The study was conducted on 40 female subjects in the age group of 40-45 years for a period of 8 weeks. The subjects were asked to follow the treatment regimen of twice daily application. The assessments were made at Day 14, Day 28 and Day 56. To compare the difference in skin attributes due to age i.e. year wise comparison, the data obtained from the study population was compared with the data of 80 subjects of age group 30-35 yrs. This evaluation helped understand if the product application for 8 weeks could change the skin attributes of subjects in the age group 40- 45 years to bring it closer to the skin attributes seen in 10 years younger age group.
This study thus evaluated the product efficacy over a period of 8 weeks in comparison to baseline data and change in skin attributes with reference to data of 10 years younger age group.
Primary end points were reduction in fine lines and wrinkles and improvement in age spots, pigmentation, at a defined time point, in comparison to baseline. Whereas secondary end points were improvement in skin firmness, skin texture and visible pores at a defined time point, in comparison to baseline.
Towards monitoring subject compliance, proper instructions on TP usage were given to subjects during product dispense. On every follow up visit, the study coordinator enquired and verified the product compliance as per the given instructions. Subject diary was provided to all the subjects. After every usage, the subjects were instructed to fill the time of TP usage in the appropriate date/day column. Subjects were instructed to capture any irritation experienced during the course of product usage. Subject diary was maintained in order to ensure and record product compliance. The subject diary was reviewed at every follow up visit to document site application reaction.
During the visit to the site the subjects were acclimatized at a temperature of 22°C +/- 5° C and relative humidity of 50% +/- 10% for 10-15 min after face wash.
Dermatological assessment was done on visit 1, 2, 3 and visit 4. Parameters for assessment include: shade card assessment, skin firmness/laxity, overall wrinkles over face, crow’s feet grading scale in unanimated face (photo numeric scale), nasolabial folds, frown lines: as per photo numerical scale, homogenecity of age-spots/hyperpigmentation, pores, skin clarity, skin smoothness, evenness of skin tone and hydration of skin.
Subjects were asked to assess their skin and score the skin attributes as per self-assessment questionnaire on visit 1, 2, 3 and 4. Parameters for assessment include: skin tone, skin firmness, fine lines & wrinkles, skin clarity, spots and pigmentation, pores, evenness of skin tone, hydration of skin and roughness/smoothness.
Instrumental measurements were carried out using chromameter, corneometer, cutometer and visioscan.
Assessment for application site reaction was done by dermatological assessment for local skin irritation at product application site erythema, oedema, dryness, urticaria, acneiform eruption, allergic reactions and any other specific reactions. Each parameter was assigned a score as: None = 0, Mild = 1, Moderate = 2, Severe = 3, Very severe = 4. The reactions were documented in dermatological assessment form for application site reaction.
Subject assessment was done at each follow up visit based on the following parameters: redness, dryness, itching, burning, rashes, pimple like reaction, sweating, oiliness and any other specific reactions. Each parameter was assessed scores similar to that of assessment for site application reaction by dermatologist.
The subjects were asked to observe the changes in their skin conditions throughout the study term and inform the same to the investigator on their site visits. The subjects were also advised to make a note of any kind of skin irritation experienced during product usage in the subject diary provided. The study personnel reviewed the subject diary and any skin irritation noted in the subject diary was recorded under subject assessment form for application site reaction on all follow up visits with severity grade after enquiry with the subject.
Descriptive statistical analysis was carried out. Results on continuous measurements were presented on Mean ± SD and results on categorical measurements were presented in frequency expressed as Number (%). Significance was assessed at 5 % level of significance. Paired t test was performed to find out efficacy in comparison to baseline on continuous scales such as dermatological assessment and instrumental assessments. T-test has also been used for comparison with benchmark data. Statistical figures at 95% confidence level were considered suggestively significant at p-value ≤0.10 and statistically significant at p-value ≤0.05.
In Indian subcontinent (skin of colour) as the skin ages, pigmentation related problems increases. Wrinkling is seen much later than various concerns associated with pigmentation as signs of aging. As skin ages, there is tanning, unevenness, blotchiness and age spots. The scope of improvement and expectation is also higher for these parameters.
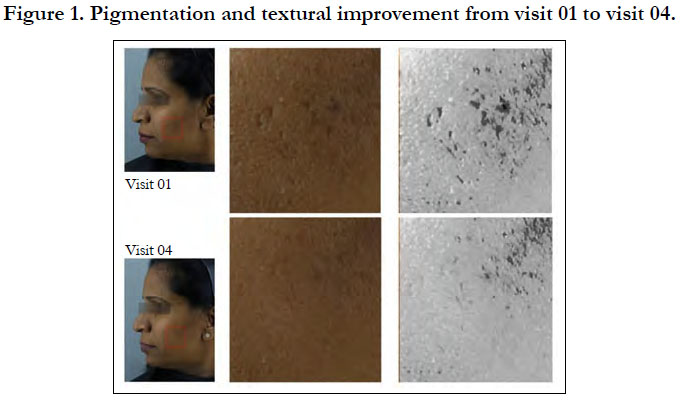
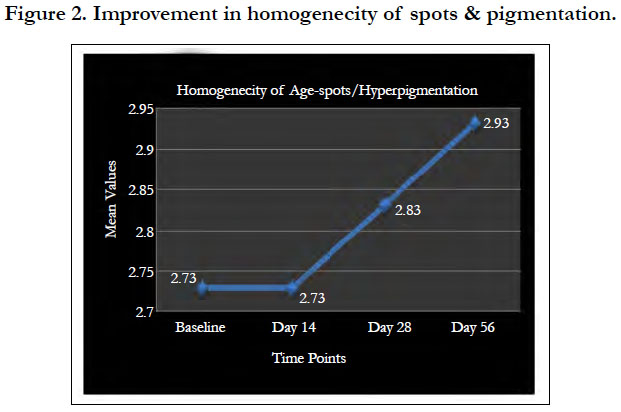
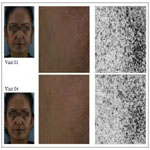
With 8 weeks of product usage, the improvement in the skin pigmentation rendered the population with skin complexion better than the skin pigmentation noted in the 10 years younger age group. Visible improvement in the skin texture with younger looking skin is evident from Figure 1. As per dermatological visual assessment by Image comparison, up to 25% of improvement was seen in 47.5% of study population and 50% of improvement was seen in 25% of the study population. One subject showed up to 75% improvement at the end of the study. The significant improvement in homogeneity of spots and pigmentation is shown in Figure 2.
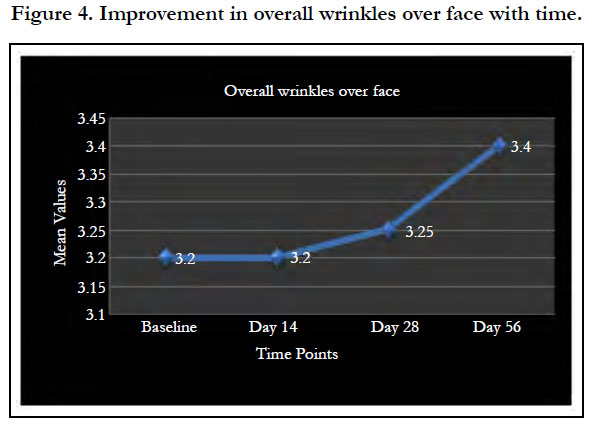
Statistically significant improvement was observed in the fine lines and wrinkles across the face with 8 weeks of product usage as per the dermatological assessment. The improvement was progressive. Dermatological assessment represents a visual improvement captured on a linear scale. A more sensitive method of evaluation using Visioscan assessment for skin typology showed statistically significant improvement in skin wrinkle value seen from Day 14 onwards and progressive with time.
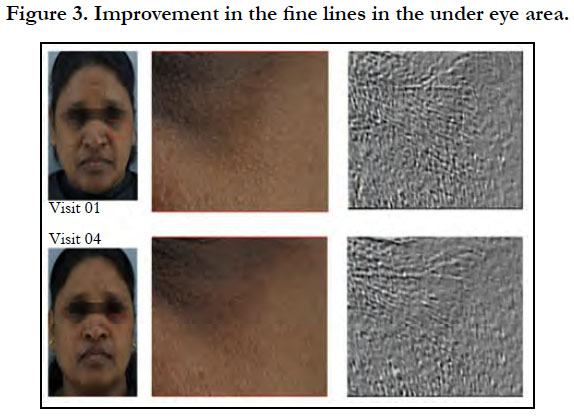
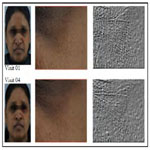
Significant visible improvement in the fine lines in the unde eye area was observed from visit 01 to visit 04 (Figure 3). When compared to reference benchmark data, the product was able to render up to 10 years younger skin attribute for fine lines and wrinkles with 8 weeks of product usage as per the evaluation using Visioscan.
As per dermatological visual assessment by Image comparison, up to 25% of improvement was seen in 45% of study population and 50% of improvement was seen in 5% of the study population. One subject showed up to 75% improvement at the end of the study.
The improvement in skin wrinkles with product usage for 8 weeks rendered the skin wrinkle condition of subjects better than the condition seen in reference age group with statistically significant difference on Skin wrinkle value (SeW) by Visoscan. It thus implies the skin looks younger by 10 years after product usage. Significant improvement in overall wrinkles over face with time is shown in Figure 4.
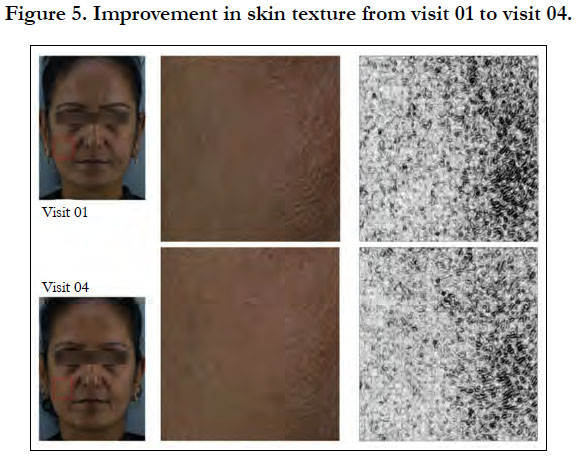
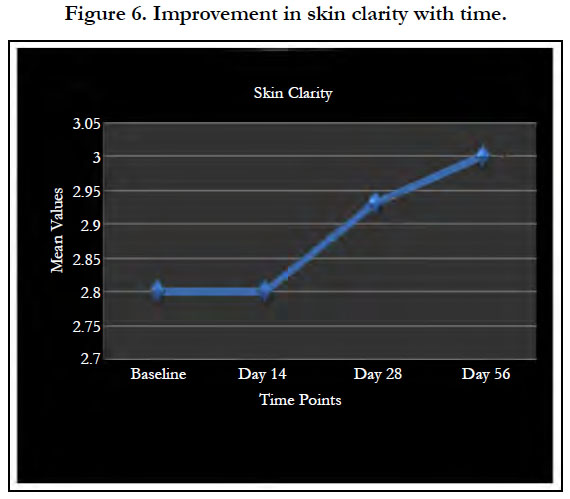
Younger skin appears healthier, supple and tighter with less visibility of pores (Figure 5). The product showed significant improvement in the skin condition (Figure 5) and the skin looked visibly younger with 8 weeks of product usage. The significant improvement in clarity in the dermatological assessment can be seen in Figure 6.
These results demonstrated that TP is highly efficacious in imparting anti aging attributes. It gave younger skin attributes in 8 weeks of product usage. The efficacy was comparable with up to 10 years younger looking skin. As per dermatological visual assessment by Image comparison, up to 25% of improvement was seen in 47.5% of study population and 50% of improvement was seen in 40% of the study population.
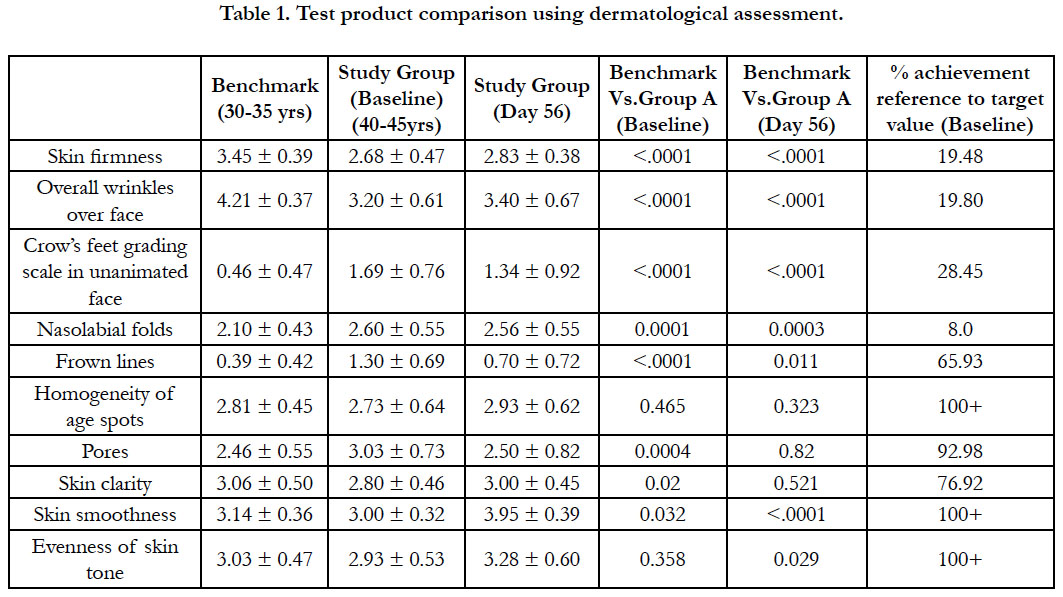
The TP comparison using dermatological assessment regarding other skin parameters is elaborated in Table 1. It was observed that after TP application for 56 days results into statistically significant improvement in the skin attributes under the investigation such as skin clarity, evenness of skin tone, skin smoothness, skin firmness, pores frown lines, hydration of skin and reduction in the spots and pigmentation. At the same time it has achieved target for age spots, even skin tone, hydration, smoothness and the value was better than bench mark. In case of Fine lines and wrinkle; about 20% improvements in wrinkle condition, about 28% improvement in crow’s feet and about 66% improvement in frown lines was evident.
While regarding pigmentations; TP had achieved target for age spots and skin tone more effectively than bench mark. About 77% improvement in skin clarity was observed.
TP has achieved target for hydration and smoothness and the values were better than bench mark. About 20% improvement in skin elasticity was evident.
Significant and progressive improvement was observed with time in all parameter at the end of the study in comparison to baseline mean score. At day 56, 68% improvement in skin clarity and above 40% improvement in skin tone, smoothness and evenness of skin tone over baseline were evident at the end of the study. At day 56, 37.55% reduction of spots and pigmentation and 38.34% improvement in fine lines and wrinkles from baseline mean score were observed at the end of the study.
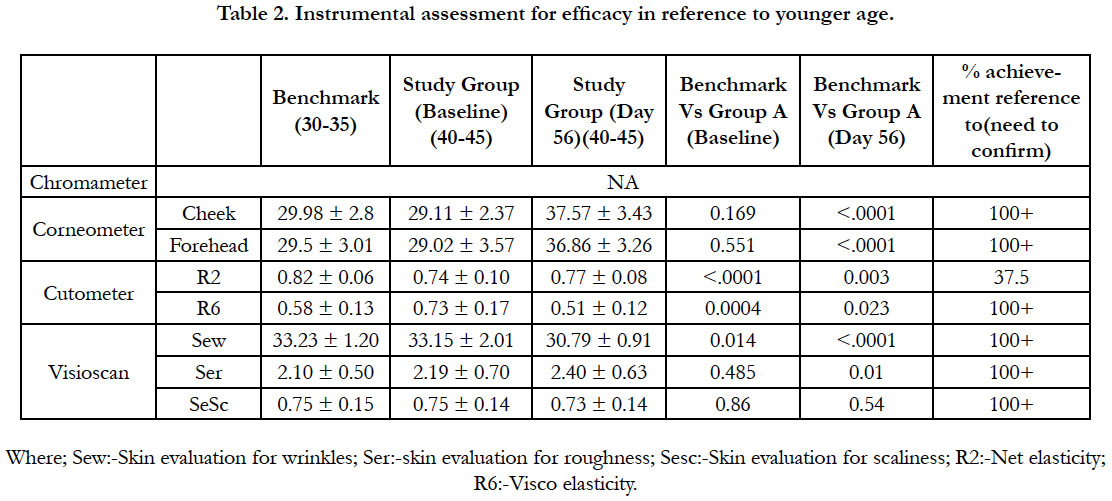
A summary of instrumental analysis is explained in Table 2. Corneometer results on both the sites (Cheeks & forehead) demonstrated significant high moisturized skin value in contrast to baseline & benchmark. Significant improvement in skin elasticity (R2) was evident when compared to baseline. R6 value stands for viscoelasticity of skin. Higher value is sign of tired skin. Decreasing value is a sign of improvement. Value of viscoelasticity decreased significantly in comparison to baseline and benchmark. Results from visioscan exhibited significant decrease in wrinkles and scaliness in comparison to baseline and benchmark.
One subject experienced moderate dryness at day 14 which was resolved by day 28. Mild acneiform eruption was observed in two subjects at baseline and it was resolved by day 14. There were no erythema, oedema, urticaria and allergic reaction noted during the study. The frequency and severity of other reactions was clinically insignificant and did not require any clinical intervention as per the investigator’s discretion. The TP was overall safe.
Mild dryness was experienced by one subject at day 14 which was resolved by day 28. In one subject mild sweating was observed at day 28 and day 56. There were no signs of redness, itching, burning, rashes, pimple like reaction and oiliness noted during the study. Product safety was evaluated by subjects at all the time points. The occurrence, its severity and resolution without any medical intervention implies the product to be overall safe.
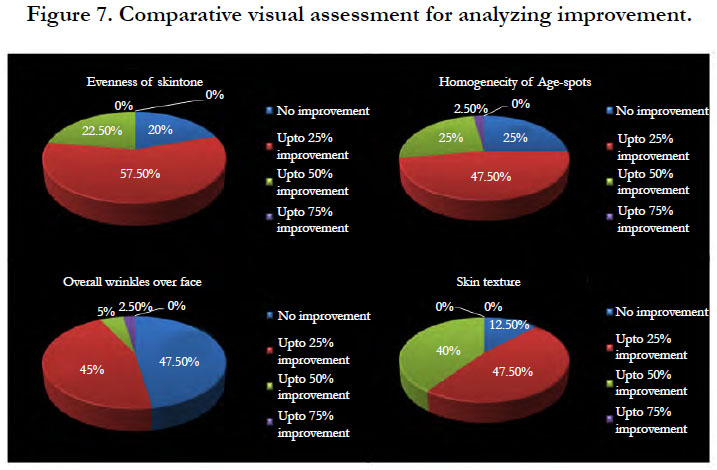
Dermatological assessment in an objective scale does not show more improvement. More over the evaluation is done on a fixed scale where the descriptive change between the grades is difficult. In a comparative image assessment subtle changes on a higher resolution could be identified (Figure 7).
At the end of study, up to 25% of improvement in evenness of skin tone, homogeneity of age spot, overall wrinkles and skin texture was observed in 57.5%, 47.5%, 45% and 47.5% of study population, respectively.
An improvement of 50% in evenness of skin tone, homogeneity of age spot, overall wrinkles and skin texture was observed in 22.5%, 25%, 5% and 40% of the study population, respectively. There was also an improvement 75% in homogeneity of age spots and overall wrinkles in 2.5% of study population.
The data of the study population was compared with the benchmark data collected from a younger age group (by 10 years) to see the changes brought by the product on the age based variability. The comparison was carried for both dermatological as well as instrumental data.
The skin attributes such as evenness of skin tone, clarity, hydration and texture were significantly better. The improvement in overall wrinkles was nearly 20% and improvement in frown lines by 60%. The skin conditions at the end of the study, such as smoothness, hydration and pores were better than the benchmark value. The improvement in skin elasticity and wrinkle by instrumental evaluation showed statistical significance and better value than younger age group.
Conclusion
With the increasing longevity of the population worldwide, there has been an increased interest in anti ageing preparations and their purported ability to enhance a person's more youthful appearance. Skin aging is a part of a natural human “aging mosaic” which becomes evident and follows different trajectories in different organs, tissues and cells with time. Aging signs of internal organs may remain hidden from the ambient “eyes,” but they make their first obvious marks of the passing time on skin.
Skin is a feature that may make all the difference in how old an individual looks. If skin is leathery or wrinkly, it can make you look a decade older than you really are. In contrast to this, smooth and supple skin can take years off your appearance right away. Though chronological aging is genetically determined, photoaging can be prevented using anti aging creams.
The TP was found highly efficacious in imparting anti ageing attributes such as reduction in fine lines, wrinkles, spots and pigmentation and improvement in skin firmness, skin texture, evenness of skin tone and hydration effect in healthy female subjects with Fitzpatrick skin type III-V. The TP was over all well tolerated as there were no untoward events.
The benchmarking analysis demonstrated that TP application for 8 weeks could change the skin attributes of subjects in the age group 40-45 years to bring it closer to the skin attributes seen in 10 years younger age group. Therefore it can conclude that an advanced sign anti aging cream is efficacious in imparting anti aging attributes reversing the years.
References
- Ganceviciene R, Liakou AI, Theodoridis A, Makrantonaki E, Zouboulis CC. Skin anti-aging strategies. Dermatoendocrinol. 2012 Jul 1;4(3):308-19. PubMed PMID: 23467476.
- Cevenini E, Invidia L, Lescai F, Salvioli S, Tieri P, Castellani G, et al. Human models of aging and longevity. Expert Opin Biol Ther. 2008 Sep;8(9):1393-405. PubMed PMID: 18694357.
- Sharma B, Sharma A. Future prospect of nanotechnology in development of anti-ageing formulations. Int J Pharm Pharm Sci. 2012;4(3):57-66.
- Ramos-e-Silva M, Celem LR, Ramos-e-Silva S, Fucci-da-Costa AP. Antiaging cosmetics: facts and controversies. Clin Dermatol. 2013 Nov-Dec;31(6):750-8. PubMed PMID: 24160281.
- Farage MA, Miller KW, Elsner P, Maibach HI. Intrinsic and extrinsic factors in skin ageing: a review. Int J Cosmet Sci. 2008 Apr;30(2):87-95. PubMed PMID: 18377617.
- Kohl E1, Steinbauer J, Landthaler M, Szeimies RM. Skin ageing. J Eur Acad Dermatol Venereol. 2011 Aug;25(8):873-84. PubMed PMID: 21261751.
- Baumann L. Skin ageing and its treatment. J Pathol. 2007 Jan;211(2):241-51. PubMed PMID: 17200942.
- Gilchrest BA. Skin aging and photoaging: an overview. J Am Acad Dermatol. 1989 Sep;21(3 Pt 2):610-3. PubMed PMID: 2476468.
- Fisher GJ. The pathophysiology of photoaging of the skin. Cutis. 2005 Feb;75(2 Suppl):5-8. PubMed PMID: 15773537.
- Hashizume H. Skin aging and dry skin. J Dermatol. 2004 Aug;31(8):603-9. PubMed PMID: 15492432.
- Wulf HC, Sandby-Møller J, Kobayasi T, Gniadecki R. Skin aging and natural photoprotection. Micron. 2004 Apr 1;35(3):185-91. PubMed PMID: 15036273.
- Duraivel S, Shaheda A, Basha R, Pasha E. Jilani.“Formulation and Evaluation of Antiwrinkle Activity of Cream and Nano Emulsion of Moringa Oleifera Seed Oil”. IOSR J Pharm Biol Sci. 2014;9(4):58-73.