Sedation for Upper Gastrointestinal Endoscopy, whatever the Agents does it Matter
Walid Y. Kamel1*, Elbaz AA2, Shimaa Y. Kamel2
1 Department of Anesthesia, Intensive Care and Pain Management, Ain Shams University, Abbasiya, Cairo, Egypt.
2 Tropical Medicine, Gastroenterology, and Hepatology; Ain Shams University, Abbasiya, Cairo, Egypt.
*Corresponding Author
Walid Y. Kamel, MD,
Department of Anesthesia, Intensive Care and Pain Management,
Ain Shams University, Abbasiya, Cairo, Egypt.
Tel: 00201006305703
E-mail: walid_yousofkamel@yahoo.com
Received: December 14, 2019; Accepted: January 06, 2020; Published: January 07, 2020
Citation: Walid Y. Kamel, Elbaz AA, Shimaa Y. Kamel. Sedation for Upper Gastrointestinal Endoscopy, whatever the Agents does it Matter. Int J Anesth Res. 2020;S1:001:1-5. doi: dx.doi.org/10.19070/2332-2780-SI01001
Copyright: Walid Y. Kamel© 2020. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Propofol is an essential agent for sedation for GI endoscopy. Opioids are administered during endoscopy to provide analgesia, suppress cough, and reduce the dose of propofol. Opioids with rapid onset and short duration are generally preferred. The most commonly used opioids in this setting is fentanyl. Ketamine also produces a dissociative state with amnesia, intense analgesia and minimal respiratory depression at sedative doses which may be administered along with propofol.
The aim of study is to compare Propofol-ketamine vs propofol-fentanyl combinations for sedation during gastrointestinal endoscopy to reach a safe and satisfactory level of sedation for patients and operators with the least effective drugs as being a day case procedure with fast track criteria.
Methods: The study was conducted on 88 patients scheduled for diagnostic upper endoscopy. In group K; the patients in this group were injected with a combination of ketamine 0.25 mg/kg and propofol 1 mg/kg. In group F, the patients in this group were injected with a combination of Fentanyl 1ug/kg and propofol 1 mg/kg.
Results: The mean procedure time in the 2 groups was (13 min ± 4.7 min in group K vs 11.76 min ± 3.8 min in group F). The volume of the drug injected was 12.3 ± 3.63 ml in group K vs 13.29 ± 4.2 ml in group F to achieve the targeted level of sedation, however the level of sedation was significantly higher in the fentofol group (RASS -5 in 20% vs. 84.4% in group K and F respectively). The level of sedation was achieved in shorter time in group K (10.8 ± 4.5 min) vs. (12.62 ± 2.92 min) in group F. The total top up dose was significantly lower in group F vs group K (6.3 ± 4.17 ml vs. 8.2 ± 3.84 ml respectively). The recovery time was significantly earlier in group F than in group K (1.83 ± 2.08 min vs. 3.5± 2.64 min). The patient’s satisfaction as well as the operator satisfaction was much higher in group F than in group K.
Discussion: The recurrence reports vary in the literature. The most recent review by Garcia-Cano E, et al., showed no recurrence whatsoever; Ramer and colleagues reported only 5 cases of recurrence. However, only 39 cases out of 68 were followed up. In this systematic review out of 84 patients, 4 (4.7%) presented recurrence. From these, 3 cases were treated by enucleation and one by curettage.
Conclusion: Fentanyl-propofol combination in the aforementioned dose is a good choice for upper GI endoscopy with a little hemodynamic change, respiratory events and earlier patients discharge.
2.Introduction
3.Aim of Study
4.Patients and Methods
4.1 Statistical analysis
5.Results
6.Discussion
7.Conclusion
8.References
Keywords
Sedation; Propofol; Ketamine; Fentanyl; Gastrointestinal Endoscopy.
Introduction
There has been a rapid increase in the gastrointestinal (GI) endoscopic procedures performed during the last decade [1].
The role of anesthetist ranges from providing anesthesia for procedures that require sedation or general anesthesia to provide only monitoring for patients with significant co-morbidities, for whom advanced endoscopic procedures are often performed as alternatives to open surgery [2].
The patient undergoing gastrointestinal endoscopy should be assessed for conditions that increase sensitivity to sedative and analgesic medications (e.g., older age; obstructive sleep apnea; advanced chronic lung disease; pulmonary hypertension; coronary artery, liver, or renal diseases; anxiety disorders; chronic pain; use of opioids, sedatives, or recreational drugs) to allow appropriate drug dosing and administration [2].
Patients should follow the preoperative fasting guidelines. For those with impaired gastric emptying or with a high risk of aspiration and in emergencies situations, the potential for aspiration makes the endotracheal intubation the best choice for those patients. However, some clinicians intubate all or most patients who undergo complex endoscopic procedures (e.g., endoscopic retrograde cholangiopancreatography (ERCP) [3].
Moderate or deep sedation is commonly used for patients without risk factors for aspiration. Deep sedation can easily become general anesthesia, whenever propofol is administered [4].
Moderate sedation refers to a level of sedation in which patients respond purposefully to verbal commands and maintain spontaneous ventilation without support. Patients under deep sedation cannot be easily aroused, but respond purposefully to painful stimulation, and may require assistance in maintaining a patent airway [2].
The depth of sedation may affect the rate of complications during GI endoscopy, with deeper sedation (usually with propofol) [4], there is increased risk of respiratory and cardiopulmonary complications [5, 6], as well as a risk of colonic perforation during colonoscopy [7].
Respiratory events, including hypoxemia, hypercarbia, and respiratory arrest, are among the most common complications of anesthesia for GI endoscopy. So, ventilation should be monitored with capnography especially during moderate or deep sedation. Capnography facilitates early detection of apnea and airway obstruction [8], predicts the development of hypoxemia and may reduce patient injury related to respiratory depression [9].
The medications used for GI endoscopy should be based on patient factors, clinician preference and experience, the depth of sedation, the pharmacodynamic of the drugs used [2].
Propofol is an essential agent for sedation for GI endoscopy. Advantages of propofol are its rapid effect, short elimination half-time even after prolonged infusion, rapid recovery without residual psychomotor effects and improved patient satisfaction during endoscopy, compared with standard sedation. In addition, nausea and vomiting were less common and it is efficient in case of difficulty with sedation with other medications [3, 9].
However, propofol has a narrow therapeutic index (i.e. patients may rapidly have a deeper level of sedation or even general anesthesia with its consequence as apnea, airway obstruction, hypoxemia, and/or hypotension). In addition, the depth of sedation may be unpredictable, especially in older patients, and if opioids are added [10, 11].
Opioids are administered during endoscopy to provide analgesia, suppress cough, and reduce the dose of propofol. Opioids with rapid onset and short duration are generally preferred. The most commonly used opioids in this setting are fentanyl and remifentanil. Fentanyl is typically administered in small, intermittent IV boluses of 50 to 100 mcg, with reduced doses in elderly [12].
Ketamine produces a dissociative state with amnesia, intense analgesia and minimal respiratory depression at sedative doses (ketamine 0.25 to 0.5 mg/kg IV). This small dose may be administered along with propofol [13] or dexmedetomidine [14] to reduce the required doses and cardiovascular effects of those medications, enhance analgesia and reduce the need for opioids.
Aim of Study
The aim is to compare of Propofol-ketamine vs propofol-fentanyl combinations for sedation during gastrointestinal endoscopy to reach a safe and satisfactory level of sedation for patients and operators with the least effective drugs as being a day case procedure with fast track criteria.
Patients and Methods
The study is a double blinded clinical trial that was conducted in Ain shams University hospital on 90 patients scheduled for diagnostic upper endoscopy. The study was approved by the medical ethics committee and conducted in accordance with the principles of the declaration of Helsinki. All patients provided written informed consent before enrolment. Any patient with risk of aspiration, obstructive sleep apnea was excluded from the study. The patients were randomly divided into two equal groups group K and group F.
The patients in the two groups laid down in supine position, the standard monitors including ECG, pulse oximeter, non-invasive blood pressure and capnography were attached to the patients. 20 G cannula is inserted. The patients are then asked to lie in the lateral position.
In group K; the patients in this group were injected with a combination of ketamine 0.25 mg/kg (Ketalar Pfizer 50mg/ml) and propofol 1 mg/kg (Deprivan Astra Zenica 1%).
In group F, the patients in this group were injected with a combination of Fentanyl 1ug/kg (fentanyl citrate janssen 100mcg/2ml) and propofol 1 mg/kg (Deprivan Astra Zenica 1%).
The medications in both group were injected by anesthetists. The time interval from the induction till the accepted level of sedation were recorded, the accepted level of sedation was RASS ≤ -4 (Table 1). If the accepted level of sedation was not achieved within 2 minutes from the induction another 50 mg propofol were given or whenever the level of sedation decreased to an extent interfering with the continuation of the process and the total doses were recorded.
Monitoring and supplemental oxygen (O2) should be maintained during the patient recovery from the effects of the sedative. Patient should be transferred to phase I recovery area (postanesthesia care unit [PACU]) for early detection of respiratory or cardiovascular compromise, whether they have received general anesthesia or sedation. Patients who have completely recovered (i.e., breathing spontaneously without airway support, alert, speaking, obeying, and hemodynamically stable) can be fasttracked to the phase II recovery area (predischarge unit).
The time of the procedure was recorded as well as the time of full recovery (the time by which the patients become alert and obeying), any mishaps during the procedure were recorded including respiratory events (apnea and subsequent desaturation, laryngeospasm, aspiration) and hemodynamic instability.
The patients were then discharged to the recovery room and the patients’ satisfaction as well as the operator’s satisfaction and the time interval for discharge were also recorded. the patients and the operator’s satisfaction were recorded by yes/no questions of 4 questions, whenever the score is equal to or more than 3, the patients/ operators are reported as being satisfied. The stability of the haemodynamics was given a point for each the intraoperative and the postoperative (no more than 15% of the base line).
Statistical analysis was done using Statistical package for Social Science program version 20. (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp). Quantitative variables are expressed as mean and SD or as median and interquartile range (IQR) in cases of nonparametric variables. Student t test or Mann Whitney Test were used to compare a continuous variable between two study groups according to data distribution. Chi square and Fisher’s exact tests were used to examine the relationship between categorical variables. P-value < 0.05 was considered statistically significant.
Results
There is no significant statistical difference in the geographic data among the patients in the two groups regarding the gender, age groups as well as the ASA classification as shown in Table (2).
The mean procedure time in the 2 groups was (13 min ± 4.7 min in group K vs 11.76 min ± 3.8 min in group F). The volume of the drug injected was 12.3 ± 3.63 ml in group K vs 13.29 ± 4.2 ml in group F to achieve the targeted level of sedation, however the level of sedation was significantly higher in the fentofol group (RASS -5 in 20% vs. 84.4% in group K and F respectively).
The level of sedation was achieved in shorter time in group K (10.8 ±4.5 min) vs. (12.62 ± 2.92 min) in group F.
The coughing and gaging reflex were significantly abolished in group F than in K. The total top up dose was significantly lower in group F vs group K (6.3 ± 4.17 ml vs. 8.2 ± 3.84 ml respectively). There was no significant statistical change in the oxygen saturation, hemodynamic abnormalities, respiratory events in the form of obstruction, aspiration…etc. or other adverse events apart.
The recovery time was significantly earlier in group F than in group K (1.83 ± 2.08 min vs. 3.5 ± 2.64 min). All the patients were discharged within the first 2 hour from the end of the procedures in both groups Table (3).
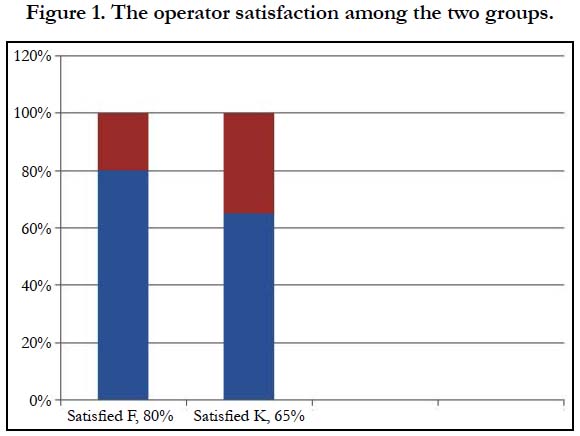
The patient’s satisfaction as well as the operator satisfaction was much higher in group F than in group K (Table 3 & Figure 1).
Discussion
Anesthesia for GI endoscopy may be associated with a higher risk of complications than anesthesia for many other procedures. The reasons for this association are multifactorial, and likely include the fact that most of these procedures are performed in out-ofoperating room locations, increasingly complicated procedures and patients, and the anesthetic technique. Many complications of sedation for GI endoscopy involve respiratory events; cardiac arrest is most commonly preceded by hypoxemia.
In practice, propofol is often combined with a low dose of midazolam to enhance amnesia, as well as a low dose of opioid (e.g. fentanyl or remifentanil) to provide analgesia and suppress cough. Moderate or deep sedation is commonly used for patients without risk factors for aspiration [4]. With moderate sedation, the patients respond purposefully to verbal commands and maintain spontaneous ventilation while at deep sedation the patients can’t be easily aroused but respond purposefully to painful stimulation and usually require assistance in maintaining a patent airway.
Respiratory event including hypoxemia, hypercarbia, and respiratory arrest, are the most common complications of anesthesia for GI endoscopy. Monitoring with capnography during moderate or deep sedation is mandatory for early detection of apnea and airway obstruction [8], predicts the development of hypoxemia [15].
The total top up dose was significantly lower in group F vs group K (6.3 ± 4.17 ml vs 8.2 ± 3.84 ml respectively). This results coincide with a study where the combination of fentanyl (1 mcg/ kg IV) or remifentanil (0.05 mcg/kg/min IV) with propofol (1.5 mg/kg IV followed by 1 mg/kg/hour IV) for sedation/ analgesia for ERCP reduced the required dose of propofol and increased hemodynamic stability, compared with propofol alone [6]. But this were against the results of a study done by Riham H & Wael S where the total dose of propofol needed to achieve a deep sedation level was lower in the ketofol group (57.71 ± 16.97) than in the fentanyl-propofol group (97.08 ± 23.31), which contributed to the lower incidence of propofol sedation-related adverse effects [16].
Despite an out-of-operating room, location is a risk factor for complications of anesthesia. Contributing factors may include unfamiliar procedure rooms and personnel, inadequate availability and space for routine anesthesia equipment, a dark environment, and inadequate monitoring. But different retrospective reviews and analysis of malpractice claims had reported that most complications result from over sedation and inadequate oxygenation during monitored anesthesia care [17].
The current study reveals that the rate of complications was almost the same in the two groups. there is no difference in the hemodynamic abnormalities or respiratory events in the form of obstruction, aspiration …etc.
The recovery time was significantly earlier in group F (1.83 ± 2.08 min vs 3.5 ± 2.64 min). This result were going with the results of the study done by Riham H & Wael S where the recovery time and time to discharge from the recovery room in the ketofol group (11.19 ± 2.59) and (13.28 ± 5.14), was slightly longer than that of group fentanyl-propofol (9.43 ± 1.23) and (12.58 ± 5.41) [16].
The patient’s satisfaction as well as the operator satisfaction was much higher in group F than in group K. All the patients were discharged within 2 hours from the end of the procedures in both groups.
Conclusion
The choice of the agents used for sedation is better to be tailored based upon the procedure as Fentanyl-propofol combination in the aforementioned dose is a good choice for upper GI endoscopy with a little hemodynamic change, respiratory events and earlier patients discharge.
References
- Goudra BG, Singh PM, Penugonda LC, Speck RM, Sinha AC. Significantly reduced hypoxemic events in morbidly obese patients undergoing gastrointestinal endoscopy: Predictors and practice effect. J Anaesthesiol Clin Pharmacol. 2014 Jan;30(1):71-7. PubMed PMID: 24574597.
- Basavana G, Anesthesia for gastrointestinal endoscopy in adults, uptodate, 2018.
- Singh H, Poluha W, Cheung M, Choptain N, Baron KI, Taback SP. Propofol for sedation during colonoscopy. Cochrane Database Syst Rev. 2008 Oct 8;(4):CD006268. PubMed PMID: 18843709.
- Goudra B, Singh PM, Gouda G, Borle A, Carlin A, Yadwad A. Propofol and non-propofol based sedation for outpatient colonoscopy-prospective comparison of depth of sedation using an EEG based SEDLine monitor. J Clin Monit Comput. 2016 Oct;30(5):551-7. PubMed PMID: 26364193.
- Goudra B, Nuzat A, Singh PM, Borle A, Carlin A, Gouda G. Association between Type of Sedation and the Adverse Events Associated with Gastrointestinal Endoscopy: An Analysis of 5 Years' Data from a Tertiary Center in the USA. Clin Endosc 2017; 50:161. PubMed PMID: 27126387.
- Goudra B, Nuzat A, Singh PM, Gouda GB, Carlin A, Manjunath AK. Cardiac arrests in patients undergoing gastrointestinal endoscopy: A retrospective analysis of 73,029 procedures. Saudi J Gastroenterol. 2015 Nov-Dec;21(6):400-11. PubMed PMID: 26655137.
- Wernli KJ, Brenner AT, Rutter CM, Inadomi JM. Risks Associated with Anesthesia Services During Colonoscopy. Gastroenterology. 2016 Apr;150(4):888-94. PubMed PMID: 26709032.
- Srinivasa V, Kodali BS. Capnometry in the spontaneously breathing patient. Curr Opin Anaesthesiol. 2004 Dec;17(6):517-20. PubMed PMID: 17031088.
- Padmanabhan A, Frangopoulos C, Shaffer LET. Patient Satisfaction with Propofol for Outpatient Colonoscopy: A Prospective, Randomized, Double- Blind Study. Dis Colon Rectum. 2017 Oct;60(10):1102-1108. PubMed PMID: 28891855.
- Vuyk JT. TCI: supplementation and drug interactions. Anaesthesia. 1998 Apr;53:35-41.
- Vuyk J. Pharmacokinetic and pharmacodynamic interactions between opioids and propofol. J Clin Anesth. 1997 Sep;9(6 Suppl):23S-26S. PubMed PMID: 9278851.
- Haytural C, Aydınlı B, Demir B, Bozkurt E, Parlak E, Dişibeyaz S, et al. Comparison of Propofol, Propofol-Remifentanil, and Propofol-Fentanyl Administrations with Each Other Used for the Sedation of Patients to Undergo ERCP. Biomed Res Int. 2015; 2015:465465. PMID: 26576424.
- Akhondzadeh R, Ghomeishi A, Nesioonpour S, Nourizade S. A comparison between the effects of propofol-fentanyl with propofol-ketamine for sedation in patients undergoing endoscopic retrograde cholangiopancreatography outside the operating room. Biomed J. 2016 Apr; 39(2):145-9. PMID: 27372170.
- Goyal R, Hasnain S, Mittal S, Shreevastava S. A randomized, controlled trial to compare the efficacy and safety profile of a dexmedetomidine-ketamine combination with a propofol-fentanyl combination for ERCP. Gastrointest Endosc. 2016 May; 83(5): 928-33. PMID: 26364968.
- Bhananker SM, Posner KL, Cheney FW, Caplan RA, Lee LA, Domino KB. Injury and liability associated with monitored anesthesia care: a closed claims analysis. Anesthesiology. 2006 Feb;104(2): 228-34. PMID: 16436839.
- Hasanein R, El-Sayed W. Ketamine/propofol versus fentanyl/propofol for sedating obese patients undergoing endoscopic retrograde cholangiopancreatography (ERCP). Egyptian Journal of Anaesthesia. 2013 Jul 1; 29(3): 207-11.
- Metzner J, Posner KL, Domino KB. The risk and safety of anesthesia at remote locations: the US closed claims analysis. Curr Opin Anaesthesiol. 2009 Aug; 22(4):502-8. PMID: 19506473.