Efficacy of APOQUEL® for the Control of Otitis Externa Secondary to Allergic Skin Disease in Client-Owned Dogs
Gotthelf LN*
Animal Hospital of Montgomery, Atlanta Highway, Montgomery, AL, USA.
*Corresponding Author
Louis N. Gotthelf,
Animal Hospital of Montgomery,
3310 Atlanta Highway, Montgomery, AL 36109, USA.
Tel: (334) 272-2200
Fax: (334) 272-4188
E-mail: louegee@aol.com
Received: May 24, 2017; Accepted: August 28, 2017; Published: August 30, 2017
Citation: Gotthelf LN. Efficacy of APOQUEL® for the Control of Otitis Externa Secondary to Allergic Skin Disease in Client-Owned Dogs. Int J Vet Health Sci Res. 2017;5(7):208-212. dx.doi.org/10.19070/2332-2748-1700041
Copyright: Gotthelf LN© 2017. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: This single-arm, unmasked, single-site study was conducted to evaluate the efficacy of oclacitinib (APOQUEL ®) administered twice daily for 14 days with a topical anti-infective, when indicated, for the control of otitis externa secondary to allergic skin disease in dogs. Thirteen (13) client-owned dogs were enrolled; three (3) dogs were withdrawn for owner non-compliance. Dogs were required to be ≥1 year old, weigh 3.0-80.0 kg, have uncomplicated otitis externa that the Investigator attributed to allergic skin disease, have an Otitis Index Score of ≥6 and been withdrawn from any systemic or topical treatments with antipruritic, anti-inflammatory or anti-infective properties that could interfere with assessments. Cytology samples were collected from each ear. Ears with otitis externa were cleaned at enrollment. Oclacitinib was administered orally twice daily; in dogs with evidence of infection, enrofloxacin/silver sulfadiazine (Baytril® Otic) was administered topically twice daily in affected ears. Owner assessments were performed daily and Investigator assessments were performed on Days 7 and 14.
Results: Seven (7) female and three (3) male dogs completed the study. Ages ranged from 3.0-12.0 years and body weight ranged from 3.6-47.0 kg. Administration of oclacitinib and enrofloxacin/silver sulfadiazine twice daily for 14 days resulted in mean Investigator otitis scores improving from 19.7 on Day 0 to 3.4 by Day 14, for a mean reduction of 81.7%. Mean owner assessment improved by 94.7% (left ear) and 80.6% (right ear) during the 14-day study. Cytologic examination of the ears showed bacteria in seven left ears and seven right ears at enrollment compared to only one left ear and no right ears on Day 14. One dog had no bacteria or yeast on Day 0 compared to nine dogs with no bacteria on Day 14. Yeast was present in two left and two right ears at enrollment and in only one right ear on Day 14.
Conclusions: The results of this single-arm, single-site, unmasked study suggest that oral administration of oclacitinib with a topical anti-infective applied to affected ears twice daily for 14 days is effective for the management of otitis externa secondary to allergic skin disease in dogs.
2.Abbreviations
3.Background
4.Methods
5.Results
5.1 Study Population
5.2 Physical Examination Findings
5.3 Investigator Otitis Index Scores (OTIS)
5.4 Owner VAS Scores
5.5 Ear Cytology
5.6 Safety
6.Discussion
7.Conclusions
8.Ethics Approval and Consent to Participate
9.Funding
10.Acknowledgments
11.References
Keywords
Otitis Externa; Canine; Oclacitinib; APOQUEL.
Abbreviations
ND (Not Done); NSAID (Non-Steroidal Anti-Inflammatory); OTIS (Otitis Index Score); USA (United States of America); VAS (Visual Analog Scale).
Background
Otitis externa, an inflammation of the skin of the external ear canal, is a common problem in domestic dogs. Clinical signs of otitis externa include erythema, edema, pain, odor, pruritus, head shaking and excoriations [1, 2]. The prevalence of otitis externa has been estimated to be between 7.3 and 10.2% of cases presenting to veterinary clinics [3-6]. Primary triggers for otitis externa in dogs include atopic dermatitis, food allergy, parasites and foreign bodies, while predisposing factors include conformation of the ear, lifestyle factors causing excessive moisture in the ear canal and inappropriate treatment [7, 8]. In a retrospective study that evaluated primary causative factors for otitis externa in patients presented to a veterinary school, allergic dermatitis was the most common primary factor and was observed in 43% of the otitis externa cases evaluated [9]. A similar study found that 60% of dogs presenting with atopic dermatitis had otitis externa [10]. Bacterial infections associated with otitis externa are generally considered opportunistic and not primary pathogens [2] and can become perpetuating factors in otitis externa if not appropriately treated [8]. Treatment of otitis externa has historically focused on controlling the causative factors contributing to the individual animal’s condition [11]. Since dogs with allergic skin disease make up a large proportion of the dogs with otitis externa, controlling allergic skin disease becomes important in the management of otitis externa.
Oclacitinib (APOQUEL®) is a novel Janus kinase (JAK) inhibitor that has been shown to inhibit cytokines involved in pruritus (IL-31) and allergy and inflammation (IL-2, IL-4, IL-6 and IL- 13) [12]. Oclacitinib is currently approved for the control of pruritus associated with allergic dermatitis and for the control of atopic dermatitis in dogs that are at least 12 months of age [13]. Oclacitinib has been shown to be safe and effective for long-term use in dogs with atopic and allergic skin disease [14]. The present study was designed to evaluate the potential for oclacitinib to aid in the control of otitis externa in dogs with allergic skin disease. Due to the importance of controlling secondary bacterial infections in successful treatment of otitis externa, the present study included twice daily administration of an anti-infective in the affected ears if infection was present.
Methods
This single-site study was conducted as an exploratory, clinical study to assess the efficacy of oclacitinib (APOQUEL®) for the control of otitis externa secondary to allergic skin disease in dogs, when administered in combination with a topical anti-infective (enrofloxacin/silver sulfadiazine; Baytril® Otic). The study was conducted in client-owned dogs at a private veterinary practice in Montgomery, Alabama, USA, and each owner provided written informed consent before any study procedures were performed on their dog. The protocol was approved by the Zoetis Ethical Review Board in April 2015 before the start of the study.
Healthy dogs of any gender, breed or mix of breeds presenting with otitis externa that the investigator determined was secondary to allergic skin disease were evaluated for enrollment. The otitis externa could be in one or both ears. Dogs could not be pregnant or lactating, intended for breeding, and had to weigh 3.0-80.0 kg and be at least 12 months of age. On the day of enrollment, a thorough physical examination was performed, including an assessment of the tympanic membrane. The Investigator determined the Otitis Index Score (OTIS) by assessing the horizontal and vertical ear canals in each ear for erythema, edema/ swelling, erosion/ulceration and exudate using a 0-3 scale for each variable [1]. The odor, pain and severity of otitis externa were also assessed by the investigator for each ear. A total score of 6 in at least one ear was required for enrollment. At enrollment, the owner was instructed on the use of a visual analog scale (VAS) for the severity of otitis. The clinical signs considered included odor, discharge, redness, heat, pain, swelling, itching, irritation, crusts/scales, scratching and/or rubbing. A vertical line was present on the form for each ear, and the owner was to mark a single horizontal cross mark on the vertical line at the point at which the owner thought best reflected the condition of that ear. After the assessments were completed, an ear cytology sample was collected from each ear with clinical signs of otitis externa for evaluation in the clinic’s laboratory. Separate non-sterile cottontipped applicators were used to collect a sample from each ear. Slides were prepared immediately by rolling the swab on a new pre-cleaned slide lengthwise. The left ear sample was placed on one side of the slide and the right ear sample was placed on the other side of the slide. The slides were appropriately labeled R, L and the name of the patient with pencil. Each slide was heat fixed for 1 minute and then stained with Diff Quick solution (Jorgensen Labs) according to manufacturer’s instructions. Slides were evaluated under immersion oil and the presence or absence of bacteria and/or yeast was recorded for each of five random high power fields for each ear. After cytology sample collection was complete, the affected ears were cleaned using an ear cleanser solution (Epi-Otic®, Virbac).
Dogs with incidental health conditions requiring treatment were allowed to enroll if they had been on the same treatment regimen (including hypoallergenic diets) for at least 6 weeks before enrollment and continued those treatments/diets throughout the study. All dogs were free of fleas at enrollment, and there was no restriction on administration of flea control products. No dogs enrolled in this study were receiving allergen-specific immunotherapy. Any systemic or topical treatments with known or suspected antipruritic and/or anti-inflammatory properties that could confound the assessment of the otitis externa secondary to allergic skin disease were prohibited or discontinued for periods prior to entry into the study similar to those recommended for drug withdrawal prior to intradermal testing [15]. Dogs that had previously been treated with oclacitinib were excluded.
Oclacitinib and enrofloxacin/silver sulfadiazine were both administered at the approved label doses in this study. The dose of oclacitinib (0.4 to 0.6 mg oclacitinib/kg body weight twice daily) was determined using the dosing chart on the package insert [14]. The oclacitinib dose was administered directly into the mouth or in a small amount of food. The enrofloxacin/silver sulfadiazine dose was 5-10 drops/treatment for dogs weighing 35 lb (15.9 kg) or less and 10-15 drops/treatment for dogs weighing more than 35 lb twice daily, according to the package insert [16]. Following treatment, the ear was gently massaged to ensure complete and uniform distribution of the medication throughout the external ear canal. At enrollment, the dispenser placed the appropriate number of the correct size oclacitinib tablets (3.6 mg, 5.4 mg or 16 mg) for 14 (±2) days of dosing into a pill container which was dispensed to the owner, along with a bottle of enrofloxacin/silver sulfadiazine. The owner was provided instructions on how to administer the oclacitinib (orally) and the enrofloxacin/silver sulfadiazine (topically in the ear). The owner was also instructed on how to record dose administration and was provided with the appropriate forms for recording dosing and VAS assessment data. Any abnormal health events were to be documented.
At the Day 7 (±2 days) evaluation, the investigator performed a physical examination, completed an OTIS score for each ear, performed cytology as described for the enrollment visit, and affected ears were cleaned with an ear cleansing solution (Epi- Otic®). Day 14 (±2 days) was the final study evaluation. The Investigator performed a physical examination, determined the OTIS score for each ear and collected a cytology sample from each ear as described for the enrollment visit. The owner returned all study forms and remaining study medications. Owner forms and drug accountability were reviewed.
Frequency distributions of gender, spayed/neutered and breed were calculated. Descriptive statistics were calculated for age and body weight at each time point. A frequency distribution of study completion was calculated. The efficacy variables were the Investigator OTIS score and the Owner VAS. The percent reduction from baseline for each variable was determined for each dog. Only the most severely affected ear at the enrollment visit was used for the OTIS summaries. Descriptive statistics were calculated for all four efficacy variables at each time point.
A total of 13 dogs were enrolled in this study. The owners of three of the dogs did not return for subsequent visits after the enrollment visit, and these three cases were considered lost to follow-up. The data summaries include data from the 10 dogs that completed the study. Breeds represented include mixed breed (n=4), dachshund (n=2), Labrador retriever (n=2), pit bull (n=1) and West Highland white terrier (n=1). There were four spayed females, three intact females, one neutered male and two intact males. The ages of the dogs that completed the study ranged from 3 to 12 years (mean=6.7 years; median=6.0 years) and the body weights at enrollment ranged from 3.6 kg to 47 kg (mean body weight=19.7 kg; median body weight =14.0 kg). The Day 7 and Day 14 visits occurred between Days 5-12 (mean day 7.5) and Days 14-26 (mean day 15.7), respectively.
All dogs enrolled in this study showed clinical signs of otitis externa secondary to active allergic skin disease. At enrollment, clinical signs of allergic otitis externa observed included pruritus, hyperplasia, wax accumulation, erythema and stenosis. Clinical signs of otitis externa improved in five dogs by Day 7 and had completely resolved in three dogs at that time. By the end of the study, clinical signs of otitis externa had completely resolved in seven of the 10 dogs.
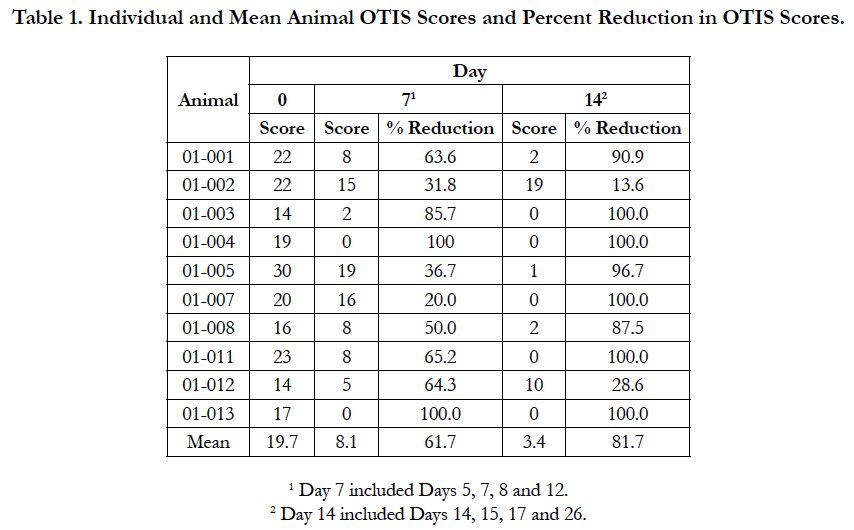
The individual animal OTIS scores for the ear that was most severely affected on Day 0 and the percent reduction in score from Day 0 are presented in Table 1. From Day 0 to Day 7, the OTIS scores decreased for all dogs, with the percent reduction in score ranging from 20.0 to 100%. From Day 7 to Day 14, the OTIS scores decreased for eight dogs, but increased for the remaining two dogs. The Day 14 OTIS scores for these two dogs were numerically lower than their Day 0 scores, indicating improvement from baseline. The percent reduction in Day 14 OTIS scores compared to the Day 0 scores ranged from 13.6% to 100%.
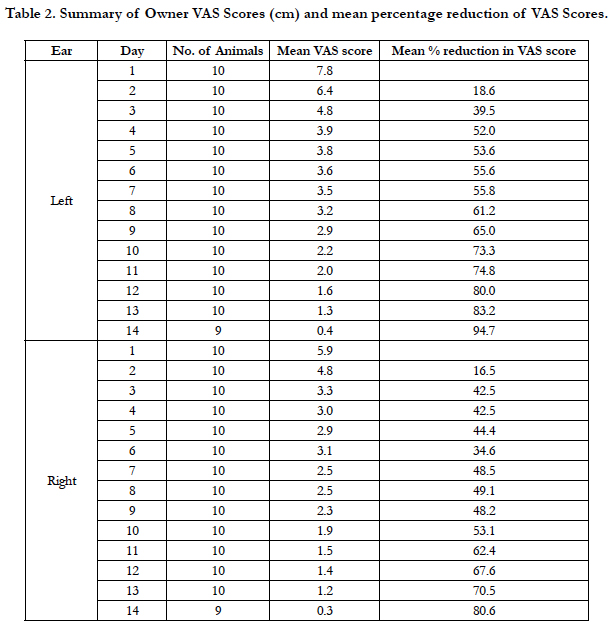
Each owner assigned VAS scores for each of their dog’s ears daily during the study (Table 2). Mean owner VAS scores for the left ear decreased each day throughout the study, while the mean scores for the right ear decreased daily except for Day 6 and 8. A summary of the percentage reduction in owner VAS scores is presented in Table 2. A 50% reduction in the owner VAS scores for the left ear was achieved on day 4; a 50% reduction in the owner VAS score for the right ear was not observed until Day 10. On Day 14, owner VAS scores were reduced 94.7% for the left ear and 80.6% for the right ear.
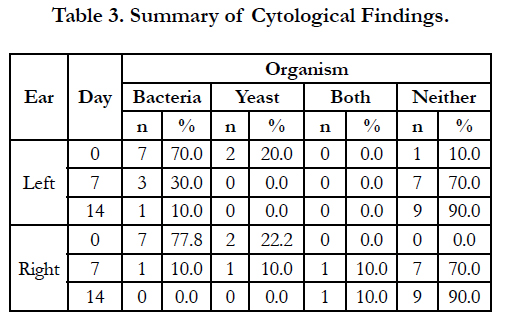
A summary of cytology findings is presented in Table 3. At enrollment, bacteria alone were found in 7/10 left ears and 7/9 right ears, while yeast was found in 2/10 left ears and 2/9 of right ears. No dog had both bacteria and yeast in either ear on Day 0. By Day 14, bacteria alone were found in 1/10 left ears and none of the right ears, while bacteria and yeast were again found in 1/10 right ears. Yeast alone was not found in any ears on Day 14.
No adverse events were reported during this study. Some loose stools were noted in one dog at study exit on Day 26, but as this was 12 days after the end of dosing, it was not considered an adverse event to medications used in the study.
Discussion
The results of this study showed that administration of oclacitinib with a topical anti-infective was effective for the management of otitis externa in dogs with allergic skin disease. In the present study, the OTIS scores showed mean percent reduction in the most severely affected ear of 61.7% on Day 7 and 81.7% on Day 14. Half of the dogs achieved OTIS scores of 0 on Day 14, confirming complete recovery from clinical signs of otitis externa. Two dogs showed improvement on Day 7, but then a worsening of clinical signs on Day 14. Both had modest improvement on Day 14 compared to Day 0.
In addition to the Investigator OTIS scores, the owners also completed VAS assessment for each ear on each day of the study. Although the percentage improvements in the VAS scores were comparable to percent improvements in the OTIS scores, improvements for individual animals did not always correlate between the scales. The two dogs that showed modest improvement on the OTIS scale had 75 and 80% reduction in VAS scores on Day 14, while other dogs that showed improvement in OTIS scores did not show improvement in VAS scores. In contrast to OTIS score, the owner VAS was a single subjective assessment that included all of the animal’s clinical signs in one global score. Thus it is not surprising that there was not perfect correlation between the 2 scores.
Historically, otitis externa has been managed through ear cleaning and application of topical anti-infective products (antibiotics and antifungals) and NSAIDs [17]. Systemic glucocorticoids and antibiotics are administered in more severe cases [11, 17]. Oclacitinib may become an additional effective tool for the management of the clinical signs of otitis externa secondary to allergic skin disease. It is important to emphasize that oclacitinib would not be indicated in dogs with otitis externa not associated with primary allergic disease, where the primary underlying disease may instead be parasitic infestations, foreign bodies, or endocrinopathies.
Oclacitinib is approved for the control of pruritus associated with allergic dermatitis and for the control of atopic dermatitis in dogs. In a study of oclacitinib for the control of pruritus and skin lesions associated with allergic dermatitis, there was already a 30% reduction in pruritus VAS after 24 h, and successful reduction of pruritus (2 cm decrease in the VAS) was achieved in 44% of oclacitinib-treated dogs after 24 h [18]. The onset of action for oclacitinib was rapid in the present study as well, when oclacitinib was administered for the treatment of otitis externa secondary to allergic skin disease. Owners reported Day 3 VAS reductions of 39.5% in the left ear and 42.5% in the right ear.
No adverse events were observed by the owners or reported to the investigator in this study, providing supportive evidence of safety of oclacitinib in this population of dogs [18].
The small size of this study limits the conclusions that may be drawn. While the cases enrolled had all been diagnosed with allergic skin disease, the small number of animals limits the variety and severity of cases that were enrolled. Only one dog had been diagnosed with food allergies before being enrolled in this study. This dog responded well to treatment, and was one of the dogs with an OTIS score of 0 on Day 14. The percentage of dogs with food allergies in the present study was consistent with previous findings that found 7% of the cases with atopy had prediagnosed food allergies [10]. Another possible limitation was the low percentage of cases that had yeast infections at enrollment. In the present study, yeast was observed in 20% of the left ears and 22.2% of the right ears at enrollment. In contrast, Malassezia pachydermatis was isolated from 73% of samples from cases with bilateral otitis externa when each ear was sampled independently [19]. It is not known how these differences may have impacted the outcome of the study.
Conclusions
The results of this single-arm single-site unmasked study suggest that oral administration of oclacitinib orally together with a topical anti-infective applied to affected ears twice daily for 14 days is effective for the management of otitis externa secondary to allergic skin disease in dogs. Additional larger controlled, blinded studies are warranted to further evaluate this potential indication for oclacitinib.
Ethics Approval and Consent to Participate
The study protocol was reviewed by the Zoetis Kalamazoo Ethical Review Board prior to study initiation. Each owner provided written informed consent for their dog’s participation in the study.
Funding
Zoetis provided funding to conduct the study as well as APOQUEL tablets.
Acknowledgements
The author would like to thank Beth Oman (Beth Oman Clinical Consulting, LLC) for assistance with manuscript preparation.
References
- Nuttall T, Bensignor E. A pilot study to develop an objective clinical score for canine otitis externa. Vet Dermatol. 2014 Dec 1;25(6):530. doi:10.1111/vde.12163.
- Rosser EJ Jr. Causes of otitis externa. Vet Clin of North Am: Small Anim prac. 2004 Mar 1;34(2):459-68.
- Bartlett PC, Van Buren JW, Neterer M, Zhou C. Disease surveillance and referral bias in the veterinary medical database. Prev Vet Med. 2010 May 1;94(3-4):264-71.
- O’Neill DG, Church DB, McGreevy PD, Thomson PC, Brodbelt DC. Prevalence of disorders recorded in dogs attending primary-care veterinary practices in England. PLoS One. 2014 Mar 4;9(3):e90501. doi:10.1371/journal/pone.0090501.
- Summers JF, O'Neill DG, Church DB, Thomson PC, McGreevy PD, Brodbelt DC. Prevalence of disorders recorded in Cavalier King Charles Spaniels attending primary-care veterinary practices in England. Canine Genet Epidemiol. 2015 Apr 18;2(1):4. doi:10.1186/s40575-015-0016-7.
- O’Neill DG, Darwent EC, Church DB, Brodbelt DC. Demography and health of pugs under primary veterinary care in England. Canine Genet Epidemiol. 2016 Jun 10;3(1):5. doi:10.1186/s40575-016-0035-z.
- Paterson S, Tobias K. Atlas of Ear Diseases of the Dog and Cat. Chichester, West Sussex: Wiley-Blackwell. 2013. 97-102.
- Murphy KM. A review of techniques for the investigation of otitis externa and otitis media. Clin Tech in Small Anim Pract. 2001 Nov 1;16(4):236-41.
- Saridomichelakis MN, Farmaki R, Leontides LS, Koutinas AF. Aetiology of canine otitis externa: a retrospective study of 100 cases. Vet Dermatol. 2007 Oct 1;18(5):341-7.
- Zur G, Ihrke PJ, White SD, Kass PH. Canine atopic dermatitis: a retrospective study of 266 cases examined at the University of California, Davis, 1992-1998. Part I. Clinical features and allergy testing results. Vet Dermatol. 2002 Apr 1;13(2):89-102.
- Gotthelf LN. Perpetuating Factors and Treatment of Otitis Externa. In: Small Animal Ear Diseases. 2nd ed. St. Louis, MO: Elsevier/Saunders; 2005. 173-185.
- Gonzales AJ, Bowman JW, Fici GJ, Zhang M, Mann DW, Mitton-Fry M. Oclacitinib (APOQUEL®) is a novel Janus kinase inhibitor with activity against cytokines involved in allergy. J Vet Pharmacol Ther. 2014 Aug 1;37(4):317-24. doi:10.1111/jvp.12101.
- Zoetis[Internet]. APOQUEL US Product Label. 2013 Feb - [cited 2017 Aug 1 ]. Available from: https://www.zoetisus.com/products/dogs/apoquel/ downloads/final_apoquel_pi_030116.pdf
- Cosgrove SB, Cleaver DM, King VL, Gilmer AR, Daniels AE, Wren JA, et al. Long-term compassionate use of oclacitinib in dogs with atopic and allergic skin disease: safety, efficacy and quality of life. Vet Dermatol. 2015 Jun 1;26(3):171. doi:10.1111/vde.12194.
- Miller WH, Griffin CE, Campbell K. Hypersensitivity Disorders. In: Muller and Kirk’s Small Animal Dermatology. 7th edn. St. Louis, MO: Elsevier/ Saunders; 2013 Aug 13. 376-377.
- Bayer[Internet]. Baytril® Otic US Product Label. 2012-[cited 2017 Aug 1]. Available from: https://bayer.naccvp.com/product/view/ basic/1040010?u=bayer
- Rosychuk RAW. Management of otitis externa. Vet clin of North Am: Small Anim Pract. 1994 Sep 1;24(5):921-52.
- Cosgrove SB, Wren JA, Cleaver DM, Martin DD, Walsh KF, Harfst JA, et al. Efficacy and safety of oclacitinib for the control of pruritus and associated skin lesions in dogs with canine allergic dermatitis. Vet Dermatol. 2013 Oct 1;24(5):479-e114.
- Oliveira LC, Leite CAL, Brilhante RSN, Carvalho CB. Comparative study of the microbial profile from bilateral canine otitis externa. Can Vet J. 2008 Aug;49(8):785-8.