Polymerized-Type I Collagen Induces a High Quality Cartilage Repair in a Rat Model of Osteoarthritis
Almonte-Becerril M1, Enríquez AB2, Kourí JB1, Furuzawa-Carballeda J2*
1 Department of Infectomic and Molecular Pathogenesis, Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional. Av.
Instituto Politécnico Nacional, Col. San Pedro Zacatenco, Apdo.Postal 14740, Mexico City, Mexico.
2 Department of Immunology and Rheumatology, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Vasco de Quiroga No. 15, Col. Belisario Domínguez, Sección XVI, Alcaldía Tlalpan, C.P.14080, Mexico City, Mexico.
*Corresponding Author
Janette Furuzawa-Carballeda, MSc, PhD,
Department of Immunology and Rheumatology,
Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán,
Vasco de Quiroga No. 15, Col Belisario Domínguez, Sección XVI,
Alcaldía Tlalpan, C.P.14080, Mexico City, Mexico.
Tel: +52 55 54850766
Fax: +52 55 55732096
E-mail: jfuruzawa@gmail.com
Received: May 26, 2017; Accepted: July 14, 2017; Published: July 15, 2017
Citation: Almonte-Becerril M, Enríquez AB, Kourí JB, Furuzawa-Carballeda J (2017) Polymerized-Type I Collagen Induces a High Quality Cartilage Repair in a Rat Model of Osteoarthritis. Int J Bone Rheumatol Res. 4(2), 68-76. doi: dx.doi.org/10.19070/2470-4520-1700015
Copyright: Furuzawa-Carballeda J© 2017. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Osteoarthritis (OA) is a chronic, degenerative and inflammatory disease. It is characterized by progressive deterioration of articular cartilage. It is the most common disabling rheumatic pathology in adults older than 45 years, and there is no specific treatment.
Objectives: Based on the rationale that in vitro polymerized-type I collagen induces chondrocytes proliferation, up-regulates the cartilage extracellular matrix proteins and down-regulates proinflammatory cytokines, we decided to evaluate its effect on cartilage repair in a rat model of OA.
Methods: Thirty Wistar male rats with partial meniscectomy were subjected to daily high impact exercise during 3 weeks. Rats were randomly allocated into 5 groups a) training control, b)sham/operated control, c) toxicity control, d) OA treated with 4 intraarticular (IA) injections of placebo, and e) OA treated with 4 IA injections of polymerized-type I collagen. Weight, temperature and thickness of the knee were measured. Histological and radiological analysis was also performed. Type I and II collagen as well as, MMP13 expression was determined by immunofluorescence.
Results: Clinimorphometric analysis showed a higher temperature and thickness of the knee in OA/placebo vs. OA/polymerized-type I collagen treated rats. Radiological and histological analysis demonstrated that polymerized-type I collagen but not placebo preserved joint cavity structure and proteoglycans content and induced an increase of 2 to 4 fold type II collagenexpressing chondrocytes whereas it inhibited type I collagen and MMP13 producing chondrocytes.
Conclusion: The results suggest that polymerized-type I collagen is safe and effective chondroprotective biodrug with disease modifying effects.It induces high quality cartilage repair.
2.Introduction
2.1 Rationale
3.Animals and Methods
3.1 Rats
3.2 Induction of OA
3.3 Disease Models
3.4 Radiographic Evaluation
3.5 Safranin O Staining
3.6 Immunofluorescence
3.7 Statistical Analysis
4.Results
4.1 Demographic and Clinical Characteristics
4.2 Macroscopic Findings
4.3 Radiographic Findings
4.4 Histological Findings
4.5 Effect of Polymerized-Type I Collagen on Chondrocytes
4.6 Adverse Events
5.Discussion
6.Conclusion
7.Acknowledgments
8.References
Keywords
Polymerized-Type I Collagen; Cartilage Repair; OA Rat Model; Collagen Type II; MMP-13.
Introduction
Osteoarthritis (OA) is a degenerative joint disease that is classically described as the result of both mechanical and biochemical events that increase the degradation of articular cartilage and subchondral bone. It is characterized by morphological, biochemical, molecular and biomechanical changes producing softening, fibrillation, ulceration and loss of articular cartilage, sclerosis and eburnation of the subchondral bone, osteophytes and subchondral cysts. Patients afflicted with OA may experience joint pain and pressure, crepitation, movement limitation, stroke and varying degrees of local inflammation with deleterious effects on patient activity level and lifestyle habits [1, 2].
Knee OA is the most common of the rheumatic diseases affecting approximately 3.8% (95% CI: 3.6-4.1%) of the global population. Moreover, OA is regarded as a prevalent cause of morbidity and disability worldwide. Prevalence is higher in females than males. OA of the hip and knee contributes the most to OA burden, often resulting in joint replacement surgery [3].
However, despite the prevalence and severity of the disease, relatively little is known about its exact etio- and pathophysiology. Recent compelling investigations have attributed the onset of OA to age, female sex, genetic factors, adiposity, obesity, and diet and joint-level factors such as injury, malalignment, and joint biomechanics [2, 4]. Latest studies suggest that patients exhibit inflammatory infiltration of synovial tissue membranes [5-8].
Moreover, the factors involved in joint destruction in OA including a) chondrocyte and synovial tissue cells that synthesize proinflammatory cytokines (interleukin (IL)-1β, IL-6, IL-15, IL-18, oncostatin M and tumor necrosis factor (TNF)-α), adipokines (leptin, visfatin, adipsin), and/or chemokines (IL-8, CCchemokine ligand 5 (CCL5) and CCL19); b) the inflammatory mediators, such as prostaglandin E2(PGE2), nitric oxide (NO), reactive oxygen species (ROS) and complement; c) the depletion of extracellular matrix proteins through degradation by metalloproteinase (MMP)1, MMP3, MMP8, MMP13, aggrecanases, a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)4, ADAMTS5 and cathepsins; d) the cell-derived and/ or matrix-derived products in regions of matrix depletion, such as alarmins (e.g. S100), fibronectin fragments, hyaluronic acid fragments, type II collagen cleavage epitopes, proteoglycan fragments and high mobility group box; and e) the increased of autophagy and chondroptosis [2, 4, 9-11].
Current therapeutic strategies for OA are targeted primarily at symptomatic relief, have limited effects on the underlying cascade of joint obliteration and have high incidence of side effects [12, 13]. Nonetheless, no proven structure-modifying therapy is available as yet. Local drug delivery strategies may provide for the development of more successful OA treatment outcomes that have potential to reduce local joint inflammation, reduce joint proteolysis, offer pain relief, and restore activities of daily living levels and diarthrodial joint function. For this reason, we have evaluated the effect of a γ-irradiated mixture of a telopeptidic porcine type I collagen and polyvinylpyrrolidone (polymerized-type I collagen), which has anti-inflammatory properties [14, 15]. One percent polymerized-type I collagen addition to synovial tissue cultures from non-rheumatoid arthritis (RA) and RA does not induce any change in DNA concentration or metabolism. However, polymerized collagen modifies the histological and biochemical pattern of fibrosis, without altering the total collagen content. Polymerized- type I collagen induces the recovery of type III collagen at similar levels to those detected in normal synovial tissue. This biodrug diminishes the accumulation of dense and tightly packed type I collagen fibers and contributes to establish normal tissue architecture. Moreover, polymerized-type I collagen down-regulates pro-inflammatory cytokines (IL-1β, and TNF-α); adhesion molecule expression [endothelial leucocyte adhesion molecule (ELAM)-1, vascular cell adhesion molecule (VCAM)-1, intercellular adhesion molecule (ICAM)-1], cyclooxygenase (Cox)-1 as well as collagenolytic activity and up-regulates tissue inhibitor of metalloproteinase (TIMP)-1 production [16, 17]. Likewise, 1% polymerized-type I collagen addition to cartilage and synovial tissue co-cultures obtained from total knee replacement of patients with OA up-regulates chondrocyte proliferation (Ki-67 antigen), the synthesis of highly sulphated proteoglycans, COMP and type II collagen, modifying extracellular matrix turnover in cartilage and restoring normal tissue architecture [18]. Furthermore, down-regulation of TNF-α and IL-1β seems to stimulate selectively activated synovial cell death via apoptosis in synovium cultures [16-18]. Finally, polymerized-collagen has demonstrated to be clinically safe and efficient for the treatment of RA [19, 20]. Thus, we performed this study to evaluate the effect IA administration of polymerized-type I collagen on cartilage repair in partial meniscectomized rat model of OA.
The rationale to inject IA polymerized-type I collagen was based on the encouragement from previous experience related to the biodrug effects (anti-inflammatory and tissue regenerator effects) observed during the course of the treatment of hypertrophic scars, scleroderma (SSc) skin lesions and RA patients [14-21].
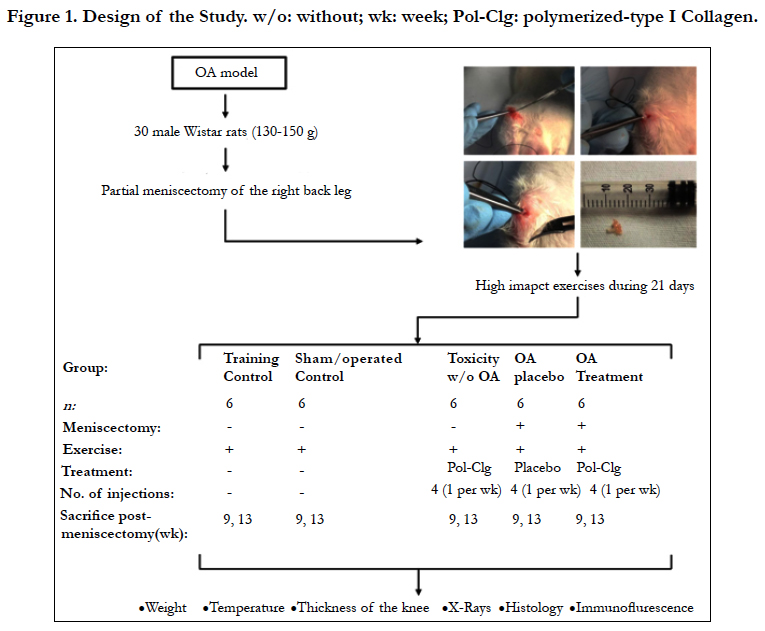
Thirty Wistar male rats (130-150 g) were kept under specific pathogen-free conditions. All animals were set aside under standard conditions in 12 h day/night rhythm with access to food and fresh water ad libitum. All animal procedures and experiments were carried according to international guidelines (ICLAS-WHO) and Mexican official regulatory guideline NOM 062-ZOO-1999. The protocol of this study was approved by the Animal Care Research Committee of the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (CINVA:1093).
Rats were anesthetized with intraperitoneal injection of ketamine (50 mg/kg PISA) 0.09 ml and xylazine (5mg/kg) 0.02 ml. The right back leg was shaved. Under sterile surgical conditions an antero medial incision was done in the knee's internal region, parallel to the leg long axis. The soft tissues were dissected parallel to the bones until the internal ligaments were viewed. The ligaments were sectioned perpendicular to the long axis and the joint capsule was excised to expose the medial compartment of the knee. The meniscus was clearly seen after a 90-degree flexion of the knee. It was carefully separated from the internal condyles and tibia plateau with a pointed knife scalpel. Special precautions were taken to avoid damaging the cartilage. A blunt curved needle was used to pass a surgical thread through the meniscus, and then it was gently pulled upwards and held with fine pointed pliers. Then it was sectioned with a sickle-shaped scalpel in order to remove 30-50% of the meniscus (Figure 1). Tissues were closed with number 4.0 silk suture. Postoperatory casts were not used. Animals were maintained within their cages where they moved freely. Ambulation started the day after surgery, with gentle and then with progressive intensity according to the possibility for using the injured joint. After three days, the animals used both legs entirely and they were subjected to high impact exercise for 15 minutes each day during 21 days. Five animals were placed in a box of 33 cm wide X 60 cm high X 33cm high deep with a bed of sawdust. During the first week 3 cycles of daily high impact exercise were performed, as follows: 1) the box with rats was balanced back and front for 2 min (approximately 100 displacements per animal), 2) then each rat was grasped and released from a height of 30 cm (approximately 15 times/rat); and lastly 3) the box was shaken for one minute (approximately 80 continuous jumps up to 10 cm in height). During the second week, rats were grasped and released from the total height of the box. This exercise stimulated the muscles, joints and bones by working flexion and extension and compression of the limbs [9, 22-24].
Figure 1. Design of the Study. w/o: without; wk: week; Polymerized type-I Clg: polymerized-type I Collagen.
The inflammatory progression in each animal was assessed every week post training by measuring the thickness of the ankles (vernier), temperature, and weight. At 9 (early disease) or 13 (longterm disease) weeks rats were sacrificed. To test the effect of Polymerized-type I collagen in rats with OA, 50 μL was injected via intra articular (IA) once a week for 4 weeks, previous sedation with sevoflurane (sevoflurane, ABBOT).
Six rats without partial meniscectomy and without treatment were subjected to high impact exercise for 15 minutes each day during 3 weeks and were sacrificed at the 9th (n=3) and 13th weeks (n=3).
Six rats in which the capsule and synovial membrane were cut but the meniscus was not removed and without treatment were subjected to high impact exercise for 15 minutes each day during 3 weeks and sacrificed at the 9th (n=3) and 13th weeks (n=3) post incisional wound on the right back leg.
Six rats without partial meniscectomy were subjected to high impact exercise for 15 minutes each day during 3 weeks and then were treated by 4 IA administrations, once a week for 4 weeks, with 50 μL of polymerized-type I collagen (FibroquelMR). Three rats were sacrificed at the 9th and three rats at the 13th.
Six rats with partial meniscectomy were subjected to high impact exercise for 15 minutes each day during 3 weeks and were treated by 4 IA administrations, once a week during 4 weeks, with 50 μL of citric/citrate buffer (vehicle control). Three rats were sacrificed at the 9th (early disease) and three rats at the 13th post-meniscectomy (long-term disease).
Six rats with partial meniscectomy were subjected to high impact exercise for 15 minutes each day during 3 weeks and were treated by 4 IA administrations, one per week during 4 weeks, with 50 μL of polymerized-type I collagen. Three rats were sacrificed at the 9th (early disease) and three rats at the 13th post-meniscectomy (long-term disease).
After the sacrifice, a radiographic AP and lateral record was made in order to evaluate joint space, the presence of osteophytes, sclerosis and the integrity of hyaline cartilage (Tryumph X-ray apparatus). Measurement of bony features of knee was rated on a scale of 0 (absent) to 3 (highly advanced), according to the consensus of two blinded readers [25].
Cartilage proteoglycans content was detected by the Safranin O staining method, according to standard protocols [26, 27]. Briefly, cryosections were stained with Weigert’s iron hematoxylin for 10 min, then with fast green (Sigma, St. Louis, MO) for 5 min, rinsed with 1% acetic acid solution for 10 seconds and stained with 0.1% Safranin O (Sigma) for 5 min. Finally, sections were dehydrated, mounted in Poly-Mount and evaluated by optical microscopy.
Cartilage samples from normal and OA knee of male rats were obtained at the 9th and 13th post-meniscectomy. Cartilages of all joints were obtained from the weight bearing areas of the condyles. All samples were immediately fixed in 4% PBS-paraformaldehyde during 24 hours at room temperature [22-24]. Fixed samples were cryosectioned (Leica CM 1100; Heerbrugg, Switzerland) and mounted on gelatin coated slides. Tissues were processed for IF as has been described elsewhere [28]. In order to identify type I Collagen, type II Collagen and MMP13 in chondrocytes, independent samples were incubated with rabbit (Abcam pcl, CA, UK). Nuclei were counterstained with DAPI (Sigma) and cover-slipped in Vectashield mounting medium (Vector Laboratories, Burlingame, CA). As secondary antibody FITC-tagged donkey anti-rabbit (Jackson Immuno Research Laboratories, Inc, WestGrove) was used. Then, all samples were observed through a Leica confocal microscope (TCS-SP5-DMI6000B with Plan Neor Fluor at 63X objective)[10, 28].
To quantify the immunolabeling, Leica LAS AL light confocal program was used, in which fluorescence intensities were measured in each zone by the number of pixels per area (μm). To perform the microscopy analysis, each zone was selected according to the morphological characteristics expressed in each area as has been described elsewhere [10, 28]. As an internal control, sham samples were also compared with normal cartilage; negative controls were included, omitting the primary antibody.
It was performed using the SigmaStat 11 program (Aspire Software International, Leesburg, VA, USA) by one way analysis of variance on Ranks and by Holm-Sidak method for all pairwise multiple comparison procedures, as well as Tukey-Kramer multiple comparison test. Data were expressed as the mean ± SD. The P-values smaller than or equal to 0.05 were considered as significant.
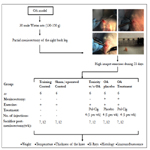
Except for the group of trained rats with partial meniscectomy and OA, the other groups of rats presented a significant increase in weight at 8 week. No statistically significant differences were determined between the group of rats with early and long-term disease treated by 4 IA administrations, once a week for 4 weeks, with 50 μL of polymerized-type I collagen when compared withtraining or sham/operated control groups (Figure 2A).
In regards to temperature, only the group of rats with early and long-term OA treated with placebo had an increase of temperature at 8 week when compared with the groups of toxicity, sham, training and OA treated with polymerized-type I collagen. No statistically significant differences were found between the sham/ operated control group, the polymerized-type I collagen toxicity assessment and the group of early and long-term OA treated with the polymerized-type I collagen (Figure 2B).
The back leg knee thickness of long-term OA treated with placebo had significant increase at 10 week as compared with the groups of toxicity, sham, exercise and OA treated with polymerized- type I collagen. No statistically significant differences were found between the sham/operated control group, the polymerized- type I collagen toxicity assessment group and the group of early and long-term OA treated with (Figure 2C).
Figure 2. Effects of polymerized-type I collagen in early and long-term rat model of OA. Fifty microliters of Polymerizedtype I collagen (toxicity model and OA/polymerized-type I collagen treatment) or citric/citrate buffer (OA/placebo model) were administered intraarticular once a week during 4 weeks. (A) Weight of the rats throughout the study. (B) Temperature of the rats during the study. (C) Right back knee thickness in early (9th week) and long-term OA (13th week). Data represent mean ± SEM (each group, n = 6). *P < 0.05.
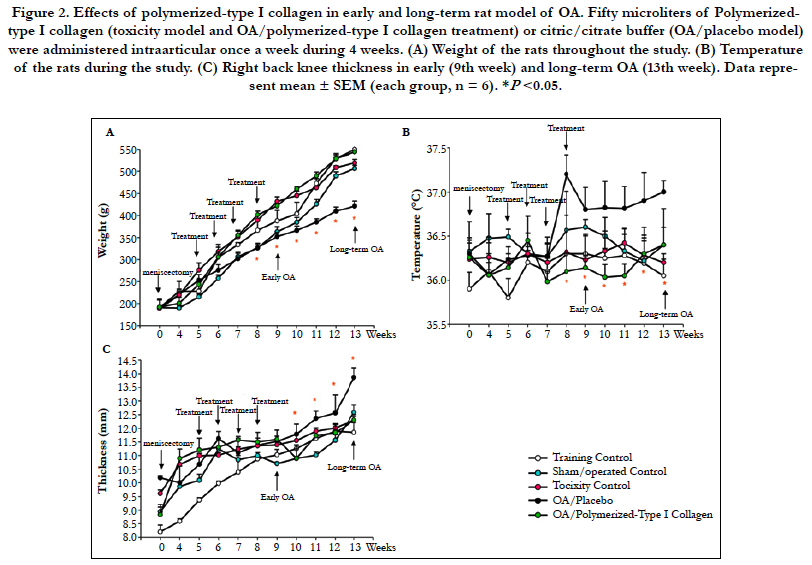
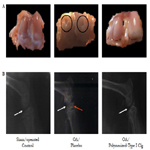
Knee cartilage from training control (data not shown), the polymerized-type I collagen toxicity assessment (data not shown) and sham/operated control displayed a white, smooth, glossy surface (Figure 3A, left panel). OA cartilage in early disease showed a less glossy, more opalescent, and a depression was frequently observed in the weight-bearing region of the condyles. OA cartilage in long-term disease in trained ratswas softened, irregular and displayed a fibrillated surface and ulcerations, all animals showed a thinner, irregular and in many cases absent cartilage. Frequently the subchondral bone was exposed to the surface (Figure 3A, middle panel). The group of early and long-term OA treated by 4 IA administrations, once a week for 4 weeks, with 50 μL of polymerized-type I collagen exhibited a cartilage of similar characteristics to those observed in the control groups (Figure 3A, right panel).
Based on the radiographic assessment the index was grade 0 regarding to OA severity in training control group, sham/operatedcontrol (Figure 3B, left panel), and the polymerized-type I collagen toxicity assessment groups (data not shown). The group of early and long-term OA treated with 50 μL of polymerized-type I collagen exhibited grade 0 and grade 1 regarding to OA severity. The OA in early and long-term disease in trained rats exhibited grade 3 in OA severity index. OA was accompanied by increases in the volume, thickness and contour of the cortical plate, alterations in the state of bone mineralization and material properties, changes in the subchondral trabecular bone architecture and bone mass, the formation of bone cysts, the appearance of osteophytes and joint space narrowing. Tilting with bone contact was also observed (Figure 3B, middle panel). Meanwhile, rats with OA treated with polymerized-type I collagen showed joints similar to control groups, avoiding the joint narrowing and preserving the articular structure (Figure 3B, right panel).
Figure 3. Macroscopic and radiographic characteristics of OA rat model. (A) Cartilage from training control group shows condyles with a white, smooth and glossy surface (left panel). Long-term OA cartilage display opalescent, opaque and rough surface condyles. Cartilage loss is also observed in some regions (black circle; middle panel). Long-term OA cartilage treated by 4 intraarticular administrations, once a week for 4 weeks, with 50 μL of polymerized-type I collagen (right panel). (B) Radiography of the right back knee showing the joint space (white arrow; left and right panel) and thecharacteristic narrowing of the joint space (white arrow; middle panel) chondrocalcinosis (orange arrow; middle panel).
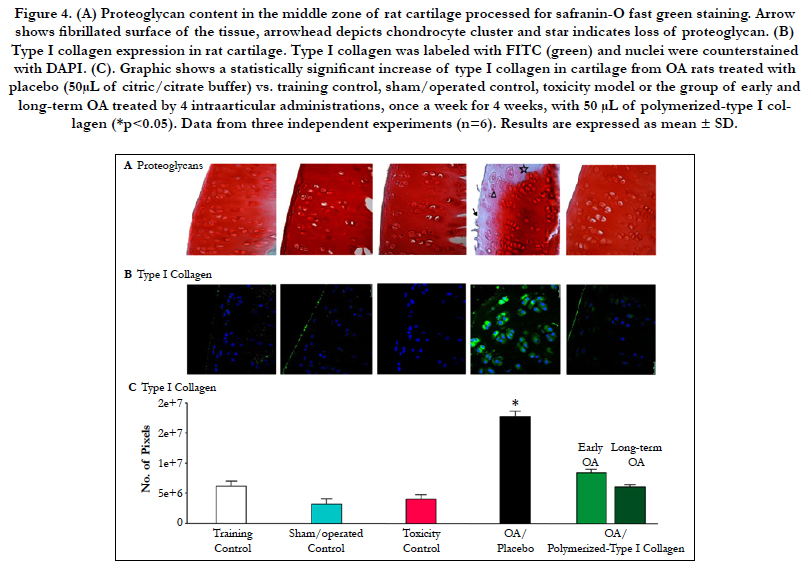
Knee cartilage from control groups (training, sham/operated and the toxicity assessment of polymerized-type I Collagen) showed the typical chondrocytes from the three main zones of the tissue, flattened chondrocytes from the superficial zone, the rounded secretory chondrocytes from the middle zone and hypertrophic chondrocytes from the deep zone. Expression of proteoglycans was observed homogeneous, covering the three zones of the cartilage Figure 4A).
OA cartilage in early disease in trained animals was thickened. Some chondrocytes from the superficial andupper-middle zones of the cartilage had started to contract, they had increased in number and were frequently organized in clusters. Changes in OA cartilage in long-term disease were more evident. The surface of the cartilage was observed with deep fibrillation and the superficial zone was lost in some regions of the cartilage. The number of fibroblasts and fibers tended to increase. Also, the number of chondrocytes diminished, whereas hypertrophic, densely-contracted chondrocytes and empty lacunae were increased. Furthermore, a progressive and severe reduction of proteoglycans content was observed. The extracellular matrix displayed a disorganized architecture and fibrillated surface. The tidemark was observed as a fuzzy region and in many cases it was not visible (Figure 4A).
The group of early and long-term OA treated with 50 μL of polymerized-type I collagen exhibited a cartilage of similar histological characteristics to those observed in the control groups (Figure 4A).
It is important to mention that contra-lateral leg of these trained rats did not show OA degeneration (data not shown).
The content of type I collagen in cartilage from control groups (training, sham/ operated and the toxicity assessment of polymerized-type I collagen) was scarce, as it was observed in immunofluorescence. Nonetheless, cartilage from rats with OA had a conspicuous number of type I collagen-expressing chondrocytes (Figure 4B and C). The group of early and long-term OA treated by 4 IA administrations of 50 μL of polymerized-type I collagen showed similar number of type I collagen-expressing chondrocytes to those observed in the control groups (Figure 4B and C).
Figure 4. (A) Proteoglycan content in the middle zone of rat cartilage processed for safranin-O fast green staining. Arrow shows fibrillated surface of the tissue, arrowhead depicts chondrocyte cluster and star indicates loss of proteoglycan. (B) Type I collagen expression in rat cartilage. Type I collagen was labeled with FITC (green) and nuclei were counterstained with DAPI. (C). Graphic shows a statistically significant increase of type I collagen in cartilage from OA rats treated with placebo (50μL of citric/citrate buffer) vs. training control, sham/operated control, toxicity model or the group of early and long-term OA treated by 4 intraarticular administrations, once a week for 4 weeks, with 50 μL of polymerized-type I collagen (*p<0.05). Data from three independent experiments (n=6). Results are expressed as mean ± SD.
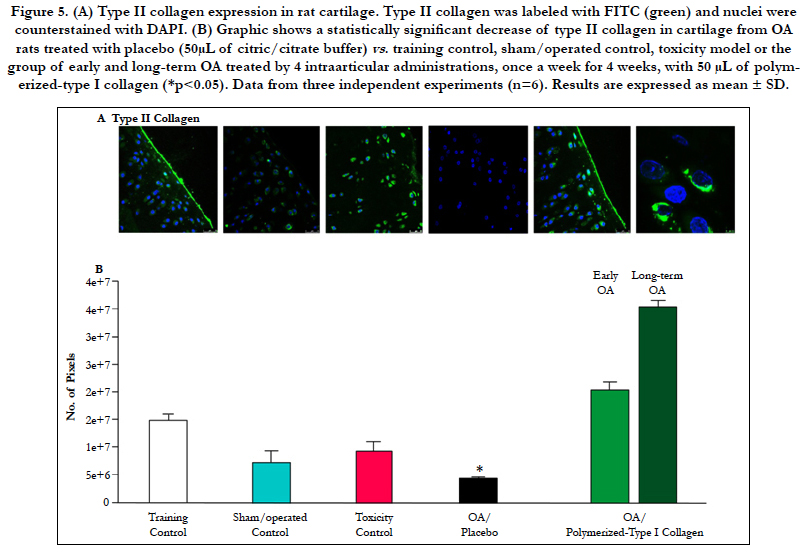
Type II collagen-expressing chondrocytes in cartilage from control groups (exercise, surgery/sham and the toxicity assessment of polymerizedtype I Collagen) were higher and statistically significant when compared with cartilage from rats with OA (Figure 5A and B). The group of early and long-term OA treated by 4 IA administrations of 50 μL of polymerized-type I collagen showed similar number of type II collagen-expressing chondrocytes to those observed in the control groups (Figure 5A and B).
Figure 5. (A) Type II collagen expression in rat cartilage. Type II collagen was labeled with FITC (green) and nuclei were counterstained with DAPI. (B) Graphic shows a statistically significant decrease of type II collagen in cartilage from OA rats treated with placebo (50μL of citric/citrate buffer) vs. training control, sham/operated control, toxicity model or the group of early and long-term OA treated by 4 intraarticular administrations, once a week for 4 weeks, with 50 μL of polymerized- type I collagen (*p<0.05). Data from three independent experiments (n=6). Results are expressed as mean ± SD.
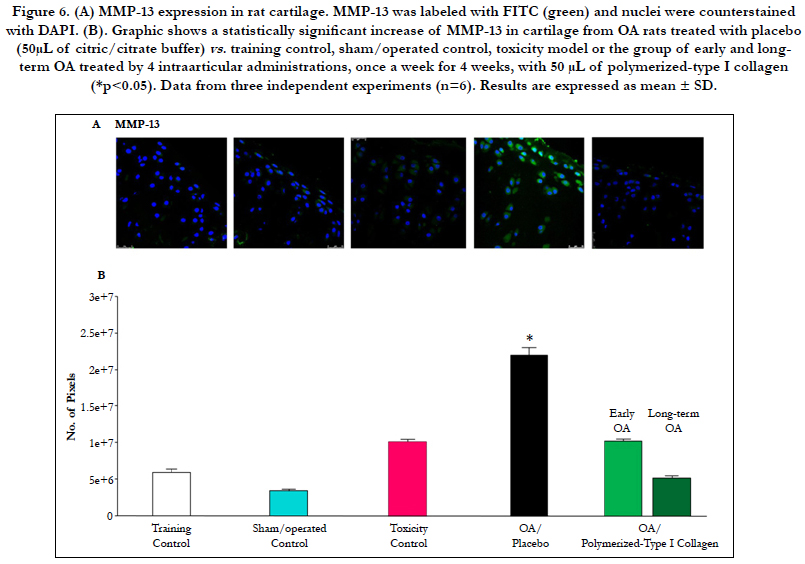
MMP13-expressing chondrocytes in cartilage from control groups (training, sham/operated and the toxicity assessment of polymerized-type I Collagen) were limited. Nonetheless, cartilage from rats with OA had a higher number of MMP13-expressing chondrocytes when compared with OA treated with polymerized-type I collagen (Figure 6A and B). The group of early and long-term OA treated with 50 μL of polymerized-type I collagen showed similar number of MMP13-expressing chondrocytes to those observed in the control groups (Figure 6A and B).
Figure 6. (A) MMP13 expression in rat cartilage. MMP13 was labeled with FITC (green) and nuclei were counterstained with DAPI. (B). Graphic shows a statistically significant increase of MMP13 in cartilage from OA rats treated with placebo (50μL of citric/citrate buffer) vs. training control, sham/operated control, toxicity model or the group of early and longterm OA treated by 4 intraarticular administrations, once a week for 4 weeks, with 50 μL of polymerized-type I collagen (*p<0.05). Data from three independent experiments (n=6). Results are expressed as mean ± SD.
There were no evidence of adverse events after the injection of placebo or polymerized-type I collagen with lingering effects still absent out to 13 weeks. Adverse events evaluation included determination of pseudoseptic reaction, crystalline arthritis, swelling, local skin reactions, etc. The only adverse event observed was pain lasting less than 15 min in the injection site.
Discussion
At the present time is widely accepted that OA results from an admixture of changes occurring throughout the joint tissues, including cartilage, synovium, ligament, and bone and that inflammation is a key player in the progression of cartilage destruction and joint disease [29, 30]. Increased levels of proinflammatory cytokines are frequently present in the synovial fluid and tissues of OA-affected joints, particularly, IL-1β and TNF-α directly contribute to reduced anabolic and enhanced catabolic activities in OA-affected joints through regulation of proteases expression that acts to degrade cartilage [2, 24, 31]. Hence, a chronic cycle of joint destruction ensues with inflammation, increasing proteolytic activity that changes local tissue mechanics and generates extracellular matrix fragment release, factors that further promote inflammation. Therapeutic agents aiming to interrupt or modify this local proinflammatory, catabolic joint environment are of great interest for the potential to alter the progression of joint destruction and reduce the functional and symptomatic consequences of OA [32].
Numerous disease-modifying OA drugs have been proposed. They are focus on antagonizing the production or activity of inflammatory mediators, such as IL-1β and TNF-α and proteolytic enzymes [33]. The goal of these compounds is to block or modify the local proinflammatory, such as MMP inhibitors, catabolic environment found in the OA affected joint [34, 35]. Although several advances have been made in the area of arthritis drug development, only a very small fraction of these have been investigated for local drug delivery applications in the treatment of osteoarthritis. Therefore, the present study outlines efficacy of IA injections of Polymerized-type I collagen in a rat model of early and long-term knee OA at clinical, radiological, histological, and molecular level. Four intraarticular administrations of the biodrug were an effective treatment modifying the progression of OA, inducing chondroprotection and a high quality cartilage repair (hyaline cartilage). Biodrug was innocuous as determined in the toxicity model. Polymerized-type I collagen was well tolerated during therapy and adverse events were not detected, except pain lasting less than 15 min in the injection site [36].
Clinical evaluation such as weight, joint thickness and temperature showed significant differences between the group with early and long-term OA treated with polymerized-type I collagen compared with the group with OA under placebo treatment. The weight was higher in the group of trained rats that underwent meniscectomy and were treated with polymerized-type I collagen. The increase in the weight of rats treated with the biodrug may be due to the reduction of the inflammatory process that is reflected in the animal's ability to move, to feed and in the general well-being. This is reinforced by the fact that the groups of treated rats had a lower thickness in the joint compared with the group of rats with OA. Furthermore, based on its role as inducer of tissue repair, polymerized-type I collagen reduced degenerative damages in the cartilage structure. Articular surface did show neither fibrillation nor irregularity. Additionally, less MMP13 and type I collagen content and the increase of immunoreactive cell percentage for type II collagen and proteoglycans in trained rats with OA under treatment with polymerized-type I collagen indicates a high quality cartilage repair (hyaline cartilage) [37]. In contrast, matrix degradation was found with regards to MMP13 immunolabeling increased in the superficial and the middle zone of OA cartilage [37]. The considerable reduction of proteoglycans in cartilage of trained rats with OA under placebo treatment was accompanied by presence of type I collagen inducing the substitution of hyaline cartilage by fibrohyaline cartilage.
Conclusion
Summing up, our data suggest that the biological role of polymerized-type I collagen is not only restricted to providing protection, lubrication and mechanical stability to the collagen network, cells and joint surfaces, but also to up-regulate the synthesis of highly sulphated proteoglycans and type II collagen and to downregulate type I collagen and collagenolytic activity.
Polymerized-type I collagen could be a successful treatment for OA, is a safe and effective for the treatment of early and longterm disease and that the improvement was sustained during follow up. It is a chondroprotective biodrug with disease-modifying effects. Polymerized-type I collagen induces high quality cartilage repair maintaining proteoglycan content, increasing type II collagen synthesis and decreasing type I collagen production and MMP13 expression. Adverse events (pseudoseptic reaction, crystalline arthritis, swelling, local skin reactions, etc.) were not detected, except pain lasting less than 15 min in the injection site.
Acknowledgments
We are grateful to Dr. Griselda Salmeron and Camila Rueda-Furuzawa for their assistance with animal handling.
References
- Hart DJ, Spector TD (1995) The classification and assessment of osteoarthritis. Baillieres Clin Rheumatol. 9(2): 407-432.
- Martel-Pelletier J, Barr AJ, Cicuttini FM, Conaghan PG, Cooper C, et al., (2016) Osteoarthritis. Nat Rev Dis Primers. 2: 16072.
- Cross M, Smith E, Hoy D, Nolte S, Ackerman I, et al., (2014) The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 73(7): 1323-1330.
- Azamar-Llamas D, Hernández-Molina G, Ramos-Ávalos B, Furuzawa-carballeda J (2017) Adipokine contribution to the pathogenesis of osteoarthritis. Med Inflamm. 2017: 546823.
- Furuzawa-Carballeda J, Macip-Rodríguez PM, Cabral AR (2008) Osteoarthritis and rheumatoid arthritis pannus have similar qualitative metabolic characteristics and pro-inflammatory cytokine response. Clin Exp Rheumatol. 26(4): 554-560.
- de Lange-Brokaar BJ, Ioan-Facsinay A, van Osch GJ, Zuurmond AM, Schoones J, et al., (2012) Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthritis Cartilage. 20(12): 1484-1499.
- Scanzello CR, Goldring SR (2012) The role of synovitis in osteoarthritis athogenesis. Bone. 51(2): 249–257.
- Felson DT, Niu J, Neogi T, Goggins J, Nevitt MC, et al., (2016) Synovitis and the risk of knee osteoarthritis: the MOST study. Osteoarthritis Cartilage. 24(3): 458-464.
- Kourí-Flores JB, Abbud-Lozoya KA, Roja-Morales L (2002) Kinetics of the ultrastructural changes in apoptotic chondrocytes from an Osteoarthrosis rat model: a window of comparison to the cellular mechanism of apoptosis in human chondrocytes. Ultrastruct Pathol. 26(1): 33-40.
- Almonte-Becerril M, Navarro-García F, González-Robles A, Vega-López MA, Lavalle C, et al., (2010) Cell death of chondrocytes is a combination between apoptosis and autophagy during the pathogenesis of Osteoarthritis within an experimental model. Apoptosis. 15(5): 631-638.
- Wojdasiewicz P, Poniatowski LA, Szukiewicz D (2014) The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 561459.
- Burgos-Vargas R, Cardiel MH, Loyola-Sánchez A, Mendes De Abreu M, Pons-Estel BA, et al., (2014) Characterization of knee osteoarthritis in Latin America. A comparative analysis of clinical and health care utilization in Argentina, Brazil, and Mexico. Rheumatol Clin. 10(3): 152-159.
- Smith SR, Deshpande BR, Collins JE, Katz JN, Losina E (2016) Comparative pain reduction of oral non-steroidal anti-inflammatory drugs and opioids for knee osteoarthritis: Systematic analytic review. Osteoarthritis Cartilage. 24(4): 962-972.
- Krötzsch-Gómez FE, Furuzawa-Carballeda J, Reyes Márquez R, Quiróz-Hernández E, Díaz de León L (1998) Cytokine expression is down regulated by collagen-polyvinylpyrrolidone in hypertrophic scars. J Invest Dermatol. 111(5): 828-834.
- Furuzawa-Carballeda J, Krötzsch-Gómez FE, Espinosa-Morales R, Alcalá M, Barile-Fabris L (2005) Subcutaneous administration of collagen-polyvinylpyrrolidone down-regulates IL-1beta, TNF-alpha, TGF-beta1, ELAM-1 and VCAM-1 expression in scleroderma skin lesions. Clin Exp Dermatol. 30(1): 83-86.
- Furuzawa-Carballeda J, Ortíz-Ávalos M, Lima G, Jurado-Santa Cruz F, Llorente L (2012) Subcutaneous administration of polymerized type I collagen downregulates interleukin (IL)-17A, IL-22 and transforming growth factor-β1 expression, and increases Foxp3-expressing cells in localized scleroderma. Clin Exp Dermatol. 37(6): 599-609.
- Furuzawa-Carballeda J, Alcocer-Varela J, Díaz de León L (1999) Collagen- PVP decreases collagen turnover in synovial tissue cultures from rheumatoid arthritis patients. Ann N Y Acad Sci. 878: 508-602.
- Furuzawa-Carballeda J, Rodríguez-Calderón R, Alcocer-Varela J, Díaz de León L (2002) Mediators of inflammation are down- regulated while apoptosis is up-regulated in rheumatoid arthritis synovial tissue by polymerizedcollagen. Clin Exp Immunol. 130(1): 140-149.
- Furuzawa-Carballeda J, Muñoz-Chable OA, Barrios-Payán J, Hernández- Pando R (2009) Effect of Polymerized-Type I Collagen in knee osteoarthritis. I. In vitro Study. Eur J Clin Invest. 39(7): 591- 597.
- Furuzawa-Carballeda J, Cabral AR, Zapata-Zuñiga M, Alcocer-Varela J (2003) Subcutaneous administration of polymerized-type I collagen for the treatment of patients with rheumatoid arthritis. An open-label pilot trial. J Rheumatol. 30(2): 256–259.
- Furuzawa-Carballeda J, Fenutria-Ausmequet R, Gil-Espinosa V, Lozano- Soto F, Teliz-Meneses MA, et al., (2006) Polymerized-type I collagen for the treatment of patients with rheumatoid arthritis. Effect of intramuscular administration in a double blind placebo-controlled clinical trial. Clin Exp Rheumatol. 24(5): 521–528.
- Abbud-Lozoya K, Kouri-Flores J (2000) A novel rat Osteoarthrosis model to assess apoptosis and matrix degradation. Pathol Res Pract. 196(11): 729–745.
- Kouri JB, Rojas L, Pérez E, Abbud-Lozoya KA (2002) Modifications of Golgi complex in chondrocytes from osteoarthritic (OA) rat cartilage. J Histochem Cytochem. 50(10): 1333–1340.
- Roja-Ortega M, Cruz R, Vega-López MA, Cabrera-González M, Hernández- Hernández MA, et al., (2015) Exercise modulates the expression of IL- 1β and IL-10 in the articular cartilage of normal and osteoarthritis-induced rats. Pathol Res Pract. 211(6): 435-443.
- Mazzuca SA, Brandt KD, German NC, Buckwalter KA, Lane KA, et al., (2003) Development of radiographic changes of osteoarthritis in the “Chingford knee” reflects progression of disease or non-standardised positioning of the joint rather than incident disease. Ann Rheum Dis. 62(11): 1061-1065.
- Kirivanta I, Jurvelin J, Tammi M, Säämänen A-M, Helminen HJ (1985) Microspectrophotometric quantitation of glycosaminoglycans in articular cartilage sections stained with Safranin O. Histochemistry. 82(3): 249-255.
- Gerwin N, Bendele AM, Glasson S, Carlson CS (2010) The OARSI histopathology initiative – recommendations for histological assessments of osteoarthritis in the rat. Osteoarthritis Cartilage. 18(2): S24-S34.
- Almonte-Becerril M, Costell M, Kouri JB (2014) Changes in the integrins expression are related with the osteoarthritis severity in an experimental animal model in rats. J Orthop Res. 32(9): 1161-1166.
- McMasters J, Poh S, Lin JB, Panitch A (2017) Delivery of anti-inflammatory peptides from hollow PEGylated poly(NIPAM) nanoparticles reduces inflammation in an ex vivo osteoarthritis model. J Control Release. 258: 161-170.
- Li YS, Luo W, Zhu SA, Lei GH (2017) T cells in osteoarthritis: alterations and beyond. Front Immunol. 8: 356.
- Furuzawa-Carballeda J, Alcocer-Varela J (1999) Interleukin-8, Interleukin-10, Intracellular Adhesion Molecule-1 and Vascular Cell Adhesion Molecule-1 Expression Levels are higher in Synovial Tissue from patients with Rheumatoid Arthritis than in Osteoarthritis. Scand J Immunol. 50 (2): 215-222.
- Allen KD, Adams SB, Setton LA (2010) Evaluating intra-articular drug delivery for the treatment of osteoarthritis in a rat model. Tissue Eng Part B Rev. 16(1): 81-92.
- Mobasheri A (2013) The future of osteoarthritis therapeutics: emerging biological therapy. Curr Rheumatol Rep. 15(12): 385.
- Mapp PI, Walsh DA, Bowyer J, Maciewicz RA (2010) Effects of a metalloproteinase inhibitor on osteochondral angiogenesis, chondropathy and pain behavior in a rat model of osteoarthritis. Osteoarthritis Cartilage. 18(4): 593-600.
- Azizi S, Kheirandish R, Farsinejad A, Rasouli N (2017) The effect of intraarticular serum rich in growth factors (SRGF) on knee osteoarthritis in the rat model. Transfus Apher Sci. 56(3): 371-375.
- Furuzawa-Carballeda J, Rojas E, Valverde M, Castillo I, Díaz de León L, et al., (2003) Cellular and humoral responses to collagen-polyvinylpyrrolidone administered during short and long periods in humans. Can J Physiol Pharmacol. 818(11): 1029-1035.
- Roberts S, Menage J, Sandell LJ, Evans EH, Richardson JB (2009) Immunohistochemical study of collagen types I and II and procollagen IIA in human cartilage repair tissue following autologous chondrocyte implantation. Knee. 16(5): 398-404.
- Li Z, Liu B, Zhao D, Wang B, Liu Y, et al., (2017) Protective effects of Nebivolol against interleukin-1β (IL-1β)-induced type II collagen destruction mediated by matrix metalloproteinase-13 (MMP-13). Cell Stress Chaperones. Doi: 10.1007/s12192-017-0805-x.