Coconut Oil as an Adjuvant to Magnesium Sulphate Therapy in Acute Aluminum Phosphide Consumption
Dayananda VP1, Satish Kumar M N2*, Rakesh Kumar B3
1 Associate Professor, Anaesthesia Department, Bangalore Medical College and Research Institute, Karnataka, India.
2 Assistant Professor, Anaesthesia Department, Bangalore Medical College and Research Institute, Karnataka, India.
3 Resident, Anaesthesia Department, Bangalore Medical College and Research Institute, Karnataka, India.
*Corresponding Author
Dr. Satish Kumar M N,
Assistant Professor Anaesthesia Department,
Bangalore Medical College and Research Institute,
Bangalore, Karnataka, India.
E-mail: drsats007@gmail.com
Received: January 27, 2018; Accepted: February 24, 2018; Published: February 27, 2018
Citation: Dayananda VP, Satish Kumar M N, Rakesh Kumar B. Coconut Oil as an Adjuvant to Magnesium Sulphate Therapy in Acute Aluminum Phosphide Consumption. Int J Anesth Res. 2018;6(2):509-514. doi: dx.doi.org/10.19070/2332-2780-18000102
Copyright: Satish Kumar M N© 2018. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Objective: Aluminium phosphide (ALP) poisoning is becoming a serious health care problem in India. It has high mortality rate in spite of aggressive intensive management.
Aim: This study is designed to assess the effect of prolonged and multiple dose of coconut oil as adjuvant to magnesium sulphate along with supportive therapy in ALP poisoning patients.
Method: Prospective randomized clinical trial on ALP poisoning patients admitted in ICU of tertiary care institution. 50 (25 cases in each group) patients of ALP poisoning, confirmed by history and symptoms of ALP poisoning were included in the studied. Group A received multiple dose of coconut oil through ryles tube for 48 hours along with magnesium sulphate (MgSO4) infusion, Group B received only MgSO4 infusion with supportive therapy in both the groups. Patients were assessed for number of tablets consumed, delay in reaching hospital, cardiac symptoms, acidosis (Ph), requirement of vasoactive drugs, need of mechanical ventilation and outcome of patients.
Results: Mean dose of aluminium phosphate was 1.7gm and delay in reaching hospital ranging from 3.16 - 4.16 hrs (p= 0.036). Cardiac symptoms were lower in Group A (74 %) Comparing to Group B (100%) (p=0.001), Less acidosis seen in Group A Compared to Group B (p=0.001). The mortality rate in Group A (30%) was less comparison to Group B (60%).
Conclusion: Multiple dose of coconut oil for 48 hours through ryles tube along with MGSO4 infusion in ALP poisoning patients has beneficial effect on the management and ultimate favourable outcome of the patients.
2.Introduction
3.Materials and Methods
4.Result
5.Discussion
6.Conclusion
7.References
Keywords
Aluminium Phosphide; Coconut Oil.
Introduction
Aluminium phosphide (ALP) poisoning is common poisoning seen in developing country like India, Iran and Tharana. Release of phosphine gas exposes to increased risk for major morbidity and mortality. Aluminium phosphide (ALP) is widely used as a rodenticide across India [1]. Its consumption may be accidental, occupational or homicidal. Poisoning accounts for one-third of the world's suicide rate [2]. In India pesticide consumption causes more deaths than infection [3, 4]. ALP is being used as a common outdoor and indoor pesticide in developing countries as it is cheap, effective, free from toxic residue. Ashu et al., concluded in their clinical study that aluminium Phosphide is an extremely toxic compound and resulted in a high mortality rate of 59.5% [5].
Oral intake of ALP releases phosphine gas in stomach. Phosphine gas inhibits mitochondrial cytochrome oxidase and cellular oxidation, similar to cyanide poisoning. The cellular superoxide and peroxide radicals in cell damages the cellular components. Tissue cell effected are cardiac myocytes and vascular tissues leading to cardiac failure, circulatory collapse and respiratory failure. Hence increasing level of superoxide dismutase (SOD), malondialdehyde (MAD) and catalase in post mortem report of ALP indicate mortality is mainly due to above mention mechanism [6].
A specific antidote which neutralises the phosphine gas is not available. Several modalities like stomach wash and supportive therapy has been tried for ALP poison. Stomach wash done with potassium permanganate, activated charcoal, sorbitol, liquid paraffin, coconut oil and antacids. Supportive therapy done with vasoactive drugs, magnesium sulphate (MgSO4), soda bicorbonate and ventilator support [7]. In present study our aim is to prevent the absorption of phosphine from stomach by administering multiple dose of coconut oil for longer period and removal of free radical produced by aluminium with infusion of MgSO4.
Literature review revealed very few studies assessing the effect of single dose of coconut oil and intermittent dose of MgSO4 infusion of ALP poison patients. In our prospective randomized study undertaken “to asses coconut oil for 48 hours through ryles tube in divided dose and continues magnesium sulphate in treatment of ALP poison” along with supportive therapy.
Materials and Methods
The prospective randomized study was conducted on patients with history and clinical presentation suggestive of ALP poisoning admitted to intensive care unit of a tertiary hospital during period of march 2014 to august 2016 after institutional ethical committee approval. Informed written consent was obtained from the patients relatives.
All patients of age group of 18 to 60 years and either sex with oral in take of ALP poison were included in study. Patients with comorbid diseases, multiple poisoning and post cardiac arrest cases if any were are excluded from study.
Patients were randomly divided into two groups with 25 patients each group, GROUP A: received coconut oil for 48 hours as in divided dose through ryles tube and MgSO4 infusion with supportive therapy, GROUP B: were treated with only MgSO4 and supportive therapy. In case of doubt, diagnosis was made by simple silver nitrate-impregnated paper test on gastric content or on breath. Chemical analysis for phosphine in blood or urine is not recommended as phosphine is rapidly oxidised to phosphite and hypophosphite.
After initial resuscitation and gastric lavage with potassium permanganate a baseline electrocardiogram was recorded and blood samples for biochemical and haematological investigations were sent from the emergency room within an hour of admission to the hospital. Infusion of vasoactive agents and mechanical ventilator support were instituted where indicated. Clinical data’s like age, gender, nature of poisoning (suicidal/accidental), the dose and characteristics of the poison consumed (exposed/unexposed form) and the delay in presentation to hospital were recorded from patients close relatives.
The severity of the poisoning was assessed by the extent of organ damage (cardiovascular, gastrointestinal renal, hepatic, neurological etc.). Treatment was started with the symptomatic and supportive therapy depending upon the presenting sign and symptoms also for hypotension, bradycardia, cardiac arrhythmias, nausea and vomiting.
In Group A 100 ml of coconut oil given through ryles tube immediately after gastric lavage and closed for 12 hours. Coconut oil was continued up to 48 hours in same manner. We used the normal coconut oil accepted by food and drug administration of India. MgSO4 infusion started from first day onwards at the dosage of 2 gm bolus and latter 1 gm every 6 hourly. Sign and symptoms of MgSO4 toxicity were strictly noted i.e. respiratory rate, urine output ,deep tendon reflex. Same treatment protocol followed during the ICU stay of the patients.
In Group B patients treated without coconut oil , with only MgSO4 infusion same manner as given in Group A with supportive therapy. Patients were monitored for hypotension, arrhythmias, acidosis, ECG changes and respiratory failure. Throughout ICU stay patients are monitored with NIBP, continuous ECG monitoring in lead II, Heart rate, SpO2, Respiratory rate, Etco2. ABG regularly once a day, Blood magnesium level noted once in every two days once, 12 leads ECG if patient having arrhythmia or cardiac irregularities.
Statistical analysis was performed using SAS 9.2, SPSS 15.0, Stata 10.1, MedCalc 9.0.1, Systat 12.0 and R environment ver.2.11.1. Quantitative parameters were performed using student’s t-test whereas qualitative parameters are compared using Chi square test and fisher exact test. P value as less than 0.05 was considered statistically significant.
Study Design
A Comparative two group study.
Result
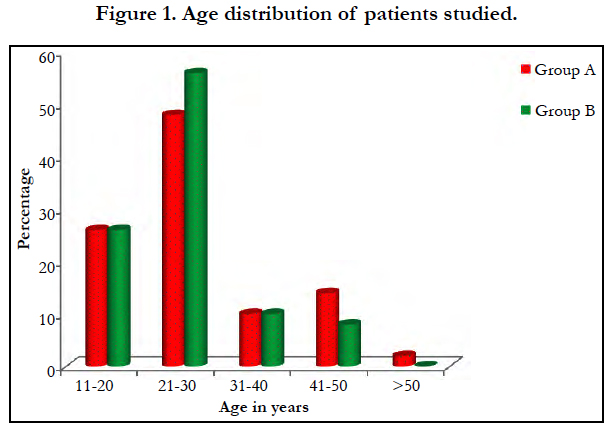
The study group comprised 50 patients. Mean age 28+/-9.6 and 25.5+/-7.92 years group A and group B respectively [Figure 1]. Male to female ratio is 1:1.27.
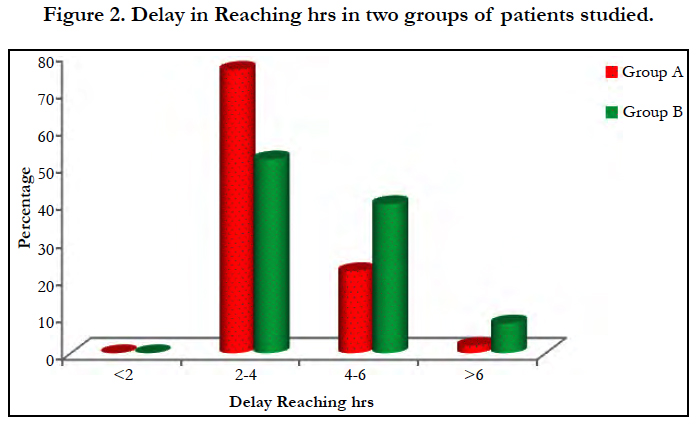
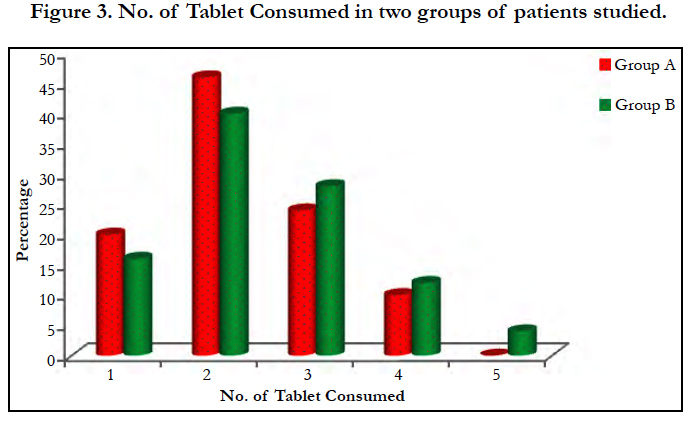
Most of the cases were of suicidal consumption of the poison (96%) while 70% of the poison were consumed in the unexposed form of tablet [Figure 3]. An average of 2.24 grams of drug was consumed by group A and 2.48 grams by group B. There is a mean delay of 3.6 and 4.6 hours before presenting to the hospital in group A and B respectively [Figure 2]. No significant association (p>0.05) noted between the dose of poison consumed or the time delay in presentation to the hospital.
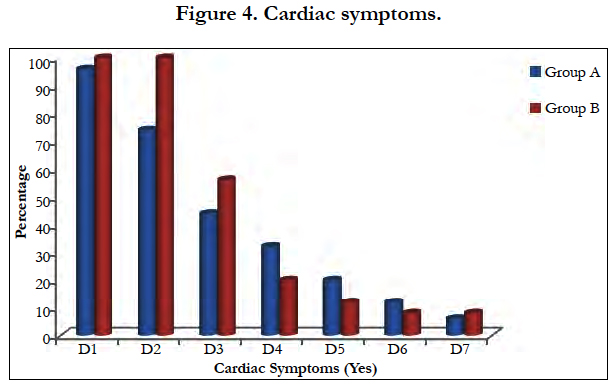
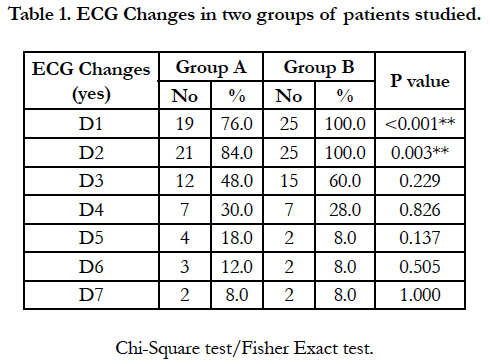
In present study 80% and 90% of patients presented with cardiac symptoms with ECG changes seen in group A and group B respectively was clinically and statistically significant (p>0.001) [Figure 4, Table 1].
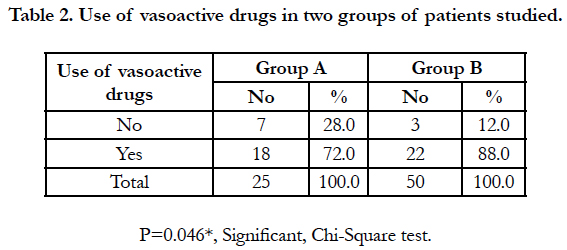
It was found that more than 95% patients presented with GIT symptoms (nausea, vomiting) in both the groups. The requirement of vasoactive drugs (noradrenaline, dopamine, dobutamine etc.) is more than twice in group B compared to group A [Table 2] and is statistically significant (p>0.001).
In ABG analysis, group B patients were more acidotic when compared to group A patients. This was observed particularly more after 24 hours of ICU stay, and it is also statistically significant (p>0.046) [day 2].
The requirement of mechanical ventilation is more intense in group B (64%) than group A (44%), and is clinically significant.
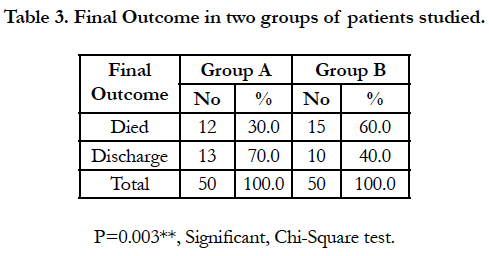
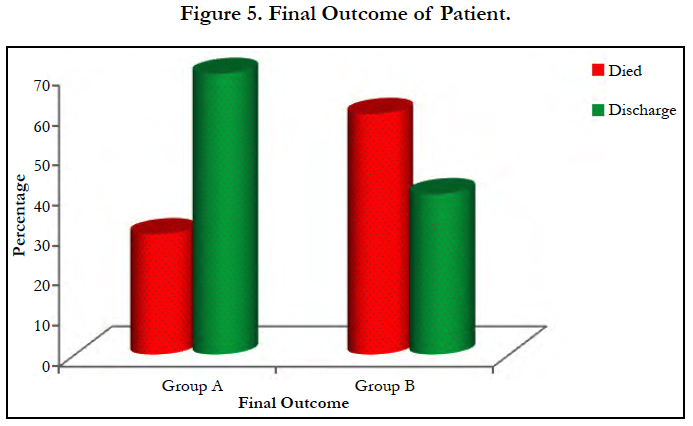
Mortality is double in group B (60%) when compared to group A (30%) [Table 3, Figure 5]. This is statistically significant with p value p>0.03.
Discussion
Treatment of APL poisoning aims towards the prevention of complication, early resuscitation and institution of supportive measures.
The main line of treatment in ALP poisoning is to achieve effective oxygenation with ventilation, prevent circulatory collapse and promote excretion of phosphine gas from body. Maryam et al., consider the high mortality rate with conventional treatment strategies in absence of specific antidote for ALP toxicity have largely disappointed the scientist [8].
To prevent the absorption of phosphine from stomach we administered coconut oil for 48 hours through ryles tube in divide doses and to combat free radical produced by aluminium poison, MgSO4 was infused.
According to the study done by Siwach et al., the lethal dose of aluminium is 1-1.5 grams but death was reported even at level low of 150-500 mgs [1, 2]. In our study, group A 1.17 gms and group B 1.5 gms of aluminium phosphate was consumed which is equivalent lethal dose [4].
Singh et al., reported better outcome in patients who presented within 4h [9]. In present study patients reached the hospital by 3.64 hr and 4.16 hrs in group A and group B respectively.
In ALP poisoning stomach wash was done with potassium permanganate, activated charcoal, sorbitol, vegetable oil, liquid paraffin and coconut oil to reduce the absorption of phosphine. Goswamin et al., used liquid paraffin for ALP poisoning through ryles tube for stomach wash [10]. Chyka et al., applied sorbitol for stomach wash in ALP poisoning [11]. Siwach et al., reported that coconut oil is use full even after 6 hour of ingestion of ALP [12].
Coconut oil is high in saturated fatty acids helps in two phases, firstly oil prevents absorption of phosphine by forming a layer on mucous membrane and secondly it dilutes the HCL and prevents break down of phosphine from pellets [13, 14].
ALP poisoning mainly causes cardiac manifestations with ECG changes due to phosphine gas toxicity. Reversible myocardial injury has been described as well. Death is mainly due to myocardial ischemia leading to hyperkinesia and akinesia. These changes were due to toxic injury of myocardial muscle [15].
The free radical scavenging action of MgSO4 helps in protection of cardiac myocytes from the huge amount of free radical generated by phosphine gas [16, 17].
Goela et al., used MgSO4 for only one day of ICU admission [18]. Chugh et al., conducted study with multiple dose of magnesium sulphate for 5 to 6 days of ICU admission. In present study also we used continuous low dose magnesium sulphate similar to Chugh et al., study [19]. In present study we estimated serum magnesium level once in two days and observed for clinical signs and symptoms of hyper magnesium.
The most common cardiac manifestation reported by Chugh et al., and Singh et al., is hypotension and is seen in 70-100% patients. The most common ECG changes are st-t segment changes with progression to arrhythmia [19, 20].
In present study of ALP poisoning hypo magnesium was noticed with hypotension and abnormal ECG changes seen in both the groups on 1st day of ICU. But we observed less cardiac symptoms on 2nd day of ICU stay in Group A compared to Group B which was statistically significant (p value of 0.001). It may be due to the ombination of prolonged use of coconut oil and continues low dose of MgSO4 infusion.
Chugh et al., opined that magnesium therapy results in better out come in patients with AALP poisoning [19]. However Singh et al., reported no benefit from magnesium therapy in aluminium phosphate poisoning, but rather itself observed that it causes hyper magnesium [20].
In our study magnesium therapy had beneficial effect in AALP poisoning by minimising circulatory collapse and better out come as seen in group A compared to the group B, similar to Chugh et al., [19].
But study conducted by Siwach et al., noticed no evidence of hypomagnesium in AALP poisoning cases nor magnesium therapy improves the survival of patients. The mortality can be reduced by intense cardiac monitoring with appropriate antiarrhythmic drugs [12]. Death result from refractory cardiogenic shock due to myocardial dysfunction. Metherpouro et al., concluded that sever AALP poisoning patients with cardiac pump failure successfully treated with intra aortic balloon pump [21].
Ashu et al., observed that 89% of ALP poisoning patients were severely hypotensive due to circulatory failure. They opined that early start of vasoactive agents prevents the circulatory collapse [5]. Omid mehro pur et al., concluded in their study that digoxin may help in managing cardiogenic shock in ALP poisoning patients [22].
In our study we notice hypotension in most of the patients on day 1 of ICU stay, 72% in group A and 88% in group B both required vasoactive agents. In group A lesser incidence of hypotension and minimal use of vasoactive agents throughout the ICU stay, may be due to prolonged use of coconut oil and continuous use of MgSO4, which is statistically significant with p value of 0.046.
The study conducted by the Jaiswal et al., summarized that acidosis is less on first day but second and third day acidosis is severe in ICU. They noticed that by giving soda bicorbonate survival rate is increased to 70% ALP poisoning [23-24]. Circulatory failure leads to anaerobic metabolism and cause lactic acidosis.
In present study on first day ph was 7.04 and 6.98 in group A and group B respectively which gradually increased to normal and almost normal on 7th day of ICU in group A with p value of 0.001. In group A less acidosis was seen is due to minimal circulatory failure hence less cardiac complication.
Louriz et al., concluded that mechanical ventilation required for 80% patients with p value of 0.03. The possible ventilation in ALP poisoning Vasodilatation with myocardial depression with shock and severe pulmonary oedema with acidosis leads to respiratory failure [25].
In our study ventilator support is equal in both group on first day, but it was 40% in group A and 60 % in group B with p value of 0.04 on second day of ICU stay which is similar to Louriz et al., study.
Shadnia S et al., done a case report methemoglobin was one of the complication of ALP poisoning but not observed in present study [26].
Ashu et al., summarized that aluminium Phosphide is an extremely toxic compound and resulted in a high mortality rate of 59.5% [5]. Chugh SN et al., concluded that 20% mortality with magnesium sulphate and 44% of mortality without magnesium sulphate therapy for ALP poisoning [19]. Siwach et al., study found no difference in mortality with or without magnesium sulphate [3].
Most of the author used single dose of coconut oil for stomach wash and intermittent dose of MgSO4 depending on the serum magnesium level resulting in variable mortality in ALP poisoning. Udismita Baruah et al., concluded that I v lipid emulsion for has extra edge over coconut oil regimen as it targets absorbed poisoning of ALP [27].
In our study mortality rate was 30% in patients treated with coconut oil through ryles tube with MgSO4 infusion (Group A) while mortality rate was 60% where patients treated without coconut oil (Group B). Also however factors like early reporting to hospital, sub lethal poisoning dose could also be contributing factor to reduce the mortality in Group A.
Conclusion
A combination of coconut oil for 48 hours in divided dose through ryles tube with low dose of MgSO4 infusion decreases the mortality ALP poisoning patients. However further study in larger number required to optimizing the treatment protocol for ALP toxicity.
References
- Shadnia S, Rahimi M, Pajoumand A, Rasouli MH, Abdollahi M. Successful treatment of acute aluminium phosphide poisoning: possible benefit of coconut oil. Hum Exp Toxicol. 2005 Apr;24(4):215-8. PubMed PMID: 15957538.
- Gupta S, Ahlawat SK. Aluminum phosphide poisoning-a review. J Toxicol Clin Toxicol. 1995;33(1):19-24. PubMed PMID: 7837309.
- Siwach SB, Yadav DR, Arora B, Dalal S. Acute aluminum phosphide poisoning-an epidemiological, clinical and histo-pathological study. J Assoc Physicians India. 1988 Oct;36(10):594-6. PubMed PMID: 3220807.
- Siwach SB, Gupta A. The profile of acute poisonings in Harayana-Rohtak Study. J Assoc Physicians India. 1995 Nov;43(11):756-9. PubMed PMID: 8773034.
- Mathai A, Bhanu MS. Acute aluminium phosphide poisoning: Can we predict mortality?. Indian J Anaesth. 2010 Jul;54(4):302-7. doi: 10.4103/0019- 5049.68372. PubMed PMID: 20882171.
- Ranga GS, Dwivedi S, Agarwal M, Kumar D. Aluminium phosphide poisoning in a young adult: a suicidal cardiotoxin simulating myocardial ischaemia. J Ind Acad Clin Med. 2004;5(4):369.
- Bajpai SR. Aluminium phosphide poisoning: Management and prevention. J Indian Acad Forensic Med. 2010;32:352-4.
- Farahani MV, Soroosh D, Marashi SM. Thoughts on the current management of acute aluminum phosphide toxicity and proposals for therapy: An evidence-based review. Indian J Crit Care Med. 2016 Dec;20(12):724-730. doi: 10.4103/0972-5229.195712. PubMed PMID: 28149031.
- Singh S, Singhi S, Sood NK, Kumar L, Walia BN. Changing pattern of childhood poisoning (1970-1989): experience of a large north Indian hospital. Indian Pediatr. 1995 Mar;32(3):331-6. PubMed PMID: 8613288.
- Goswami M, Bindal M, Sen P, Gupta SK, Avasthi R, Ram BK. Fat and oil inhibit phosphine release from aluminium phosphide--its clinical implication. Indian J Exp Biol. 1994 Sep;32(9):647-9. PubMed PMID: 7814045.
- Chyka PA, Seger D, Krenzelok EP, Vale JA. American Academy of Clinical Toxicology, European Association of Poisons Centres and Clinical Toxicologists. Position paper: single-dose activated charcoal. Clin Toxicol (Phila). 2005;43(2):61-87. PubMed PMID: 15822758.
- Siwach SB, Singh P, Ahlawat S, Dua A, Sharma D. Serum & tissue magnesium content in patients of aluminium phosphide poisoning and critical evaluation of high dose magnesium sulphate therapy in reducing mortality. J Assoc Physicians India. 1994 Feb;42(2):107-10. PubMed PMID: 7860467.
- Gupta A, Beg M, Mohammed J, Azfar SF, Akhtar N. Coconut oil and aluminium phosphide poisoning. SAFCON-2007 South Asian and VII Annual Conference of Indian Congress Forensic Medicine and Toxicology; 2007 Mar 24-25. Uttar Pradesh, INDIA.
- Shadnia S, Rahimi M, Pajoumand A, Rasouli MH, Abdollahi M. Successful treatment of acute aluminium phosphide poisoning: possible benefit of coconut oil. Hum Exp Toxicol. 2005 Apr;24(4):215-8. PubMed PMID: 15957538.
- Duenas A, Pérez-Castrillon JL, Cobos MA, Herreros V. Treatment of the cardiovascular manifestations of phosphine poisoning with trimetazidine, a new antiischemic drug. Am J Emerg Med. 1999 Mar;17(2):219-20. PubMed PMID: 10102341.
- Bolter CJ, Chefurka W. Extramitochondrial release of hydrogen peroxide from insect and mouse liver mitochondria using the respiratory inhibitors phosphine, myxothiazol, and antimycin and spectral analysis of inhibited cytochromes. Arch Biochem Biophys. 1990 Apr;278(1):65-72. PubMed PMID: 2321971.
- Shechter M. Magnesium and cardiovascular system. Magnes Res. 2010 Jun;23(2):60-72. doi: 10.1684/mrh.2010.0202. Epub 2010 Mar 31. PubMed PMID: 20353903.
- Goel A, Aggarwal P. Pesticide poisoning. Natl Med J India. 2007 Jul-Aug;20(4):182-91. PubMed PMID: 18085124.
- Chugh SN. Aluminium phosphide poisoning: present status and management. J Assoc Physicians India. 1992 Jun;40(6):401-5. PubMed PMID: 1452567.
- Singh RB, Rastogi SS, Singh DS. Cardiovascular manifestations of aluminium phosphide intoxication. J Assoc Physicians India. 1989 Sep;37(9):590-2. PubMed PMID: 2632560.
- Mehrpour O, Amouzeshi A, Dadpour B, Oghabian Z, Zamani N, Amini S, Hoffman RS. Successful treatment of cardiogenic shock with an intra aortic balloon pump following aluminium phosphide poisoning. Arh Hig Rada Toksikol. 2014 Mar;65(1):121-6. PubMed PMID: 24445229.
- Mehrpour O, Abdollahi M. Poison treatment centers in Iran. Hum Exp Toxicol. 2012 Mar;31(3):303-4. doi: 10.1177/0960327110392086. Epub 2010 Dec 7. PubMed PMID: 21138986.
- Nejad FT, Mohammadi AB, Behnoush B, Kazemifar A, Nahandi MZ, Dabiran S, et al. Predictors of poor prognosis in aluminum phosphide intoxication. Iran J Toxicol. 2012 Apr 15;6(16):610-4.
- Jaiswal S, Verma RK, Tewari N. Aluminum phosphide poisoning: Effect of correction of severe metabolic acidosis on patient outcome. Indian J Crit Care Med. 2009 Jan-Mar;13(1):21-4. doi: 10.4103/0972-5229.53111. PubMed PMID: 19881175.
- M Louriz, T Dendane, K Abidi, N Nandini, R Aboqual, AA Zeggwagh. Prognostic factors of acute ALP poisoning. Indian J Med Sci. 2009 Jun;63(6):227-34. doi: 10.4103/0019-5359.53386. PubMed PMID: 19602756.
- Shadnia S, Soltaninejad K, Hassanian-Moghadam H, Sadeghi A, Rahimzadeh H, Zamani N, et al. Methemoglobinemia in aluminum phosphide poisoning. Hum Exp Toxicol. 2011 Mar;30(3):250-3. doi: 10.1177/0960327110384287. Epub 2010 Oct 1. PubMed PMID: 20889582.
- Baruah U, Sahni A, Sachdeva HC. Successful management of aluminium phosphide poisoning using intravenous lipid emulsion: Report of two cases. Indian J Crit Care Med. 2015 Dec;19(12):735-8. doi: 10.4103/0972-5229.171412. PubMed PMID: 26816450.