Anti-Xa Assay Correlation to the Efficacy and Safety of Enoxaparin in the Treatment of Pulmonary Embolism
Abbas MS*
Department of Anesthesia, Faculty of Medicine, Ain Shams University, Cairo, Egypt.
*Corresponding Author
Mohamed S. Abbas MD
Anesthesia Department, Al Mouwasat Hospital, Dammam, KSA.
Tel: 00966138200000
E-mail: mohamed_abasy@hotmail.com
Received: April 02, 2017; Accepted: April 19, 2017; Published: April 25, 2017
Citation: Abbas MS (2017) Anti-Xa Assay Correlation to the Efficacy and Safety of Enoxaparin in the Treatment of Pulmonary Embolism. Int J Anesth Res. 5(4), 435-438. doi: http://dx.doi.org/10.19070/2332-2780-1700089
Copyright: Abbas MS© 2017. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Enoxaparin is one of the LMWH that has been used for long time in the treatment of acute pulmonary embolism. In this study, we will monitor anticoagulant therapy by anti-Xa assay and correlate its level to the efficacy and safety of enoxaparin in the treatment of pulmonary embolism.
Methods: The study was conducted on 42 patients in ICU diagnosed to have pulmonary embolism by CT pulmonary angiography and treated by subcutaneous enoxaparin 1 mg/kg every 12 hours. Anticoagulant therapy was followed by anti-Xa assay done 4 hours after the third dose of subcutaneous enoxaparin and the results were correlated to the clinical outcome of the patients as regards complications (recurrent pulmonary embolism, bleeding, low platelet count and death).
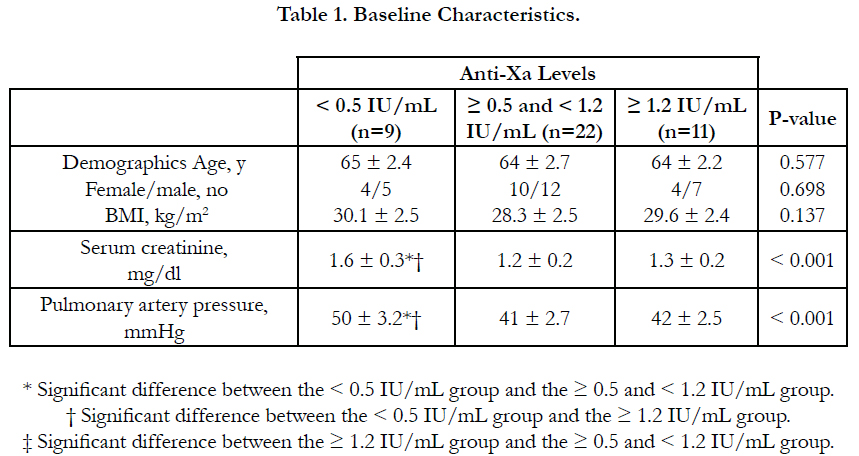
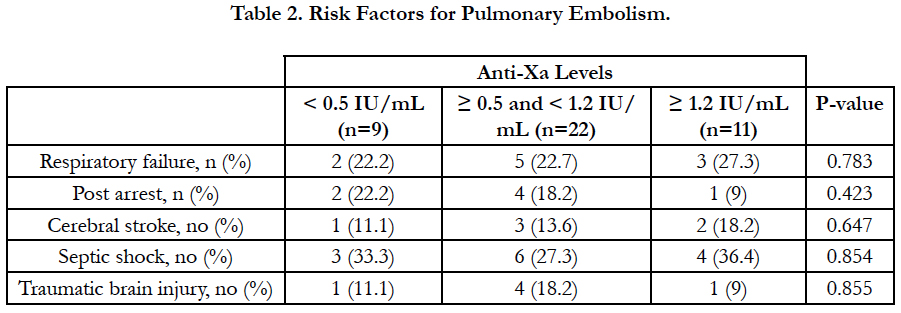
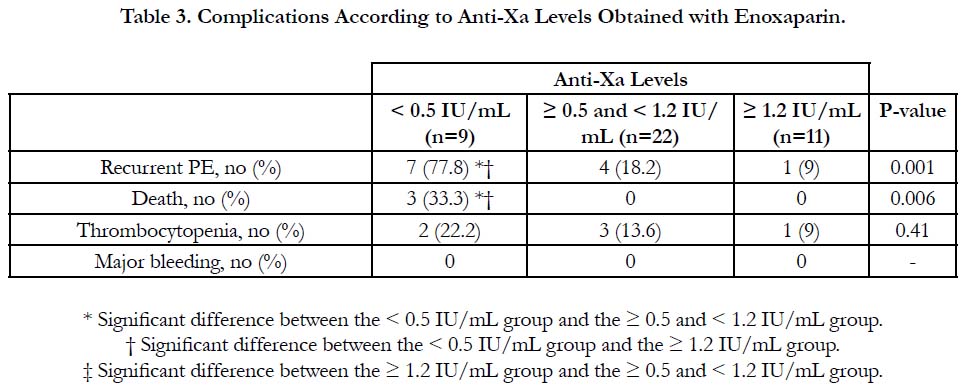
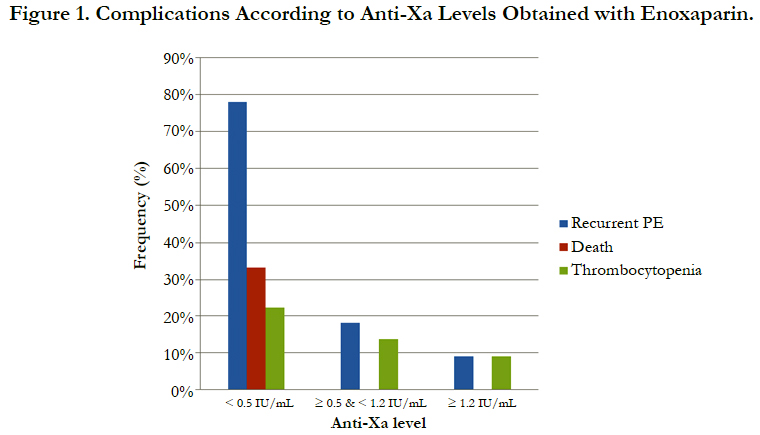
Results: According to anti-Xa assay, we divided the patients into three groups. Group I with anti-Xa level <0.5 IU/mL, group II with anti-Xa level ≥0.5 and <1.2 IU/mL and group III with anti-Xa level ≥1.2 IU/mL. 9 patients were in group I, 22 patients were in group II and 11 patients were in group III. There was no significant difference between the three groups as regards age, sex, BMI and underlying risk factors for pulmonary embolism. Serum creatinine level and pulmonary artery pressure were significantly higher in group I than in group II and III. The incidence of recurrent pulmonary embolism was significantly higher in group I than in group II and III with 7 out of 9 patients in group I had recurrent pulmonary embolism compared with only 4 out of 22 and 1 out of 9 in group II and III respectively. The mortality rate was significantly higher in group I than in group II and III with 3 recorded death cases in group I compared with zero recorded cases in group II and III. There was no significant difference between the three groups as regards thrombocytopenia and major bleeding complications.
Conclusion: Anti-Xa assay is a good and reliable test for monitoring the efficacy of LMWH therapy using enoxaparin for patients with pulmonary embolism as regards recurrence and mortality rates of pulmonary embolism but it will not monitor safety of enoxaparin therapy as regards incidence of thrombocytopenia and bleeding complications. The study was registered on www.clinicaltrials.gov, Registration ID: NCT02977013. Registered November 27, 2016.
2.Background
3.Methods
3.1 Anti-Xa Assay
3.2 Clinical Follow-Up
4.Statistical Analysis
5.Results
6.Discussion
7.Conclusion
8.References
Keywords
Pulmonary Embolism; Anti-Xa Assay; Enoxaparin.
Background
Pulmonary embolism (PE) is a detrimental life-threatening event that accounts for 5 to 10 percent of deaths among hospitalized patient [1]. Antithrombotic therapy for such disease was used to decrease early morbidity and mortality and to prevent recurrence without causing excessive bleeding. LMWH has been used for the treatment of pulmonary embolism and proved to be effective but it lacks a test for follow up the therapeutic level of the drug. The plasma anti-Xa assay is a laboratory test that indirectly measures the activity of heparins and has been used most commonly to follow up patients treated with low molecular weight heparins. The mechanism by which anti-Xa assay determines the anticoagulant activity is through measuring the ability of the heparinantithrombin (AT) complex to inhibit activated factor X [2] and by using a special chromogenic procedure involving commercially available multi calibration kits, the residual amount of factor Xa is calculated and inversely correlated to the amount of heparin in the plasma. Anti-Xa activity results are expressed as units/ml.
When using enoxaparin at a twice-daily dose, the target peak plasma concentration of anti-Xa should be kept between 0.5–1.2 IU/mL [3].
The therapeutic ranges of LMWHs using anti-Xa assay have been determined for the treatment of deep-vein thrombosis [4] and has been correlated with clinical outcome in acute Coronary Syndrome activity [5]. However no study has been done correlating anti-Xa assay with the clinical outcome of patients with pulmonary embolism treated with LMWH.
The aim of the present study is to monitor anticoagulant therapy by anti-Xa assay and correlate its level to the efficacy and safety of enoxaparin in the treatment of pulmonary embolism.
Methods
The study was registered on www.clinicaltrials.gov, Registration ID: NCT02977013, principal investigator’s name: Mohamed Sayed Mohamed Abbas, Registered November 27, 2016.
This prospective double blind observational trial was conducted on 42 patients in ICU aged 18 years or older and diagnosed to have pulmonary embolism and confirmed by the presence of intraluminal filling defect in CT pulmonary angiography and treated with subcutaneous enoxaparin 1 mg/kg/12 hours. The study was conducted from August 2013 to September 2016.
Patients were excluded from this study if they underwent thrombolysis, embolectomy or if they are unfit for anticoagulant therapy because of major bleeding or thrombocytopenia (a platelet count below 100,000 per cubic millimeter). Patients were also excluded if they had impaired kidney function with a serum creatinine level above 2 mg/dl or uncontrolled hypertension (blood pressure > 160/100 mmHg).
Written informed consent had been obtained from the patient families and the protocol was approved by the institution.
In our study, blood sampling for anti-Xa assay was done 4 hours after the third dose of subcutaneous enoxaparin. The timing of sampling was determined depending on the fact that LMWH half life in adults is ranging from 4-6 hours and the steady state concentration is reached within one day.
The conventional therapeutic range of anti-Xa levels for patients treated with LMWH enoxaparin is considered according to literatures to be between 0.5 IU/mL and 1.2 IU/ml [3].
Three groups of patients were specified according to their anti-Xa levels. The first group consisted of patients with an anti-Xa activity < 0.5 IU/mL, which was considered as sub therapeutic according to the predefined therapeutic range. The second group of patients had anti-Xa levels within the therapeutic range, ie, ≥ 0.5 IU/mL and <1.2 IU/mL. The third group consisted of patients with anti-Xa levels ≥ 1.2 IU/mL. Care was taken for the patients with anti-Xa levels ≥ 1.8 IU/ml as it might be associated with more bleeding complications [6].
All the patients were examined daily over a period of 4 weeks for symptoms and signs of recurrent pulmonary embolism, Platelet count, bleeding and death events. Recurrent pulmonary embolism was confirmed if the repeat CT angiography revealed a new sudden arterial branch cutoff or a new intraluminal filling defect that was not present on the first angiogram. Patients with platelet count less than 50,000/mm3 or those with bleeding and platelet count between 50,000 and 100,000/mm3 were considered to have severe thrombocytopenia. Bleeding is defined as major overt bleeding requiring blood transfusion > 2 units or marked hemoglobin drop > 2 gm/dl or if the bleeding was intracranial or retroperitoneal. Death is defined as all deaths occurring within the 4 weeks of the study. All these results were correlated to anti-Xa assay.
Statistical Analysis
Data were analyzed using SPSS version 16.0 computer software (Chicago, IL, USA). Numerical variables were presented as mean and standard deviation (SD), and analyzed using Kruskal–Wallis test; while categorical variables were presented as frequency (%), and analyzed using chi-square test for trend. A P-value of 0.05 was considered statistically significant.
Results
According to anti-Xa assay, we divided the patients into three groups. Group I with anti-Xa level <0.5 IU/mL, group II with anti-Xa level ≥0.5 and <1.2 IU/mL and group III with anti-Xa level ≥1.2 IU/mL. 9 patients were in group I, 22 patients were in group II and 11 patients were in group III.
There was no significant difference between the three groups as regards age, sex, BMI and underlying risk factors for pulmonary embolism. Serum creatinine level and pulmonary artery pressure were significantly higher in group I than in group II and III (Table 1, Table 2).
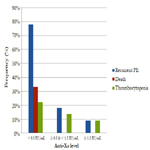
The incidence of recurrent pulmonary embolism was significantly higher in group I than in group II and III with 7 out of 9 patients in group I had recurrent pulmonary embolism compared with only 4 out of 22 and 1 out of 9 in group II and III respectively. The mortality rate was significantly higher in group I than in group II and III with 3 recorded death cases in group I compared with zero recorded cases in group II and III. There was no significant difference between the three groups as regards thrombocytopenia and major bleeding complications (Table 3, Figure 1).
Discussion
Pulmonary embolism is considered a potentially fatal disease that can cause sudden and dramatic death. With the use of anticoagulant therapy, the clinical outcome of such patients has been markedly improved [7]. Early initiation of therapeutic anticoagulation is recommended in all patients with confirmed or highly suspected PE while the diagnostic workup is still ongoing [8]. Many studies has compared the efficacy and safety of subcutaneous LMWH with those of unfractionated heparin. Of these studies, three major studies [9-11] involving a total of 1951 patients with non high risk symptomatic PE or with asymptomatic PE in association with symptomatic DVT were enrolled in a metaanalysis [12]. At the end of the study treatment, LMWH was at least as efficacious as unfractionated heparin regarding the rate of recurrent VTE and at least as safe regarding major bleeding with no difference in mortality between the two groups.
The monitoring of LMWH therapy in pulmonary embolism is crucial and helps us to avoid complications such as recurrent pulmonary embolism or complications related to anticoagulant therapy such as bleeding and thrombocytopenia. In this study, we used anti-Xa assay to monitor efficacy and safety of enoxaparin in the treatment of pulmonary embolism. Determination of the therapeutic ranges of LMWHs and its correlation to the outcome have been done for the treatment of deep-vein thrombosis [4] and acute Coronary Syndrome [5] using anti-Xa activity as a monitor of their biological activity. However, there is no study has been done correlating anti-Xa assay with the clinical outcome of patients with pulmonary embolism treated with LMWH. Anti-Xa assay has proved its efficacy compared with aPTT for monitoring of continuous heparin infusion with more expeditious achievement of therapeutic anticoagulation, less laboratory tests and heparin dosage changes and more prolonged maintenance of the therapeutic levels [13]. Also in heparin resistance, dosage escalation can be avoided without compromising efficacy, by performing a heparin assay and not increasing the dose if the heparin level is maintained in the therapeutic range [14].
In our study, it was found that sub therapeutic anti-Xa was mainly associated with inappropriate enoxaparin dose in obese patients or suboptimal enoxaparin dose in patients with mild renal impairment (serum creatinine 1.5–2 mg/dl) and in 2 patients it was unexplained lower than therapeutic level even though the patients are average body weight, with normal kidney function and receiving the proper enoxaparin dose as per body weight. In such group of patients with sub therapeutic anti-Xa, the incidence of recurrent pulmonary embolism and mortality ware significantly higher than therapeutic anti-Xa group with no significant difference as regards bleeding complications or thrombocytopenia incidence. That is to say that maintenance of anti-Xa within the therapeutic range 0.5-1.2 IU/mL was associated with significant reduction in the incidence of recurrent pulmonary embolism and mortality rate with no difference in the incidence of thrombocytopenia or bleeding complications as compared with sub therapeutic level.
These results were matching with the large unselected cohort study of patients with UA/NSTEMI treated with enoxaparin in which mortality and recurrent ischemic events have been shown to be significantly associated with low anti-Xa activity [5].
As regards the group of patients with anti-Xa level ≥ 1.2 IU/mL which included 11 patients, Anti-Xa level was surprisingly high in such group was no clear explanation same like the group of patients with unexplained low level of anti-Xa level. In this group, it was found that the recurrence rate of pulmonary embolism, mortality rate, thrombocytopenia and bleeding complications were not significantly different from the group of patients with therapeutic anti-Xa level.
Conclusion
Anti-Xa assay is a good and reliable test for monitoring the efficacy of LMWH therapy using enoxaparin for patients with pulmonary embolism as regards recurrence and mortality rates of pulmonary embolism but it will not monitor safety of enoxaparin therapy as regards incidence of thrombocytopenia and bleeding complications. That is why patients with pulmonary embolism receiving therapeutic dose of LMWH enoxaparin should be monitored with anti-Xa assay as it correlates well with the clinical outcome in such patients.
References
- Goldhaber SZ (1998) Pulmonary embolism. N Engl J Med. 339(2): 93-104.
- Ignjatovic V, Summerhayes R, Gan A, Than J, Chan A, et al., (2007) Monitoring unfractionated heparin (UFH) therapy: which anti factor Xa assay is appropriate? Thromb res. 120(3): 347-51.
- Enoxaparin Sodium Investigator Brochure. (2003) (12th edn), Bridgewater, NJ: Aventis Pharma.
- Hyers TM, Hull RD, Morris TA, Samama M, Weg TB, et al., (2001) Antithrombotic therapy for venous thromboembolic disease. Chest. 119(1): 176S–193S.
- Montalescot G, Collet JP, Tanguy ML, Ankri A, Payot L, et al., (2004) Anti-Xa activity relates to survival and efficacy in unselected acute coronary syndrome patients treated with enoxaparin. Circulation. 110(4): 392-8.
- Dose-ranging trial of enoxaparin for unstable angina patients: results of TIMI 11A. The Thrombolysis in Myocardial Infarction (TIMI) 11A Investigators (1997) J Am Coll Cardiol. 29(7): 1474–1482.
- Barritt DW, Jordan SC (1960) Anticoagulant drugs in the treatment of pulmonary embolism: a controlled trial. Lancet. 1(7138): 1309-12.
- Torbicki, Adam, Arnaud Perrier, Stavros Konstantinides, Giancarlo Agnelli, et al., (2008) Guidelines on the diagnosis and management of acute pulmonary embolism. The task force for the diagnosis and management of acute pulmonary embolism of the European society of cardiology. Eur heart j. 29(18): 2276-2315.
- A randomised trial of subcutaneous low molecular weight heparin (CY 216) compared with intravenous unfractionated heparin in the treatment of deepvein thrombosis. A collaborative European multicentre study. Thromb Haemost. 65(3): 251–256.
- Hull RD, Raskob GE, Pineo GF, Green D, Trowbridge AA, et al., (1992) Subcutaneous low-molecular-weight heparin compared with continuous intravenous heparin in the treatment of proximal-vein thrombosis. N Engl JMed. 326(15): 975–982.
- Perez de Llano LA, Baloira VA, Veres RA, Veiga F, Golpe GR, et al., (2003) Multicenter, prospective study comparing enoxaparin with unfractionated heparin in the treatment of submassive pulmonary thromboembolism. Arch Bronconeumol 39(8): 341–345.
- Quinlan DJ, Mc Quillan A, Eikelboom JW (2004) Low-molecular-weight heparin compared with intravenous unfractionated heparin for treatment of pulmonary embolism: a meta-analysis of randomized, controlled trials. Ann Intern Med. 140(3): 175–183.
- Guervil David J, Amy F Rosenberg, Almut G Winterstein, Neil S Harris, Thomas E Johns, et al., (2011) Activated partial thromboplastin time versus antifactor Xa heparin assay in monitoring unfractionated heparin by continuous intravenous infusion. Ann Pharmacother. 45(7-8) : 861-868.
- Levine Mark N, Jack Hirsh, Michael Gent, Alexander G Turpie, Moira Cruickshank, et al., (1994) A randomized trial comparing activated thromboplastin time with heparin assay in patients with acute venous thromboembolism requiring large daily doses of heparin. Arch intern med. 154(1): 49-56.