A Novel Device for Alleviating Postoperative Transient Xerostomia: Analysis of Patient-reported Outcome Measures
Zion Zibly1,2, Ehud Nagler3, Ari Zimran4, Alexander Ioscovich5,*
1 Department of Neurosurgery, Sheba Medical Center, Ramat Gan,5262000, Israel.
2 Raphael Hospital, Tel Aviv, 6158101, Israel.
3 IMD tech, Ltd, Tivon, 3604442, Israel.
4 Gaucher Clinic, ShaareZedek Medical Center, Jerusalem, 9103102, Israel.
5 Department of Anesthesiology, Perioperative Medicine, and Pain Treatment, ShaareZedek Medical Center, and Faculty of Medicine, Hebrew University of Jerusalem, 9103102, Israel.
*Corresponding Author
Alexander Ioscovich,
Department of Anesthesiology, perioperative medicine, and pain treatment, ShaareZedek Medical Center, and Faculty of Medicine, Hebrew University of Jerusalem, 9103102, Israel.
Tel: 972-50-8685052
Fax: 972-2-6555003
E-mail: aioscovich@gmail.com
Received: June 19, 2022; Accepted: October 05, 2022; Published: October 25, 2022
Citation: Zion Zibly, Ehud Nagler, Ari Zimran, Alexander Ioscovich. A Novel Device for Alleviating Postoperative Transient Xerostomia: Analysis of Patient-reported Outcome Measures. Int J Anesth Res. 2022;10(2):676-679.
Copyright: Alexander Ioscovich© 2022. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Postoperative transient xerostomia (dry mouth) is a very common phenomenon after general anesthesia, yet, no
adequate treatment is currently available. Lipsus, an FDA-approved disposableface-mounted device, was shown in a proof-ofconcept
study involving 10 patients to be safe and effective in alleviating such xerostomia. The goal of the current audit was to
investigate the effectiveness and safety of this device in a larger cohort in real-life clinical practice.
Methods: This observational audit included patients(n= 62) who underwent surgery, used the device as part of postoperative
care, and reported their xerostomia-associated discomfort before and after mounting the device using a 0-10 scale (0, no
discomfort; 10, unbearable discomfort).
Results: The median (range) age of the patients was 50 (15-77) years, 54.8% were women, and the most common surgery
was knee replacement (19.4%). Before mounting the device, 53.3% of the patients characterized their xerostomia-associated
discomfort as 10, and the mean (SD) level of reported discomfort was 8.5 (2.1). Within 15 minutes of mounting the device,
the mean (SD) dropped to 0.05 (0.2), and within 30 minutes to 0.03 (0.2). From 45 minutes on, all patients characterized their
discomfort as 0 (none).The device was safe; no adverse events were reported and no patient requested to remove it.
Conclusion: The Lipsus device is a safe and effective modalityfor alleviating postoperative transient xerostomia.
2.Introduction
3.Methodology
4.Results
5.Discussions
6.Conclusion
7.Acknowledgments
8.References
Keywords
Anesthesia; Complication; Dry Mouth; Postoperative; Xerostomia.
Introduction
Xerostomia (defined as a subjective feeling of oral dryness) has
numerous etiologies. These include adverse effects of certain
medications, radiotherapy to the head and neck, and certain autoimmune
disorders (e.g., Sjogren's syndrome, diabetes mellitus)
(reviewed in [1]). The main goal of xerostomia management is
symptom relief along with addressing the underlying cause, if
possible. Currently, treatment modalities for xerostomia include
mainly local measures such as sprays, lozenges, gels, frequent
sips of water, chewing sugar-free gum, etc [1]. In addition, a few
sialogogue drugs are currently available for xerostomia management
(e.g., pilocarpine and cevimeline), and transcutaneous electrical
nerve stimulation (TENS) has also been assessed as a treatment
modality for this condition [1, 2].
Transient xerostomia is an extremely common phenomenon in
the peri and postoperative setting occurring (at some level) in the
majority of patients [3-5]. Multiple factors potentially contribute
to postoperative xerostomia including abstaining from food/water
prior to, during, and for several hours after surgery, the use
of certain anesthetic and analgetic drugs, as well as methods of
airway management (e.g., face mask, laryngeal mask airway, and
tracheal intubation)[6, 7]. Although transient xerostomia could be as uncomfortable as non-transient xerostomia, it is not adequately
addressed by current treatment modalities. The only treatment
approach used in routine clinical practice is temporarily moistening
the lips and oral mucous membranes with warm water by
the nursing staff, who may not be immediately available to assist.
Thus, transient postoperative xerostomia clearly constitutes an
unmet clinical need.
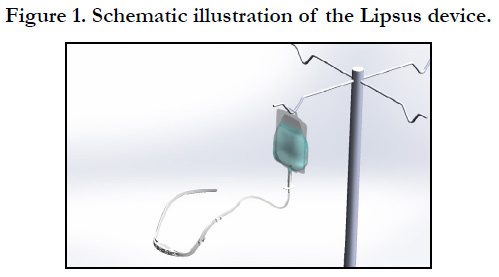
We previously described a novel treatment modality for postoperative
transient xerostomia, named the Lipsus® device (Figure
1). It is a disposable device (up to 24 h use) mounted on the face
of the patient after surgery like spectacles. The front part of the
device is made from a soft cloth and is placed above the patient's
lips touching or almost touching them. A plastic bag (300 mL)
supplies a low water flow rate (up to 12 mL/h) to the cloth. The
flow rate is low, so only the lips are wet and the patient can speak
freely [8]. In a proof-of-concept study, the device was applied in
10 patients post operatively and was found to be effective and
with no adverse events [8]. The device received approval by the
United States Food and Drug Administration (FDA) and a CE
certification in the EU (both in 2019).
The objective of the current study was to evaluate the safety and
effectiveness of the Lipsus device in alleviating transient postoperative
xerostomia, and to assess the feasibility of its use in a
hospital setting.
Methods
This observational audit involved patients who underwent surgery
in Raphael hospital between December 2020 and January
2021, and for whom Lipsus was used to alleviate postoperative
xerostomia as part of routine patient care. Since Lipsus was used
as part of its introduction into the hospital setting, patients were
asked about their experience with it and their responses were
documented. Patients were asked to indicate their level of discomfort
from xerostomia using a scale of 0 (no discomfort) to
10 (most severediscomfort), at the recovery room(post anesthesia
care unit) immediately before the Lipsus device was mounted,
and 15, 30, 45, 60, 90, 120, 180, and 240 minutes thereafter, or
until theywere able to drink. Patients were also asked about their
overall impressionof the device, using a 1 (minimally helpful) to
10 (very helpful) scale. Descriptive statistics was used to analyze
the results.
Ethical review and approval were waived for this study, as this
was an audit of pre-collected data related to FDA/CE approved
device in standard-of-care commercial use.
Results
Patient Characteristics
Overall, 62 patients are included in the current analysis. Patient
demographics are presented in Table 1. The majority of patients
were women (54.8%), the median (range) age was 50 (15-77)
years. Approximately a third of the patients were 21-40 years of
age and another third were 41-60 years of age. The cohort included
patients who underwent a variety of surgery types. The
most common type was knee replacement (19.4%), followed by
spinal surgery (16.1%), and laparoscopic cholecystectomy (9.7%).
Effect of the Lipsus device on xerostomia
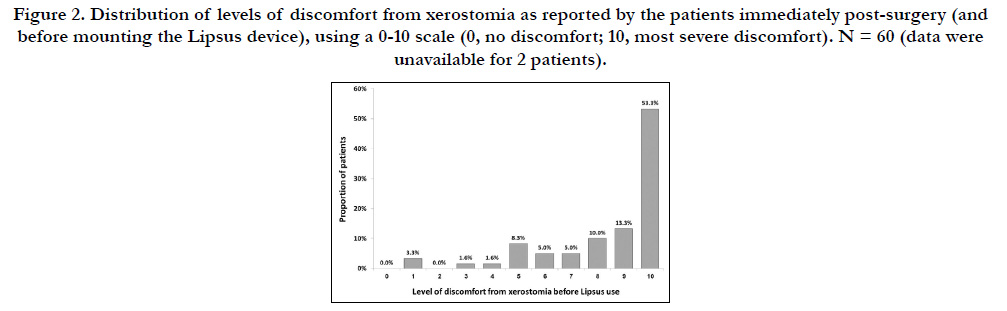
Data on the level of discomfort from postoperative xerostomia
before mounting the Lipsus device was available for 60 patients.
Thirty-two patients (53.3%) characterized their level of discomfort
as 10 (in a scale of 0-10 where 10 is the most severe discomfort
and 0 is no discomfort), 8 patients (13.3%) characterized it
as 9, and 6 (10.0%) characterized it as 8. The rest of the patients
characterized their level of discomfort between 7 and 1; none
characterized it as 0 (Figure 2).
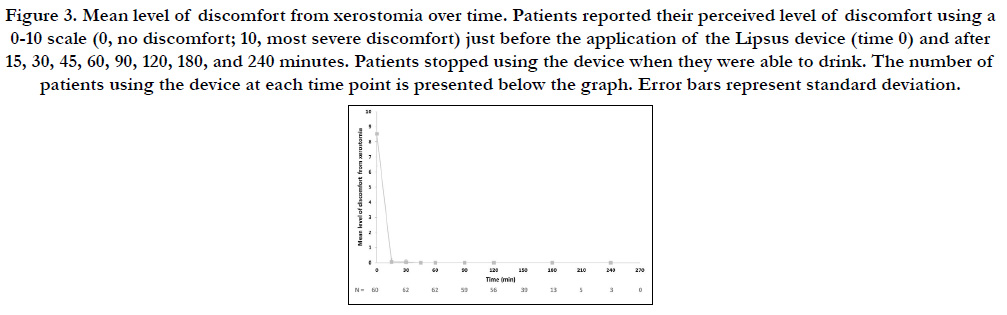
The mean (SD) level of discomfortimmediatelybefore mounting
the Lipsus device was 8.5 (2.1) (Figure 2, time 0). Within 15 minutes,
the level of discomfort dropped to a mean (SD) of 0.05
(0.2), and within 30 minutes it dropped further to a mean (SD)
of 0.03 (0.2). From 45 minutes on, all patients characterized their
discomfort as 0 (none). The number of patients using the Lipsus
device dropped over time (from 60 minutes on), as patients
started to drink normally (Figure 3).
No adverse events (e.g., irritation, allergic reaction) were reported
and no patientrequested to remove the Lipsus device.
Lastly, when asked to grade their overall impression from the device
(0-10 scale; 0, minimally helpful; 10, very helpful), 50 patients
(80.6%) graded it as 10; 2 (3.2%) as 9; 1 each (1.6%) graded it as
8 and 6, and 2 (3.2%) as 5. Responses were not available for 6
patients (9.7%).
Discussion
This observational audit demonstrated the feasibility, safety, and
effectiveness of the Lipsus device in alleviating postoperative
transient xerostomia. The findings also suggest that the device
alleviates xerostomia very quickly (within 15 minutes of mounting
it).
Notably, although postoperative xerostomia is transient, the discomfort
it causes can be substantial, as shown in the current report.
Furthermore, xerostomia could impact communication due
to difficulties in pronunciation and voice production [9] and thus
could lead to frustration, as patients may not be able to communicate
their needs clearly after their surgery.
Currently, postoperative transient xerostomia constitutes an unmet
clinical need, as the phenomenon is extremely common, yet
the routine treatment (moistening the lips/oral mucous membranes
with warm water by the nursing staff) is inadequate and
no other treatment modalities are available.To the best of our
knowledge, the only other treatment modality evaluated thus far
is a spray derived from Phyllanthus embilica (Indian gooseberry),
which was shown in a recently published randomized controlled
trial involving 64 female patients after gynecologic surgery under
general anesthesia to be significantly more effective than warm
water spray for treating postoperative xerostomia [4].
The results of our findings are consistent with an earlier proofof-
conceptstudy showing that Lipsus was safe and effective in alleviating
postoperative transient xerostomia in 10 patients (aged
21-77 years) [8]. Notably, in theproof-of-concept study, the first
time-point after mounting the Lipsus device was 60 minutes [8].
The current analysis, in which the first evaluated time-point was
15 minutesafter mounting the device, demonstrates that the device
alleviates xerostomia quickly.
Notably, healthcare-acquired infections (HAIs) constitute a major
challenge in the healthcare system, and even more so since
theemergence of the COVID-19 pandemic [10] The main HAIprevention
strategy involves increasing hand hygiene [11]. Thus,
an automated device that addresses postoperative xerostomia
without hand contact between the patient and the nursing staff
could also, indirectly, support HAI prevention.
The study is limited by the relatively small sample size, and its
unrandomized design. Also, this study evaluated the subjective
sensation of "dry mouth" as reported by the patients and no objective
measures (e.g., salivary flow, oral mucosa moisture [12])
were evaluated.
In conclusion, the Lipsus device is a safe and effective approach
to alleviating postoperative transient xerostomia.
Acknowledgments: The authors thank Avital Bareket-Samish,
PhD, for medical writing assistance which was funded by Shahak-
Tec Ltd.
Conflicts of Interest: Zion Zibly declares no conflict of interest.
Ehud Naglerreports having stock ownership and intellectual
property interest in IDM tech, Ltd. Ari Zimran and Alexander
Ioscovich declare having stock ownership in IDM tech Ltd.
Funding: This research was funded by Shahak-Tec Ltd.
References
- Talha B, Swarnkar SA, Xerostomia. In Statpearls, Treasure Island (FL), 2021.
- Sivaramakrishnan G, Sridharan K. Electrical nerve stimulation for xerostomia: A meta-analysis of randomised controlled trials. J Tradit Complement Med. 2017 Feb 14;7(4):409-413. Pubmed PMID: 29034187.
- Walker EMK, Bell M, Cook TM, Grocott MPW, Moonesinghe SR. Central SNAP-1 Organisation; National Study Groups. Patient reported outcome of adult perioperative anaesthesia in the United Kingdom: a cross-sectional observational study. Br J Anaesth. 2016 Jun 12;117(6):758-766. Pubmed PMID: 27956674.
- He H, Wen X, Chen X, Zhang G, Huang Q, Zhang Y, Lin Y. Effects of Phyllanthus emblica spray interventions on xerostomia after general anesthesia for gynecologic tracheal intubation: A randomised controlled trial. European Journal of Integrative Medicine. 2020 Jan 1;33:101035.
- Morton L, Siu ATY, Fowler S, Zhou C, Nixon C, Campbell D. A randomised controlled pilot trial of two interventions to manage dry mouth in pre-operative elective surgical patients. Pilot Feasibility Stud. 2020 Jun 24;6:89. Pubmed PMID: 32587752.
- Oh SK, Lee IO, Lim BG, Jeong H, Kim YS, Ji SG, et al. Comparison of the Analgesic Effect of Sufentanil versus Fentanyl in Intravenous Patient-Controlled Analgesia after Total Laparoscopic Hysterectomy: A Randomized, Double-blind, Prospective Study. Int J Med Sci. 2019 Sep 20;16(11):1439- 1446. Pubmed PMID: 31673234.
- Jin HS, Kim YC, Yoo Y, Lee C, Cho CW, Kim WJ. Opioid sparing effect and safety of nefopam in patient controlled analgesia after laparotomy: A randomized, double blind study. J Int Med Res. 2016 Aug;44(4):844-54. Pubmed PMID: 27358262.
- Ioscovich A, Hen Y, Zimran A. A New Modality to Alleviate Transient Xerostomia Post-Surgery. Journal of Clinical Research in Anesthesiology. 2018;1(1):1-4.
- Grinstein-Koren O, Herzog N, Amir O. Hyposalivation Affecting Womens' Voice. J Voice. 2021 Jan 28:S0892-1997(21)00027-8. Pubmed PMID: 33518475.
- Ambrosch A, Rockmann F, Klawonn F, Lampl B. Effect of a strict hygiene bundle for the prevention of nosocomial transmission of SARS-CoV-2 in the hospital: a practical approach from the field. Journal of Infection and Public Health. 2020 Dec 1;13(12):1862-7.
- Fernando SA, Gray TJ, Gottlieb T. Healthcare-acquired infections: prevention strategies. Intern Med J. 2017 Dec;47(12):1341-1351. Pubmed PMID: 29224205.
- Navazesh M, Kumar SK; University of Southern California School of Dentistry. Measuring salivary flow: challenges and opportunities. J Am Dent Assoc. 2008 May;139 Suppl:35S-40S. Pubmed PMID: 18460678.