Surgical Anatomy, Morphometry, and Histochemistry of Major Salivary Glands in Dogs: Updates and Recommendations
Gaber W1, Shymaa A. Shalaan2, Nabil A. Misk3, Ibrahim A4*
1 Department of Anatomy, Histology, and Embryology, Faculty of Veterinary Medicine, Assiut University, Assiut, Egypt.
2 Department of Care and Cure, Assiut Managemental Veterinary Center, Assiut, Egypt.
3 Department of Surgery, Anesthesiology, and Radiology, Faculty of Veterinary Medicine, Assiut University, Assiut, Egypt.
4 Veterinary Teaching Hospital, Faculty of Veterinary Medicine, Assiut University, Assiut, Egypt.
*Corresponding Author
Ahmed Ibrahim,
Veterinary Teaching Hospital, Faculty of Veterinary Medicine, Assiut University, Assiut 71526, Egypt.
Tel: +201062204009
Fax: 088-208050
E-mail: elgrah38@gmail.com
Received: February 27, 2020; Accepted: May 06, 2020; Published: May 13, 2020
Citation: Gaber W, Shymaa A. Shalaan, Nabil A. Misk, Ibrahim A. Surgical Anatomy, Morphometry, and Histochemistry of Major Salivary Glands in Dogs: Updates and Recommendations. Int J Vet Health Sci Res. 2020;8(2):252-259. doi: dx.doi.org/10.19070/2332-2748-2000048
Copyright: Ahmed Ibrahim© 2020. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
The present study aimed to highlight the characteristic anatomical, morphometrical, as well as, histochemical features of the major salivary glands (SG) in dogs. Based on the surgical anatomy of the major SG, surgical excision of the parotid SG may be the most challenge due to vital blood vessels and nerves invading its parenchyma (the branches of the facial nerve, the maxillary and superficial temporal arteries, as well as, the internal maxillary vein).Creating an incision directly over the thick fibrous capsule of the mandibular SG and dissection of the gland from the capsule may be easier to excise the gland and safer to both; the maxillary and linguofacial veins. The excision of the zygomatic SG may necessitate osteotomy or ostectomy of the zygomatic arch. Based on the histological and histochemistry of the major salivary glands, the dog that one of its salivary glands was excised, the type of its food may have to be changed according to the excised SG and upon its nature of secretion.
2.Introduction
3.Materials and Methods
4.Results
5.Discussion
6.Conclusion
7.References
Keywords
Parotid; Mandibular; Sublingual; Zygomatic; Anatomy; Histology.
Introduction
The major salivary glands (SGs) involving the parotid, mandibular, sublingual, and zygomatic glands in dogs. These glands are large and have long ducts [1, 2].
The parotid SG lies at the junction of the head and neck overlying the basal portion of the auricular cartilage. It is loosely lobulated, has a thin capsule and an outline as a V-shape with the apex directed ventrally. The mandibular SG is an ovoid gland located in the junction of the maxillary and linguo facial veins as they form the jugular vein. It is adherent cranially with the monostomatic portion of the sublingual gland and shares a common heavily fibrous capsule with that gland. The zygomatic SG is named from its location beneath the zygomatic arch of the temporal bone. It is globular to pyramidal in shape with its base directed upward and backward and lying against the ventral part of the periorbita [2].
The minimal physiological parenchymal unit ofthe salivary gland is referred to as a salivon (acinus) that is composed of pyramidal secretory cells arranged around a central lumen and surrounded by myoepithelial cells. The latter are contractile cells with numerous cell processes that embrace the basal aspect of the secretory cells of the acini. The lumen of the acinus leads to a duct system. Acini are made up of either serous or mucous cells. However, some of the mucous acini have a cap of serous cells called demilunes [1].
Surgical affections of the major salivary glands in dogs are variables and sometimes threaten the animal's life. Salivary mucocele, fistula, sialadenitis, sialadenosis, sialolithiasis, and neoplasia have been recorded in canine patients [3, 4].
Understanding of surgical anatomy, morphometry, and histochemistry of these glands is essential for a correct decision concerning medical and/or surgical intervention of such affections, and even food regime and management of the animal after surgical excision of the gland.
The aim of the present study was to throw light on the most important facts concerning the anatomy, morphometry, and histochemistry of the major salivary glands in dogs, and remarks that might be taken in consideration during and after the surgical excision of each gland.
The present study was approved by the National Ethical Committee of The Faculty of Veterinary Medicine, Assiut University, Assiut, Egypt, according to the OIE standards for use of animals in research. The study was conducted on six (n = 6) cadavers of adult mongrel dogs of both sexes (3 males and 3 non-pregnant, non-lactating females), aged between 1 - 3 years old and weighting 10 -15 kg body weight.
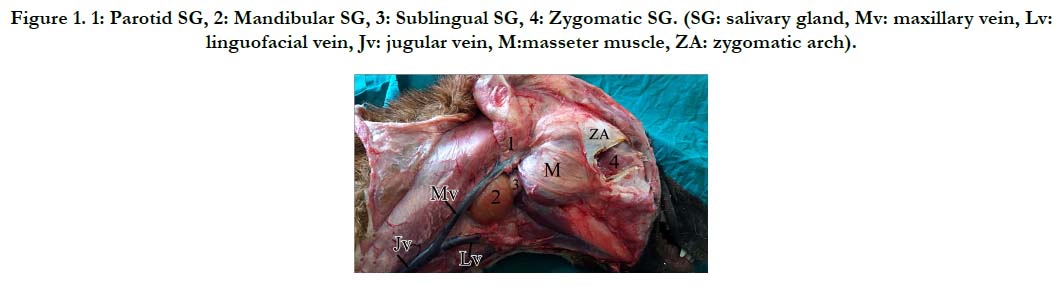
The skin over the head region was dissected for complete exposure of the parotid, mandibular, and sublingual salivary glands in situ. Ostectomy of the zygomatic arch over the zygomatic gland was needed to explore it (Figure 1). Each gland was dissected from the surrounding tissues and further anatomical, morphometrical, histological, and histochemical studies were conducted.
Figure 1. 1: Parotid SG, 2: Mandibular SG, 3: Sublingual SG, 4: Zygomatic SG. (SG: salivary gland, Mv: maxillary vein, Lv: linguofacial vein, Jv: jugular vein, M:masseter muscle, ZA: zygomatic arch).
Position, shape, as well as, relations with other structures of each gland was described.
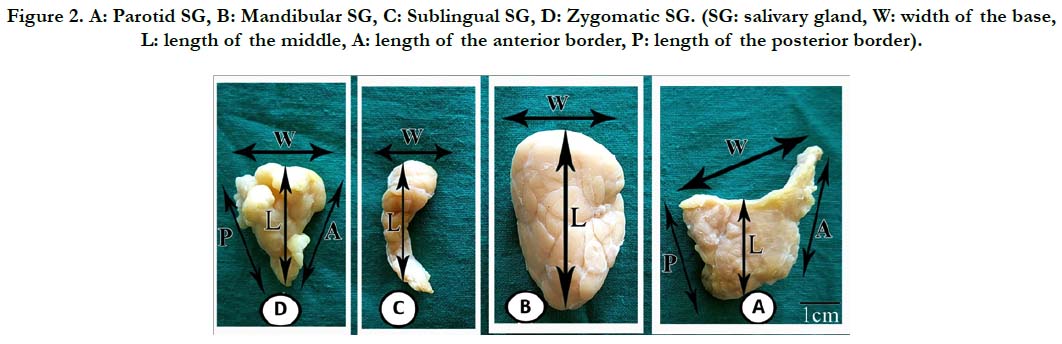
Shape, lengths (cm) of the anterior and posterior borders and at the middle of the gland, width (cm) at the base of the glands (Figure 2), thickness (mm), weight (gm), and volume (cm3) were recorded for each gland.
Figure 2. A: Parotid SG, B: Mandibular SG, C: Sublingual SG, D: Zygomatic SG. (SG: salivary gland, W: width of the base, L: length of the middle, A: length of the anterior border, P: length of the posterior border).
The length and width were measured using a graduated measuring tab. The volume of the glands was measured using a graduated cylinder contained counted amount of water (Figure. 3A). The thickness was recorded using a digital caliper (Figure. 3 B), while weight was taken by using a sensitive scale (Figure. 3C).
Figure 3. A: A graduated cylinder for measuring the volume of the SGs, B: A digital caliper for measuring of the thickness of the SGs, C: A sensitive scale for measuring of the weight of the SGs.
Samples (0.5cm × 0.5 cm) from the dissected glands, as well as, their main ducts were taken and fixed by immersion in 10 % neutral buffered formalin. The fixed specimens were dehydrated in graded alcohol series, cleared, embedded in paraffin and sectioned at 3-5 μm thick using a Richert Leica RM 2125 Microtome, Germany. The obtained sections were stained with the following stains:
1- Harris haematoxyline and eosin stain for general histological studies [5].
2- Periodic acid-Schiff (PAS) stain for detection of neutral mucopolysaccharides [6] and Periodic acid-Schiff (PAS) stain pre-treated by diastase enzyme for detection of glycogen.
3- Alcian blue stain (pH 2.5) for detection of acidic muco-polysaccharides [7].
The stained sections were examined using a Leitz Dialux 20 microscope to study the histological and histochemical characteristics of each gland as well as its main duct. Images were taken using a Canon digital camera (Candison Powershot A95).
The parotid salivary gland located in the retromandibular fossa, caudal to the ramus of the mandible and ventral to the base of the ear. It was triangular in shape with its base lied dorsally and the apex was ventrally situated. The base was concave and enclosed the base of the external ear. The gland was loosely lobulated and had a thin capsule. It possessed three angles; rostral pre-auricular angle, caudal retro-auricular angle and ventral angle, as well as, three borders; rostral, caudal, and dorsal. The rostral border (4.72cm) was longer than the caudal one (4.11 cm) and from under which the palpebral, auriculotemporal and the dorsal and ventral buccal nerves emerged. The caudal border related to the caudal auricular vein. The lateral surface of the gland was crossed vertically by the strap-like parotido-auricularis muscle. The medial surface was related to the branches of the facial nerve, the maxillary and superficial temporal arteries, and the internal maxillary vein.
The mandibular salivary gland was ovoidin shape with its broad end directed ventrally. It occupied a superficial position caudal to the ramus of the mandible and ventral to the parotid salivary gland. It lied caudal to the masseter muscle and the mandibular lymph node in the angle formed by the linguofacial and maxillary veins. Its craniodorsal pole was related to the major portion of the sublingual salivary gland with which it was enclosed by a common thick fibrous capsule.
The monostomatic sublingual salivary gland was elongated triangular in outline with its base directed caudally, resting on the craniodorsal aspect of the mandibular salivary gland. It had a large caudal portion and a tapering rostral one. The large caudal portion of the gland lied on the lateral aspect of the caudal part of the digastric muscle then it crossed the dorsal border of this muscle where the tapering rostral portion of the gland was situated between the digastric muscle and the ventral border of the masseter muscle medial to the mandible.
The zygomatic salivary gland situated in the ventral orbit, surrounded by the periorbital fat. It lied ventral to the eyeball and medial to the zygomatic arch. It was pyramidal in shape with its base directed upward and the apex was ventral and blunt.
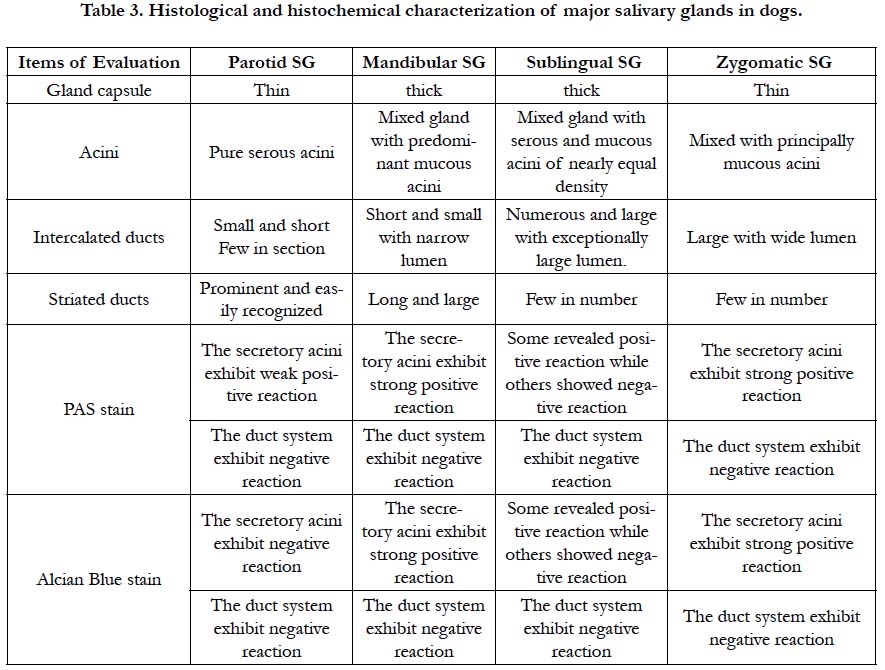
The width at the base, the length of the rostral border, caudal border and at the middle of the gland, as well as, the thickness of the studied salivary glands was summarized in Table (1). The weight and volume of the glands as well as their percentage to the total weight and volume were presented in Table (2).
Table 2. The mean weight (gm) and volume (cm³) of major salivary glands and their percentage to the total weight and volume.
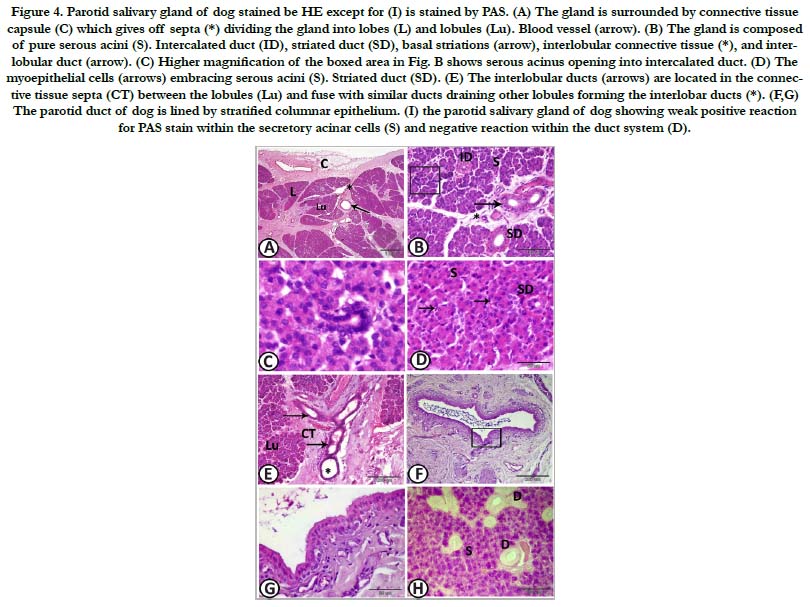
Figure 4. Parotid salivary gland of dog stained be HE except for (I) is stained by PAS. (A) The gland is surrounded by connective tissue capsule (C) which gives off septa (*) dividing the gland into lobes (L) and lobules (Lu). Blood vessel (arrow). (B) The gland is composed of pure serous acini (S). Intercalated duct (ID), striated duct (SD), basal striations (arrow), interlobular connective tissue (*), and interlobular duct (arrow). (C) Higher magnification of the boxed area in Fig. B shows serous acinus opening into intercalated duct. (D) The myoepithelial cells (arrows) embracing serous acini (S). Striated duct (SD). (E) The interlobular ducts (arrows) are located in the connective tissue septa (CT) between the lobules (Lu) and fuse with similar ducts draining other lobules forming the interlobar ducts (*). (F,G) The parotid duct of dog is lined by stratified columnar epithelium. (I) the parotid salivary gland of dog showing weak positive reaction for PAS stain within the secretory acinar cells (S) and negative reaction within the duct system (D).
Parotid salivary gland: The parotid salivary gland was surrounded by a thin connective tissue capsule, which contained large blood vessels. Septa from the capsule divided the substance of the gland into lobes. These septa, containing the large blood vessels and ducts, gave off smaller septa which divided the lobes into lobules (Figure 5A).
The parotid salivary gland of the dog was composed of pure serous acini, which were closely packed together. No mucous secretory units could be detected. The serous acini were generally spherical and they had very narrow central lumina which were surrounded by pyramidal shaped secretory cells and basely situated myoepithelial cells. The secretory cells had somewhat basely located rounded nuclei. These secretory acini were drained by intercalated ducts which lead to the striated ducts and consequently to a larger interlobular duct. The intercalated ducts were small, short, and few in section. They were composed of short highly basophilic cuboidal cells. The striated ducts wereprominent and easily recognized. They were lined by tall columnar cells with large nuclei located centrally or towards the apex of the cell. The cytoplasm stained intensely with eosin and had basal striations (Figure. 5B-D). The interlobular ducts were located in the connective tissue septa between lobules. They were lined by simple columnar epithelium, which changed to stratified cuboidal then stratified columnar as the ducts became larger and fused with similar ducts draining other lobules forming the interlobar ducts (Figure 5E). Finally, the interlobar ducts converged and joined to form the main parotid duct which is lined by stratified columnar epithelium (Figure. 5F, G). Histochemically, the secretory acinar cells exhibited weak positive reaction for PAS stain and negative reaction for alcian blue stain. The duct system revealed negative reaction for PAS stain (Figure. 5H).
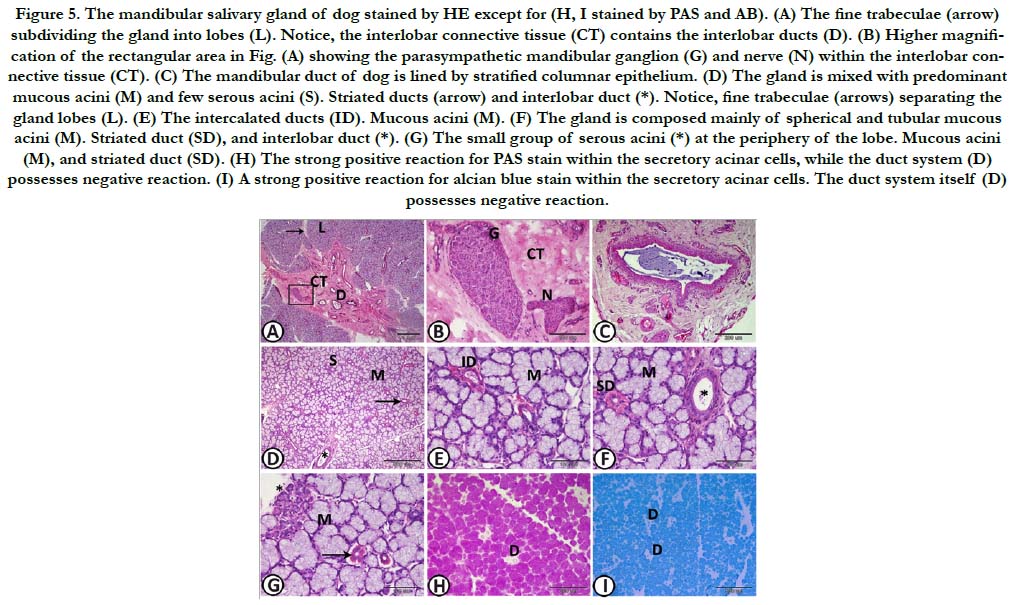
Figure 5. The mandibular salivary gland of dog stained by HE except for (H, I stained by PAS and AB). (A) The fine trabeculae (arrow) subdividing the gland into lobes (L). Notice, the interlobar connective tissue (CT) contains the interlobar ducts (D). (B) Higher magnification of the rectangular area in Fig. (A) showing the parasympathetic mandibular ganglion (G) and nerve (N) within the interlobar connective tissue (CT). (C) The mandibular duct of dog is lined by stratified columnar epithelium. (D) The gland is mixed with predominant mucous acini (M) and few serous acini (S). Striated ducts (arrow) and interlobar duct (*). Notice, fine trabeculae (arrows) separating the gland lobes (L). (E) The intercalated ducts (ID). Mucous acini (M). (F) The gland is composed mainly of spherical and tubular mucous acini (M). Striated duct (SD), and interlobar duct (*). (G) The small group of serous acini (*) at the periphery of the lobe. Mucous acini (M), and striated duct (SD). (H) The strong positive reaction for PAS stain within the secretory acinar cells, while the duct system (D) possesses negative reaction. (I) A strong positive reaction for alcian blue stain within the secretory acinar cells. The duct system itself (D) possesses negative reaction.
Mandibular salivary gland: The mandibular salivary gland was surrounded by a thick connective tissue capsule which sent fine trabeculae subdividing the gland into lobes. therefore, the supporting tissue of the gland was very weak. On the contrary, the parenchymatous tissue was very dense and compact. The connective tissue contained blood vessels and interlobar ducts. The interlobar ducts joined to form the mandibular duct which resembled the parotid duct and was lined by stratified columnar epithelium (Figure. 6A-C). The mandibular gland represented a mixed gland with predominant mucous acini in addition to few serous demilunes and sporadic serous acini. The mucous acini were spherical and sometimes tubular. They were poorly stained with H&E. Their nuclei were typically condensed and flattened against the basement membrane. The intercalated ducts were short and small with narrow lumen, while the striated ducts were long and large, similar to that of the parotid gland (Figure 6D-G). Histochemically, the secretory acinar cells showed strong positive reaction for both PAS and alcian blue stains. With diastase digestion for one hour at 37˚C, the magenta colour intensity of PAS test was slightly reduced. The duct system revealed negative reaction for both PAS and alcian blue stains. Only the luminal content of the ducts showed positive reaction for both dyes (Figure 6 H, I).
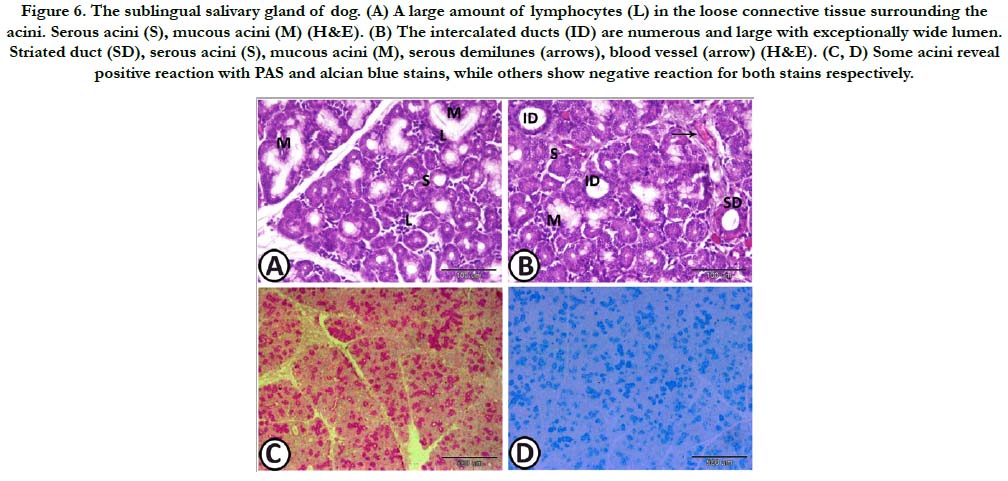
Figure 6. The sublingual salivary gland of dog. (A) A large amount of lymphocytes (L) in the loose connective tissue surrounding the acini. Serous acini (S), mucous acini (M) (H&E). (B) The intercalated ducts (ID) are numerous and large with exceptionally wide lumen. Striated duct (SD), serous acini (S), mucous acini (M), serous demilunes (arrows), blood vessel (arrow) (H&E). (C, D) Some acini reveal positive reaction with PAS and alcian blue stains, while others show negative reaction for both stains respectively.
Sublingual salivary gland: The sublingual salivary gland presented a mixed gland, its caudal part enclosed within the thick capsule of the mandibular salivary gland. It consisted of serous acini, as well as, mucous acini with serous demilunes of nearly equal density. The intercalated ducts were numerous and large with exceptionally wide lumen. The striated ducts which were few in numbers, had nearly the same diameter as the intercalated ducts with somewhat narrower lumen and higher epithelial lining. The loose connective tissue surrounding the acini and ducts contained large amount of lymphocytes (Figure 7A, B). Histochemically, some acini revealed positive reaction with PAS and alcian blue stains, while others showed negative reaction for both stains (Figure 7C, D).
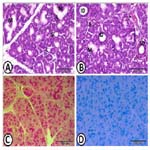
Figure 7. The zygomatic salivary gland of dog (A) showingthe gland consists principally of mucous acini (M) with small groups (S) of serous acini. Interlobular connective tissue (CT). (H&E). (B- D) Many sections of intercalated ducts (ID) in the lobule than those of the striated ducts (SD).Mucous acini (M), serous acini (S) (H&E).(E) The secretory acinar cells possess strong positive reaction for PAS stain, while the duct system (D) reveals negative reaction (PAS stain). (F) The strong positive reaction for alcian blue stain within the secretory acinar cells, while the duct system (D) exhibits negative reaction (Alcian blue stain).
Zygomatic salivary gland: The zygomatic salivary gland was a mixed gland surrounded by thin capsule, consisting principally of mucous acini. Some acini had serous demilunes. Solitary, as well as,small groups of serous acini were occasionally observed within the lobule. The intercalated ducts were large, with wide lumina. Many sections of intercalated ducts could be observed in the lobule than those of the striated ducts. The connective tissue septa between the lobules contained the interlobular ducts, which were lined by stratified cuboidal epithelium. Histochemically, the predominant mucous secretory acinar cells exhibited strong positive reaction for both PAS and alcian blue stains. With diastase digestion for one hour at 37˚C, the magenta colour intensity of PAS test was slightly reduced. The ducts displayed negative reaction for both PAS and alcian blue stains).
Discussion
The present study highlighted on the characteristic anatomical, morphometrical, as well as, histochemical features of the major salivary glands in dogs. To the best of our knowledge, neither the morphometrical nor the histological characters have been studied well in literature.
Concerning the anatomical studies of the SGs, our results were in corresponding with the findings of Thome [8], Dyce el al., [9], and Weinder et al., [10] as the parotid gland is the triangular gland that is moulded around the proximal portion of the auricular cartilage. It occupies a depression between the masseter muscle, the wing of the atlas, and the auricular cartilage. The mandibular gland is an ovoid gland in the upper neck region, contained within a strong fibrous capsule occupies a superficial position and is not covered by the parotid, with it, is fused the major sublingual gland. The zygomatic gland lies medial to the zygomatic arch.
The location of the parotid ventral to the base of the ear in dogs is similar to the location of it in other species such as human [11], rabbits [12], Koala [13], rodents [14] and rats [15]. The mandibular gland in dogs is situated caudal to the sublingual gland and such position was similarly found in the rabbits but differently, each of them has its own surrounding capsule [12]. As same as to rodent, but not to the rabbits, in dogs, from our obtained results, both mandibular and sublingual glands are enclosed within a strong fibrous capsule [10, 11]. Similar to our findings, the zygomatic gland in dogs situated ventral to the zygomatic arch [2, 9].
The morphometrical results revealed that the mandibular gland forms about 42% of the total weight of the studied salivary glands, and it is the largest and heaviest gland in dog followed by the parotid gland which forms about 34%, then comes the zygomatic gland which is about 17% and the sublingual gland forms the lowest percentage which reaches about 6%.
In contrast to other species as rabbits [12] and human [16], in which the parotid salivary gland considered the largest gland, whereas, in the dog the mandibular is the largest major salivary gland, as in the mouse [17].
Similar to the humans, the sublingual glands in dogs are found to be the smallest of the major salivary glands and also located deeply under the mucous membrane of the floor of the mouth [18].
The parenchymatous tissue of the mandibular gland in dogs is very dense and compact, while the supporting tissue is very weak resulting in indistinct lobulation of the gland. On the contrary, the parotidsalivary gland possesses distinct lobulation, thick connective tissue septa and the parenchymatous tissue is relatively loose. The mandibular gland could be considered as the master of the salivary glands in dog since it is the largest, the more parenchymatous and denser.
The surgical excision of the parotid salivary gland may be a challenge compared to the excision of the mandibular and sublingual, or zygomatic glands. This may be due to its intimately attachment to the surrounding musculature and several important neurovascular structures including; the superficial temporal artery, internal maxillary vein, rostral auricular artery and vein, buccal nerves, and facial verve and its branches. Because of the positioning of the mandibular gland between two large blood vessels; the maxillary and lingufacial veins, immobilization of the gland in situ by the index and thumb fingers, the incision over the gland directly, and dissection of the glandular tissue from the capsule may be easier to excise the gland and safer to the surrounding blood vessels. The excision of the zygomatic SG may necessitate osteotomy or ostectomy of the zygomatic arch.
In canine salivary glands, the microscopic examination of the fourc major glands revealed various proportions and distributions of serous and mucous acinar cells. Our results revealed thatthe parotidsalivary gland consists mostly of pure serous acini producing mainly a serous secretion, while the mandibular and zygomatic salivary glands are mixed glands with predominant mucous acini giving a mainly mucous secretion.
Similar findings regarding the mandibular SG were also reported in other species as in the rhesus monkeys [19], cats [20], Wistar rats [21], minipigs [22], bovine and deer [23] and European hamster [24].
EL-Kordy et al., [25] and Gomi et al., [26] recorded similar findings to ours regarding the sublingual salivary gland as a mixed gland containing serous and mucous acini of nearly the same density.
The character of purely serous parotid gland currently observed in the dog appeared similar to those previously observed in the parotid of other animal species such as rabbit [12], African palm squirrel [27], human and rodent [11], European hamster [24], deer [23], minipigs [28], goat and sheep [29], mice [30], monkeys [19], and the rat [31]. On the contrary, different results obtained from Jacob and Poddar [32] in which they described the parotid gland as mixed gland of serous and mucous acini in ferret.
Elewa et al., [33] attributed the serous type of parotid gland that observed in the herbivorous animals and the mixed one in the carnivorous could be related to the type of feeding in these animal species.
Concerning the secretion of each gland, the histochemical results of the current study showed that, the secretion of the parotid salivary gland is watery with little neutral mucin, while that of the mandibular and zygomatic salivary glands is mixed, predominantly mucous with a mixture of neutral and acid mucins. In addition, the secretion of the sublingual salivary gland is mixed; watery and mucous containing neutral and acid mucins.
The activity of the salivary glands depends on the nature of the food as a dog fed dry food produces saliva that is predominantly serous, while dogs on a meat diet secrete saliva with much more mucus. Consequently, we can suppose that, the parotid salivary gland becomes more active in dogs fed dry food since its secretion is mainly serous, while the other salivary glands especially the mandibular and the zygomatic become more active in dogs on a meat diet as their secretion is mainly mucous.Hence, in the dog that one of its salivary glands was excised, the type of its food may have to be changed according to the excised SG and upon the nature of its secretion.
Conclusion
Based on the surgical anatomy of the major SG, surgical excision of the parotid SG may be the most challenge due to vital blood vessels and nerves invading its parenchyma (the branches of the facial nerve, the maxillary and superficial temporal arteries, as well as, the internal maxillary vein). Creating an incision directly over the thick fibrous capsule of the mandibular SG and dissection of the gland from the capsule may be easier to excise the gland and safer to both; the maxillary and linguofacial veins. The excision of the zygomatic SG may necessitate osteotomy or ostectomy of the zygomatic arch.
Based on the histological and histochemistry of the major salivary glands, the dog that one of its salivary glands was excised, the type of its food may have to be changed according to the excised SG and upon its nature of secretion.
References
- Ross MH, RomrellLJ.Digestive system: oral cavity and pharynx.2ndedn. Histology Atext and Atlas.1989; 396-402.
- Evans H. The digestive apparatus and abdomen. 3rdedn. In: Miller’s anatomy of the dog. WB Saunders co, Philadelphia. 1993; 385-642.
- Splangler WL, Culbertson MR.Salivary Gland Diseases In Dogs And Cats. 245 Cases(1985-1988). J Am Vet Med Assoc. 1991 Feb 1;198(3):465-9. PMID: 2010345.
- Johnson SE.Digestive system. In: Manual Saunders small animal clinic Birchard S, editor. Roca (São Paulo).2008 ;27(6): 647.
- Harris H. On the rapid conversion of haematoxylin into haematein in staining reactions.J. Appl. Microsc. Lab. 1996: 1900(3): 777-780.
- Mc Manus JF.Histological demonstration of mucin after periodic acid. Nature. 1946; 158: 202. PMID: 20995486.
- Steedman HF. Alcian blue 8GS: a new stain for mucin. Journal of Cell Science. 1950 Dec 1;3(16):477-9.
- Thome´ H. Mundhȍhle und Schlundkopf. Textbook of the anatomy of pets.9thedn. Stuttgart, parey2004; 34: 40.
- Dyce KM, Sack WO, Wensing CJ. The head and ventral neck of the dog and cat. Textbook of Veterinary Anatomy. Fourth edition, Saunders Elsevier, St. Louis, Missouri, USA. 2010:374-406.
- Weidner S, Probst A, Kneissl S. MR anatomy of salivary glands in the dog. Anatomia, histologia, embryologia. 2012 Apr;41(2):149-53.
- Amano O, Mizobe K, Bando Y, Sakiyama K.Anatomy and histology of rodent and human major salivary glands. ActaHistochem. Cytochem. 2012; 45(5): 241- 250. PMID: 23209333.
- Al-Saffar FJ, Simawy MS. Histomorphological and histochemical study of the major salivary glands of adult local rabbits. Int. J. Adv. Res. 2014;2(11):378-402.
- Mizuno T, McKinnon A, Ichihara N, Amasaki T, Asari M, Nishita T, et al. Histological Structure and Distribution of Carbonic Anhydrase Isozymes in Major Salivary Glands in Koalas. Anatomia, histologia, embryologia. 2009 Dec;38(6):449-54.
- Jonjic S. Surgical removal of mouse salivary glands. Current protocols in immunology. 2001 Jun;43(1):1-1.
- Kimura J, Habata I, Endo H, Rerkamnuaychoke W, Kurohmaru M, Yamada J, et al. Histochemistry of complex carbohydrate in the major salivary glands of hoary bamboo rats (Rhizomyspurinosus). Anatomia, histologia, embryologia. 1998 Jun;27(3):147-53.
- Holsinger FC, Bui DT. Anatomy, function, and evaluation of the salivary glands. InSalivary gland disorders. Berlin Heidelberg: Springer-Verlag.2007; 1-16.
- Rosa F, Basso L, Valdora F, Neumaier C, Baio G. Normal mouse anatomy on 3 T MRI. European Congress of Radiology 2014.
- Saga Z, Rana R, Minhas LA, Mubarik A. Histological study of human sublingual gland with special emphasis on intercalated and striated ducts. Pakistan Armed Forces Medical Journal. 2012;62(4):606-11.
- Stephens LC, King GK, Peters LJ, Ang KK, Schultheiss TE, Jardine JH. Unique radiosensitivity of serous cells in rhesus monkey submandibular glands. The American journal of pathology. 1986 Sep;124(3):479.
- Sozmen M, Brown PJ, Cripps PJ. Quantization of histochemical staining of salivary gland mucin using image analysis in cats and dogs. Veterinary Research 1999 Jan-Feb;30(1):99-108. PMID: 10081116.
- Coire FA, Umemura AL, Cestari TM, Taga R. Increase in the cell volume of the rat submandibular gland during postnatal development. Journal of Morphological Sciences. 2017 Jan 16;20(1).
- Zhang X, Li J, Liu XY, Sun YL, Zhang CM, Wang SL. Morphological characteristics of submandibular glands of miniature pig. Chinese medical journal. 2005 Aug;118(16):1368-73.
- Adnyane IK, Zuki AB, Noordin MM, Agungpriyono S. Histological study of the parotid and mandibular glands of barking deer (Muntiacusmuntjak) with special reference to the distribution of carbohydrate content. Anat. Histol. Embryol.2010 Dec;39(6):516-20. PMID: 20682009.
- Khojasteh SM, Delashoub M. Microscopic anatomy of the parotid and submandibular salivary glands in European hamster (Cricetuscricetus L.). Int. Res. J. Appl. Basic Sci. 2012;3(7):1544-8.
- El-Kordy EA, Alanazi AD, Ali SS, Makhlouf MM, Rabah SO. Histological, histochemical and uitrastructural changes in the submandibular gland of starved young male cats. Journal of Cytology & Histology. 2014 May 20;5(4):1.
- Gomi H, Osawa H, Uno R, Yasui T, Hosaka M, Torii S, et al.Canine salivary glands: analysis of Rab and SNARE Protien Expression and SNARE Complex Formation with Diverse tissue Properties.Journal of Histochemistry and Cytochemistry. 2017; 65(11): 637-653. PMID: 28914590.
- Ekele I, Uchenna N, Okechukwu N, Isaiah A. Histology of the parotid salivary gland of the African palm squirrel (Epixerusebii). Revista de la Facultad de CienciasVeterinarias, UCV. 2013;54(1):11-6.
- Zhou J, Wang H, Yang G, Wang X, Sun Y, Song T, et al. Histological and Ultra structural characterization of developing miniature pig salivary glands. The Anatomical Record. 2010; 293: 1227-1239. PMID: 20583268.
- Elewa YH, Bareedy MH, Abuel-Atta AA, Ichii O, Otsuka S, Kanazawa T, et al. Structural characteristics of goat (Capra hircus) parotid salivary glands. Japanese Journal of Veterinary Research. 2010;58(2):121-35.
- Al Okaili AG. Histological changes of the submandibular Salirary gland of mice maintained on a liquid diet. Tikrit Journal of Pure Science. 2009;14(3):22-5.
- Kim SK.The cytochemical localization of adenylate cyclase activity in mucous and serous cells of the salivary gland. journal of Supramolecular Structure. 1976; 4(2): 185-197. PMID: 1263509.
- Jacob S, Poddar S. Gross and microscopic anatomy of the major salivary glands of the ferret. Acta. Anatomica. 1977; 98: 434-443. PMID: 883489.
- Elewa YH, Ichii O, Otsuka S, Hashimoto Y, Kon Y. Structural Changes of Goat Parotid Salivary Gland: Pre‐and Post‐Weaning Periods. Anatomia, histologia, embryologia. 2014 Aug;43(4):265-72.