Protective Effects of Hesperidin and Coenzyme Q10 on Experimental Gastrocnemius Muscle Ischemia/Reperfusion Model in Rats
Ertekin A1*, Apaydin Yildirim B2
1 Department of Biochemistry, Faculty of Veterinary Medicine, Ondokuz Mayıs University, Samsun, Turkey.
2 Department of Biochemistry, Faculty of Veterinary Medicine, Atatürk University, Erzurum, Turkey.
*Corresponding Author
Ali Ertekin,
Faculty of Veterinary Medicine, Department of Biochemistry,
Ondokuz Mayıs University, Samsun, Turkey.
Tel: +90 3624576921
Fax: +90 3624576922
E-mail: aertekin@omu.edu.tr
Received: May 30, 2018; Accepted: July 05, 2018; Published: July 06, 2018
Citation: Ertekin A, Apaydin Yildirim B. Protective Effects of Hesperidin and Coenzyme Q10 on Experimental Gastrocnemius Muscle Ischemia/Reperfusion Model in Rats. Int J Vet Health Sci Res. 2018;6(1):219-224. dx.doi.org/10.19070/2332-2748-1800043
Copyright: Ertekin A© 2018. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
In this study, we aimed to investigate the effects of hesperidin and coenzyme Q10 on muscle ischemia/reperfusion injury in a rat model. A total of 28 Sprague-Dawley male rats were divided into four groups: Control, Sham, I/R+CoQ10 and I/R+Hesperidin groups. All rats were anesthetized with xylazine and ketamine. Except for the control group, the left lower limbs of the other 3 groups were applied to 2 hours of ischemia and 2 hours of reperfusion with tourniquet. TNF-α, IL-6, GSH, MDA levels, CAT, GPx, SOD activities were measured in the plasma. The levels of MDA with XO, CAT, SOD activities were determined in tissues. Increases in TNF-α, IL-6 and MDA levels, decreases in the CAT, GPx and SOD activities were significant in the sham group according to the control group but the differences in GSH were insignificant in plasma samples. The decreases in plasma TNF-α, IL-6 and MDA levels, the increases in CAT, GPx and SOD activities were statistically significant in the experimental groups compared to the sham group, but the GSH differences were insignificant. MDA and XO levels were higher in the experimental groups than in the sham and control groups, CAT and SOD levels decreased, these differences were statistically significant. The results of this study support the possibility that hesperidin and CoQ10 may play a protective role against skeletal muscle injury caused by I/R in rats by reducing oxidative stress. Hence, hesperidin and coenzyme Q10 may be useful adjunctive therapeutic agents in some operations suspected of organ I/R damage.
2.Abbreviations
3.Introduction
4.Material and Methods
4.1 Animals and Experimental Design
4.2 Biochemical Analyzes in Plasma
4.3 Biochemical Analyzes in the Gastrocnemius Muscle
4.4 Statistical Analysis
5.Results
6.Discussions
7.References
Keywords
Antioxidants; CoQ10; Hesperidin; Ischemia/Reperfusion; Lipid Peroxidation.
Abbreviations
ROS: Reactive Oxygen Species; MDA: Malondialdehyde; SOD: Superoxide Dismutase; CAT: Catalase; GSH: Glutathione; GPx: Glutathione Peroxidase; XO: Xanthine Oxidase; SD: Standard Deviation.
Introduction
Ischemia is called as the insufficiency or stopped of blood flow to an organ for various reasons (such as surgical procedures, thrombosis, hypovolemia, transplantation). Ischemia is the first part of ischemic reperfusion injury characterized by the shift of the metabolism to the anaerobic side. The restart of oxygenation is called reperfusion. Metabolites resulting from anaerobic metabolism are oxidised by reperfusion and are confused with circulation and are responsible for distant organ damage. The destructive effects of the reperfusion process, which occurs after resumption of blood flow in the living tissue of ischemic remainder, is wider and more severe than ischemic damage. Continuous leukocyte migration to ischemic tissue after reperfusion allows the continuation and enlargement of tissue damage due to ischemia reperfusion (I/R) [15]. Reperfusion related damage in the cell is created by many factors, mostly including oxygen-derived free radicals, which are rapidly generated in the tissue as a result of reperfusion. Owing to physiological or pathological alterations, oxidative damage takes place with changes in favor of the oxidation process [38].
Hesperidin, a specific flavonoid glycoside, can be isolated in large amounts from the rinds of some citrus species. Preclinical studies and clinical trials have demonstrated hesperidin has antiinflammatory, antioxidant, antimicrobial, antitumor, antifungal, antiviral, lipid lowering and insulin sensitizing properties, contributing to its therapeutical effects in various diseases [11, 20, 25, 33]. Flavonoids are polyphenolic compounds with various pharmacological properties and act as scavengers of free radicals by OH groups in their molecular structure. Hesperidin is a compound with 3 OH groups that maintain a greater antioxidant potency and ability to activate cellular antioxidant preventing enzymes than other flavanones [18]. Coenzyme Q10 is an electron carrier proton translocator in the respiratory chain of mitochondria, and is known as a potent antioxidant either by direct removal of free radicals or indirect by regeneration of vitamin E [1].
Reactive oxygen species (ROS) mainly free radicals are directly involved in oxidative damage of cellular macromolecules such as lipids, proteins and nucleic acids in tissues. They can produce a variety of pathological changes through lipid peroxidation and DNA damage. Malondialdehyde (MDA) is the breakdown product of the major chain reactions leading to the oxidation of polyunsaturated fatty acids and thus causing oxidative stress. There are also antioxidant defense systems against different oxidants in the organism. These systems such as antioxidant vitamins, superoxide dismutase (SOD), catalase (CAT), glutathione (GSH) and glutathione peroxidase (GPx) protect the cells against lipid peroxidation [42]. The reactive oxygen species are reported to oxidise biomolecules and cause extensive lipid peroxidation in biological membranes, which lead to cell death and tissue injury. The antioxidant systems protect the cellular biomolecules against damage caused by free radicals. They involve enzymes such as SOD, GPx and CAT and non enzyme factors such as GSH and vitamins. Changes in circulating levels of the antioxidants are indications of the occurrence of oxidative stress [12]. Another source of free radicals is xanthine oxidase (XO). This enzyme exist as NAD dependent dehydrogenase and is converted into XO. XO directly transfers electrons from the oxidation of hypoxanthine to molecular oxygen, producing ROS such as superoxide and hydrogen peroxide; both of these are very toxic and can interact with free fatty acids such as arachidonic acid which via prostaglandin synthesis generates more ROS [44].
After reperfusion, cytokines such as IL-1, IL-6 and TNF-α are observed in the circulation. Using antagonists against these agents, it has been shown that both IL-1 and TNF-α contribute to vascular injury and increase endothelial adhesion molecules. Although the release of cytokines is known in I/R, it is not known whether the effects of these cytokines on permeability are directly or indirectly through cell adhesion molecule expression and neutrophil adhesion activation [15]. In this study, we investigated hesperidin and CoQ10’s protective effects in gastrocnemius muscle I/R injury.
A total of 28, Sprague–Dawley male rats (8-9 weeks), weighing 200–250g were used in the present study. The experiments were conducted according to the ethical norms approved by the Ethic Committee of Experimental Animal Teaching and Researcher Center (No: 30.03.2018, 36643897-000-E.1800090274-59). Rats were obtained from the Medical and Experimental Application and Research Center (ATADEM), Erzurum, Turkey. Throughout the animal experiments were processed following the internationally accepted ethical guidelines for the care of laboratory animals. Prior to the experiments, rats were kept in standard laboratory conditions under natural light and dark cycle (12 hours light/12 hours dark, 21°C (+/-2)) and were fed with standard food for one week, in order to adapt to the laboratory conditions. Before from the experiments, they were fasted overnight, but allowed free access to water. All rats were anesthetized with xylazine (8 mg/kg) and ketamine (60mg/kg). Except for the control group, the left lower limbs of the other 3 groups were applied to 2 hours of ischemia and 2 hours of reperfusion with tourniquet. Seven animals were used for each group of study. Rats were divided into the following groups:
Control Group: 0.5 mL 0.25% CMC was administered by gastric gavage 3 times at 8 hour intervals. Blood samples were obtained from the aorta abdominalis under anesthesia for biochemical analyzes. Tissue specimens were taken under anesthesia from the left extremity.
Sham Group: 0.5 mL 0.25% CMC was administered by gastric gavage 3 times at 8 hour intervals. Under the anesthesia, 2 hours tourniquet was applied to the lower extremity (low temperature and cyanotic claw marks the occurrence of ischemia). Subsequently, the tourniquets were opened and reperfusion was applied for 2 hours (the pinking of the claws and the increase in temperature indicate reperfusion). and afterwards, the abdomen was opened with midline incision, and blood samples for biochemical analysis were taken from Aorta abdominalis. Tissue samples were taken from the left extremity.
I/R+CoQ10 Group: Prior to ischemia formation, rats were dosed with gastric gavage at a dose of 10 mg/kg (3 times at 8 hour intervals) from 0.5 mL CoQ10 (SOLGAR) (It was dissolved in 0.25% CMC). After 30 minutes, under anesthesia, 2 hours tourniquet was applied to the lower extremity. Subsequently, the tourniquets were opened and reperfusion was applied for 2 hours, and afterwards, blood and tissue samples were taken as they were in the sham group.
I/R+Hesperidin Group: Prior to ischemia, rats were given 100 mg/kg (3 times at 8 hour intervals) from 0.5 mL Hesperidine (Sigma Aldrich St Louis, USA) (It was dissolved in 0.25% CMC) by gastric gavage. After 30 minutes, under anesthesia, 2 hours tourniquet was applied to the lower extremity. Subsequently, the tourniquets were opened and reperfusion was applied for 2 hours, and afterwards, blood and tissue samples were taken as they were in the sham group.
In the analysis of TNF-α (KHC3011, Biosource-USA) and IL-6 (Affymetrix eBioscience Rat IL-6 Platinum ELISA BMS625, Avusturya), rat-specific double antibody sandwich ELISA kit was used. GSH levels was measured according to method described by Tietze [39], malondialdehyde was analysed by the method of Yoshioka et al., [43], GPx levels were determined according to methods of Matkovics et al., [22] and catalase activity was determined with a spectrophotometric assay of hydrogen peroxide [13]. SOD activities were determined using xanthine and nitroblue tetrazolium as the substrates, and were calculated from percent inhibition of formazan production [37].
MDA [29] levels, CAT [13], SOD [37] and XO activities [16] were measured with Biotek ELISA Reader (Bio Tek μQuant MQX200 Elisa reader/USA).
The statistics of the results obtained were evaluated and the results were interpreted. For the analysis of variance, the SPSS 20.0 package program Tukey test was used. The results were expressed as mean ± standard deviation (SD).
Results
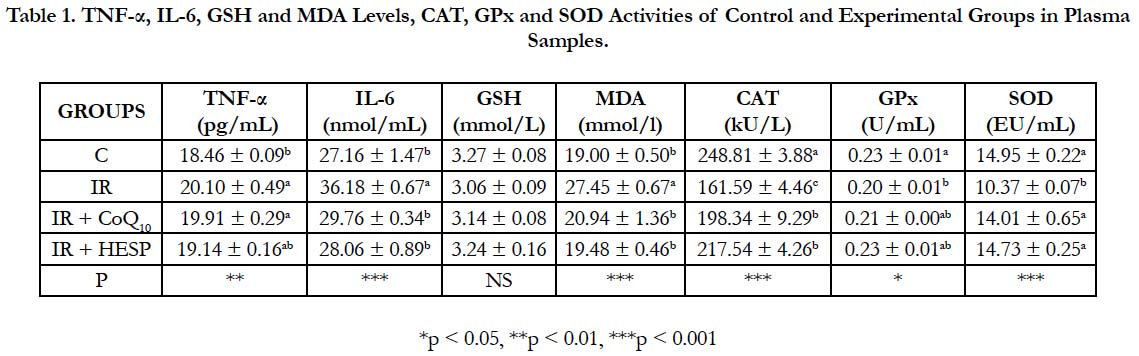
Presented in Table 1 are concentrations of TNF-α, IL-6, GSH, MDA, CAT, GPx and SOD in plasma samples of the control and experimental groups. Statistical analysis showed that, concentrations of IL-6 (p < 0.001), MDA (p < 0.001) and TNF-α (p < 0.01) were higher and CAT (p < 0.001), GPx (p < 0.05) and SOD (p < 0.001) activities were lower in the IR group, IR+CoQ10 and IR+HESP groups according to control group. There was no statistically significant differences for GSH levels in all groups to control group.
Table 1. TNF-α, IL-6, GSH and MDA Levels, CAT, GPx and SOD Activities of Control and Experimental Groups in Plasma Samples.
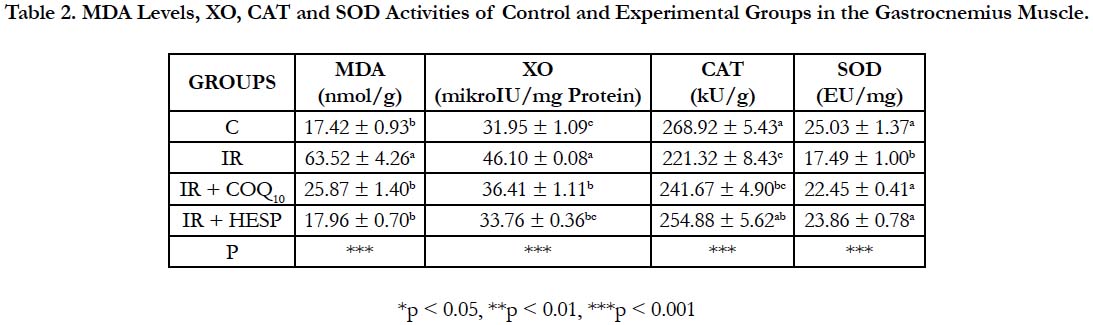
MDA levels and XO activities were markedly increased (p<0.001) and CAT and SOD activities were decreased (p<0.001) in all groups compared to control group in the gastrocnemius muscle. GSH levels did not show statistical significance in all groups (Table 2).
Table 2. MDA Levels, XO, CAT and SOD Activities of Control and Experimental Groups in the Gastrocnemius Muscle.
Discussions
The severity of the damage caused by ischemia varies according to the type of cell, differentiation, susceptibility to trauma, blood requirement and metabolism. Skeletal muscle and bowel mucosa are the most sensitive tissues to ischemia. I/R injury is common clinical table for the cerebrovascular ischemia, myocardial infarction, shock, sepsis, burns, trauma and thrombolytic therapy, coronary angioplasty, transplantation, cardiopulmonary bypass, aneurysm surgery, peripheral arterial surgery and medical and surgical interventions. Even if the cause of acute ischemia is removed and reperfusion is achieved, the risk of mortality and morbidity continues [7, 15, 34]. Reperfusion can then worsen the injury by inducing cellular infiltration and generating ROS, which leads to further cell damage. Tissues were shown to accumulate free oxygen radicals within the first few minutes of reperfusion, and postischemic endothelium is the main source of these radicals. The most significant damaging effect of free radicals on tissues is lipid peroxidation. Oxygen-free radicals cause cellular injury by inducing lipid peroxidation, which results in functional and structural cell alterations. The tissue damage caused by the production of ROS can trigger several defense mechanisms. The first-line defense mechanism includes antioxidants such as SOD, CAT, and GSH. These enzymes catalyze the conversion of ROS into less reactive species [41]. Oxidant/antioxidant imbalance caused by the increased production of ROS are the main causative factors in the oxidative damage of cellular structures and molecules. In particular, biological membranes rich in unsaturated fatty acids are cellular structures exposed to free radical attack. Therefore, increased plasma and muscle specimens MDA levels in I/R group according to control group might be due to the higher rate of oxidative metabolic activity and the higher concentration of readily oxidisable membrane polyunsaturated fatty acids. The greater extent of lipid peroxidation in the co-exposed group indicates a higher degree of free radical insult to the cellular membranes possibly due to an additive or synergistic effect of the two compounds on variety of reactive oxygen species [23]. In our study, increases in plasma (Table 1) and muscle (Table 2) samples MDA levels in CoQ10 and hesperidin groups were not as high as those in the I/R group. The lower increases in these groups may be due to the antioxidant properties of CoQ10 and hesperidin. Continuous leukocyte migration to ischemic tissue after reperfusion occurs. Activated neutrophils increase the degree of injury by further promoting oxidative injury and inflammation. In some studies, decreases in sequestration of inflammatory cells in pre-treatment with hesperidin have been observed [26, 30]. Due to its antioxidant and anti-inflammatory properties, hesperidin and CoQ10 can be recommended as a therapeutic agent in I/R induced organ damage.
Flavonoids are known for their strong antioxidant properties, protecting tissues against oxidative stress. Several reports have suggested that diseases associated with oxidative stress and inflammatory diseases may be beneficially influenced by flavonoids which can directly quench free radicals, inhibit enzymes of oxygen reduction pathways and sequester transient metal cations [27, 40]. Phytochemicals extracted from natural resources, such as vegetables, fruits and herbs, have come into the spotlight as potential treatment for inflammatory diseases. Hesperidin, a flavanone glycoside in citrus fruits, exerts anti-inflammatory, antioxidant and immune regulation actions [10]. In some previous studies, it has been shown that hesperidin reduces reactive oxygen species in ischemia-reperfusion injury and thus prevents ischemia damage [14, 32].
CoQ10 was known only as a component of the mitochondrial electron transport chain until recently, but further articles have revealed that the reduced form of CoQ10 is also a potent antioxidant substance. It prevents the peroxidation of the cell membrane and subcellular lipids, which occurs during I/R injury. After ischemic tissue is reperfused, free radicals are released and cause tissue damage through multiple mechanisms, including lipid peroxidation. CoQ10 exerts its antioxidant effect at the beginning of lipid peroxidation, and its physiological concentration is related to its activity level [5].
CAT is considered by many scientists as a significant and sensitive biomarker of oxidative stress, better than SOD. CAT also are the main enzyme of the enzymatic antioxidant defence system responsible for protection against an increase in ROS production. H2O2, formed by the catalytic reaction of SOD, is both a reactive form of oxygen and a normal cellular metabolite and it is further detoxified by GPx and CAT. The catabolism of H2O2 leads to the formation of the superoxide radical anion [31, 35]. The data of the present study show that CAT activity was significantly reduced in I/R group (p < 0.001 in plasma and muscle samples) according to control group. The reduced activity of CAT in I/R group plasma and muscle specimens could be due to depletion or inhibition as a result of the increased production of free radicals.
In the enzymatic antioxidant defence system, SOD, is copper–zinc containing enzyme that dismutase superoxide ions produced with consequent formation of H2O2. This H2O2 is then either decomposed by catalase or reduced by a GSH dependent mechanism catalysed by GPx. As a result of an autooxidation of electron transport chain components, superoxide radicals are produced in mitochondria and endoplasmic reticulum. SOD converts superoxide into H2O2 and O2 [6]. Stress conditions, in which free radical generation occurs, result in the decrease in antioxidant enzyme activity, owing to their excessive utilisation [17]. The observed decrease in SOD activity in I/R group plasma and muscle specimens could result from inactivation by H2O2. In our study, CAT, SOD and GPx activities were decreased in all groups compared to control group. This decline brings to mind the free radical production resulting in the depletion of this enzyme owing to its excessive utilisation. The antioxidant effect of hesperidin is supported by the findings that it protects against oxidative damage via inhibition of lipid peroxidation and by restoration of cytosolic catalase and glutathione peroxidase activities [9, 21].
GPx is the general name of an enzyme family with peroxidase activity whose main biological role is to protect the organism from oxidative damage. The biological function of GPx is to reduce lipid hydroperoxides conversion to their corresponding alcohols and to reduce free H2O2 reaction [2]. Declines in circulating levels of the GPx could have been resulted from the occurrence of oxidative stress.
XO is a cytosolic metallo flavoprotein that has been implicated in the pathogenesis of a wide spectrum of diseases, and is thought to be the most important source of oxygen free radicals and cell damage during reoxygenation of hypoxic tissues [3]. Its reoxidation involves molecular oxygen which acts as electron acceptor, and during this reaction, superoxide radical and H2O2 are produced. The superoxide radical is transformed into H2O2 and O2 either spontaneously or by the catalytic action of SOD. Thus, the over-activity of XO leads to the deposition of uric acid in the susceptible tissues, and this triggers the inflammatory pathways with a concomitant release of reactive oxygen species [8]. In our study, XO activity was higher in the I/R group (p < 0.001) than in the control group. XO activity was also high in CoQ10 and Hesperidin given groups, but this increase was not as high as in the I/R group.
GSH is important for the detoxification of toxicants, thus measurement of its activity is considered as a good indicator of antioxidant status or oxidative stress. GSH is a major endogenous antioxidant that participates in detoxification reactions and counterbalances free radical mediated damage by eliminating the compounds responsible for lipid peroxidation. There is an inverse relationship between oxidative stress and GSH levels due to increase in the utilisation [12]. The decline in GSH levels in all groups could be due to increased utilisation of this intracellular antioxidant by GPx or GST. In addition, this declination can also be justified either due to the inhibited synthesis of GSH or increased utilisation of GSH for detoxification of toxicant induced free radicals [4, 36]. In our study, reduced GSH levels in plasma samples of I/R, I/R+CoQ10 and I/R+Hesperidin groups appear to be a consequence of oxidant damage and may be attributed mainly to the antioxidative effects of hesperidin.
IL-6 has both proinflammatory and antiinflammatory effects. Formerly named B-cell stimulatory factor 2, IL-6 is multifunctional cytokine produced by T cells, B cells, monocytes, fibroblasts, keratinocytes, endothelial cells, mesangial cells and several tumor cells [28]. It is currently accepted that oxidation and inflammation are interlinked processes and have many feedback loops. Thus, overproduction of oxidant compounds can induce aninflammatory response, since oxidants are inflammation effectors. In fact, oxidants not only cause oxidative damage but also act as intracellular signals in inflammatory processes, particularly up-regulating pro-inflammatory genes. Indeed, ROS are considered as second messengers in the inflammatory response acting as inflammatory mediators through the activation of leukocytes, in which the expression of other mediators such as cytokines, chemokines and adhesion molecules are enhanced. Moreover, inflammatory compounds could also induce an oxidative stress situation [24]. As shown in Table 1, compared with control group IL-6 (p < 0 .01) and TNF-α (p < 0.001) showed significantly increased in I/R group. Statistically significant reductions in serum IL-6 and TNF-α were observed in CoQ10 and Hesperidin treated groups according to I/R group (Table. 1). These results showed that CoQ10 and hesperidin could reduce the inflammatory response. In a study conducted by Wang et al. on the effects of ginsenoside on cerebral I/R, were observed decreases in IL6 and TNF-α levels in the ginsenoside-treated group compared to the sham group [19].
In this study, protective effects of hesperidin and coenzym Q10 against skeletal muscle ischemia reperfusion injury have been revealed. The results of this study support the possibility that hesperidin and CoQ10 may play a protective role against skeletal muscle injury caused by I/R in rats by reducing oxidative stress. Hence, hesperidin and coenzyme Q10 may be useful adjunctive therapeutic agents in some operations suspected of organ I/R damage.
References
- Alkholy UM, Abdalmonem N, Zaki A, Elkoumi MA, Hashim MI, Basset MA, et al. The antioxidant status of coenzyme Q10 and vitamin E in children with type 1 diabetes. J Pediatr (Rio J). 2018 Feb 7. doi: 10.1016/j. jped.2017.12.005. PubMed PMID: 29425798.
- Aly N, Kawther EG, Mahmoud F, El-Sebae AK. Protective effect of vitamin C against chlorpyrifos oxidative stress in male mice. FAO. 2010 May 1;97(1):7-12.
- Mittal A, Phillips AR, Loveday B, Windsor JA. The potential role for xanthine oxidase inhibition in major intra-abdominal surgery. World J Surg. 2008 Feb;32(2):288-95. PubMed PMID: 18074171.
- Beutler E. Nutritional and metabolic aspects of glutathione. Annu Rev Nutr. 1989;9:287-302. PubMed PMID: 2669875.
- Özalp B, Elbey H, Aydın H, Tekkesin MS, Uzun H. The effect of coenzyme Q10 on venous ischemia reperfusion injury. J Surg Res. 2016 Aug;204(2):304-310. doi: 10.1016/j.jss.2016.04.075. Epub 2016 May 7. PubMed PMID: 27565065.
- Dubey N, Raina R, Khan AM. Toxic effects of deltamethrin and fluoride on antioxidant parameters in rats. Fluoride. 2012 Jul 1;45(3):242-6.
- Eltzschig HK, Collard CD. Vascular ischaemia and reperfusion injury. Br Med Bull. 2004 Oct 19;70:71-86. PubMed PMID: 15494470.
- Irondi EA, Oboh G, Agboola SO, Boligon AA, Athayde ML. Phenolics extract of Tetrapleura tetraptera fruit inhibits xanthine oxidase and Fe2+- induced lipid peroxidation in the kidney, liver, and lungs tissues of rats in vitro. FSHW. 2016 Mar 1;5(1):17-23.
- Gandhi C, Upaganalawar A, Balaraman R. Protection against in vivo focal myocardial ischemia/reperfusion injury-induced arrhythmias and apoptosis by hesperidin. Free Radic Res. 2009 Sep; 43(9):817-27. doi: 10.1080/10715760903071656. PubMed PMID: 19579067.
- Li G, Chen MJ, Wang C, Nie H, Huang WJ, Yuan TD, et al. Protective effects of hesperidin on concanavalin A-induced hepatic injury in mice. Int Immunopharmacol. 2014 Aug;21(2):406-11. doi: 10.1016/j.intimp. 2014.05.018. PubMed PMID: 24867793.
- Garg A, Garg S, Zaneveld LJ, Singla AK. Chemistry and pharmacology of the citrus bioflavonoid hesperidin. Phytother Res. 2001 Dec; 15(8):655-69. PubMed PMID: 11746857.
- Gill KK, Sandhu HS, Kaur R. Evaluation of lipid peroxidation and antioxidant status on fenvalerate, nitrate and their co-exposure in Bubalus bubalis. Pestic Biochem Physiol. 2015 Sep;123:19-23. doi: 10.1016/j. pestbp.2015.01.013. PubMed PMID: 26267048.
- Goth L. A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta. 1991 Feb 15;196(2-3):143-51. PubMed PMID: 2029780.
- Avci G, Kadioglu H, Sehirli AO, Bozkurt S, Guclu O, Arslan E, et al. Curcumin protects against ischemia/reperfusion injury in rat skeletal muscle. J Surg Res. 2012 Jan;172(1):e39-46. doi: 10.1016/j.jss.2011.08.021. PubMed PMID: 22079841.
- Güneysu E.Protective effect of iloprost in experimental ischemia-reperfusion model on to gastrocnemius muscle damage. Enemy Expertise Thesis. 2014. p.1-2.
- Hashimoto S. A new spectrophotometric assay method of xanthine oxidase in crude tissue homogenate. Anal Biochem. 1974 Dec; 62(2):426-35. PubMed PMID: 4441740.
- Hertwig B, Streb P, Feierabend J. Light dependence of catalase synthesis and degradation in leaves and the influence of interfering stress conditions. Plant Physiol. 1992 Nov;100(3):1547-53. PubMed PMID: 16653156.
- Kara S, Gencer B, Karaca T, Tufan HA, Arikan S, Ersan I, et al. Protective effect of hesperetin and naringenin against apoptosis in ischemia/reperfusioninduced retinal injury in rats. Sci World J. 2014;30:797-824.
- Wang L, Zhao H, Zhai ZZ, Qu LX. Protective effect and mechanism of ginsenoside Rg1 in cerebral ischaemia-reperfusion injury in mice. Biomed Pharmacother. 2018 Mar;99:876-882. doi: 10.1016/j.biopha.2018.01.136. PubMed PMID: 29710487.
- Li C, Schluesener H. Health-promoting effects of the citrus flavanone hesperidin. Crit Rev Food Sci Nutr. 2017 Feb 11;57(3):613-631. PubMed PMID: 25675136.
- Mahmoud AM, Ashour MB, Abdel-Moneim A, Ahmed OM. Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats. J Diabetes Complications. 2012 Nov-Dec;26(6):483-90. doi: 10.1016/j.jdiacomp.2012.06.001. PubMed PMID: 22809898.
- Matkovics B. Determination of enzyme activity in lipid peroxidation and glutathione pathways. Laboratoriumi Diagnosztika. 1988;15:248-50.
- Meral I, Mert H, Mert N, Deger Y, Yoruk I, Yetkin A, et al. Effects of 900-MHz electromagnetic field emitted from cellular phone on brain oxidative stress and some vitamin levels of guinea pigs. Brain Res. 2007 Sep 12;1169:120-4. PubMed PMID: 17674954.
- Bauer ME, Fuente Mde L. The role of oxidative and inflammatory stress and persistent viral infections in immunosenescence. Mech Ageing Dev. 2016 Sep;158:27-37. doi: 10.1016/j.mad.2016.01.001. PubMed PMID: 26773975.
- Mulvihill EE, Burke AC, Huff MW. Citrus flavonoids as regulators of lipoprotein metabolism and atherosclerosis. Annu Rev Nutr. 2016 Jul 17;36:275-99. doi: 10.1146/annurev-nutr-071715-050718. PubMed PMID: 27146015.
- Bayomy NA, Elshafey SH, ElBakary RH, Abdelaziz EZ. Protective effect of hesperidin against lung injury induced by intestinal ischemia/reperfusion in adult albino rats: histological, immunohistochemical and biochemical study. Tissue Cell. 2014 Oct;46(5):304-10. doi: 10.1016/j.tice.2014.05.009. PubMed PMID: 25063207.
- Nones J, Spohr TC, Gomes FC. Effects of the flavonoid hesperidin in cerebral cortical progenitors in vitro: indirect action through astrocytes. Int J Dev Neurosci. 2012 Jun;30(4):303-13. doi: 10.1016/j.ijdevneu.2012.01.008. PubMed PMID: 22322314.
- Özbek S. IL-6 Inhibitors: Tosylumab. Turkey Special Topics Clinical Journal of Rheumatology. 2015; 8 (3): 47-50.
- Placer ZA, Cushman LL, Johnson BC. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal Biochem. 1966 Aug;16(2):359-64. PubMed PMID: 6007581.
- Raza SS, Khan MM, Ahmad A, Ashafaq M, Khuwaja G, Tabassum R, et al. Hesperidin ameliorates functional and histological outcome and reduces neuroinflammation in experimental stroke. Brain Res. 2011 Oct 28;1420:93-105. doi: 10.1016/j.brainres.2011.08.047. PubMed PMID: 21959178.
- Regoli F, Gorbi S, Frenzilli G, Nigro M, Corsi I, Focardi S, et al. Oxidative stress in ecotoxicology: from the analysis of individual antioxidants to a more integrated approach. Mar Environ Res. 2002 Sep-Dec;54(3-5):419-23. PubMed PMID: 12408596.
- Rong Z, Pan R, Xu Y, Zhang C, Cao Y, Liu D. Hesperidin pretreatment protects hypoxia–ischemic brain injury in neonatal rat. Neuroscience. 2013;255:292-9.doi:10.1016/j.neuroscience.2013.09.030. PubMed PMID: 24076349.
- Roohbakhsh A, Parhiz H, Soltani F, Rezaee R, Iranshahi M. Molecular mechanisms behind the biological effects of hesperidin and hesperetin for the prevention of cancer and cardiovascular diseases. Life Sci. 2015 Mar 1;124:64-74. doi: 10.1016/j.lfs.2014.12.030. PubMed PMID: 25625242.
- Siemionow M, Arslan E. Ischemia/reperfusion injury: a review in relation to free tissue transfers. Microsurgery. 2004;24(6):468-75. PubMed PMID: 15378577.
- Sinclair AJ. Free radical mechanisms and vascular complications of diabetes mellitus. Diabetes Rev. 1993;2(2):7-10.
- Singh SP, Coronella JA, Beneš H, Cochrane BJ, Zimniak P. Catalytic function of Drosophila melanogaster glutathione S‐transferase DmGSTS1‐1 (GST‐2) in conjugation of lipid peroxidation end products. Eur J Biochem. 2001 May;268(10):2912-23. PubMed PMID: 11358508.
- Sun YI, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988 Mar;34(3):497-500. PubMed PMID: 3349599.
- Behroozi-Lak T, Zarei L, Moloody–Tapeh M, Farhad N, Mohammadi R. Protective effects of intraperitoneal administration of nimodipine on ischemia-reperfusion injury in ovaries: Histological and biochemical assessments in a rat model. J Pediatr Surg. 2017 Apr;52(4):602-608. doi: 10.1016/j.jpedsurg.2016.09.067. PubMed PMID: 28277298.
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502-22. PubMed PMID: 4388022.
- Verbeek R, van Tol EA, van Noort JM. Oral flavonoids delay recovery from experimental autoimmune encephalomyelitis in SJL mice. Biochem Pharmacol. 2005 Jul 15;70(2):220-8. PubMed PMID: 15946653.
- Dong X, Xing Q, Li Y, Han X, Sun L. Dexmedetomidine protects against ischemia–reperfusion injury in rat skeletal muscle. J Surg Res. 2014 Jan;186(1):240-5. doi: 10.1016/j.jss.2013.07.052. PubMed PMID: 24007817.
- Yang L, Tan GY, Fu YQ, Feng JH, Zhang MH. Effects of acute heat stress and subsequent stress removal on function of hepatic mitochondrial respiration, ROS production and lipid peroxidation in broiler chickens. Comp Biochem Physiol C Toxicol Pharmacol. 2010 Mar; 151(2):204-8. doi: 10.1016/j.cbpc.2009.10.010. PubMed PMID: 19883793.
- Yoshioka T, Kawada K, Shimada T, Mori M. Lipid peroxidation in maternal and cord blood and protective mechanism against activated-oxygen toxicity in the blood. Am J Obstet Gynecol. 1979 Oct 1; 135(3):372-6. PubMed PMID: 484629.
- Yang ZP, Dettbarn WD. Lipid peroxidation and changes in cytochrome coxidase and xanthine oxidase activity in organophosphorus anticholinesterase induced myopathy. J Physiol Paris. 1998 Jun-Aug; 92(3-4):157-61. PubMed PMID: 9789800.