Cell Proliferation Study in Canine Mammary Carcinomas
Łopuszyński W1*, Hellmén E2*
1 Faculty of Veterinary Medicine, Department of Pathological Anatomy, University of Life Sciences, Głęboka 30, 20-612 Lublin, Poland.
2 Faculty of Veterinary Medicine and Animal Science, Department of Anatomy, Physiology and Biochemistry Swedish University of Agriculture Sciences, Box 7011, 750 07 Uppsala, Sweden
*Corresponding Author
Dr Wojciech Łopuszyński,
Faculty of Veterinary Medicine, Department of Pathological Anatomy,
University of Life Sciences, Głęboka 30, 20-612 Lublin, Poland.
Tel/Fax: 081-445-61-66
E-mail:wojciech.lopuszynski@up.lublin.pl
Professor Eva Hellmén,
Faculty of Veterinary Medicine and Animal Science,
Department of Anatomy, Physiology and Biochemistry,
Swedish University of Agriculture Sciences,
Box 7011, 750 07 Uppsala, Sweden.
Tel: +4618672128
Fax: +4618672111
E-mail:eva.hellmen@slu.se
Article Type: case study
Received: December 30, 2014;Accepted: February 10, 2015;Published: February 11, 2015.
Citation: Łopuszyński W, Hellmén E (2015) Cell Proliferation Study in Canine Mammary Carcinomas. Int J Vet Health Sci Res. 3(2), 39-45. doi: dx.doi.org/10.19070/2332-2748-1500011
Copyright: Łopuszyński W, Hellmén E© 2015. This is an open-access article distributed under the terms of the Creative Commons Attribution License, whichpermits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Prognosis of postsurgical behaviour of mammary carcinomas in dogs is difficult using a routine histopathological examination alone. The aim of the present study was an assessment of prognostic value of proliferating cell nuclear antigen (PCNA) and Ki-67 antigen expression in canine mammary carcinomas. Expression was evaluated with computer assisted microscopic
image analysis in surgically removed, formalin-fixed, paraffin-embedded tissue samples by means of immunohistochemistry.
The growth fraction parameters were compared with previously described data of flow cytometric DNA analysis as well as with clinical outcome based on 24-month long postoperative observation and careful postmortem examinations. In the group of dogs which died or were euthanatized due to metastases the mean Ki-67 index value was significantly higher in comparison to the group of animals which survived the observation period without malignant process progression. Such a relation was not observed for PCNA. Both Ki-67 and PCNA index values were significantly higher in the group of dogs with neoplasms that had increased levels of cells in the S-phase. The presence of a significant correlation between the Ki-67 antigen index and the clinical course after the operation, calculated using the t-student test, with the lack of such correlation in variance analysis test suggests that it should be treated only as a prognostic marker helper. The heterogenity of staining of the cell nuclei and the lack of correlations with the clinical course in case of PCNA seems to disqualify it as a prognostic factor.
2.Introduction
3.Materials and Methods
3.1 Materials
3.2 Immunohistochemistry
3.3 Evaluation
4.Results and Discussion
5.Discussion
6.References
Keywords
Ki-67; PCNA; Dog; Mammary Tumours; Immunohistochemistry; Flow Cytometry.
Introduction
Mammary gland tumours are the most frequent cancer reported in female dogs [1-3]. Among them mammary carcinomas comprise the most numerous types [3,4]. They are characterized by a variety of histological forms creating diversified microscopic picture. It is the cause of many discrepancies between classification methods and selection of morphological features as the basis for characterization of malignancy. Moreover, a high proportion of cases with recurrence and metastases following surgical procedures suggest that additional prognostic factors are needed for disease course prognosis [4-7].
Uncontrolled proliferation is one of the main causes of the transformation of a normal cell into a malignant one. The Ki-67 and the proliferating cell nuclear antigen (PCNA) are currently being widely studied using immunohistochemical method to evaluate
proliferation activity in tumours. PCNA is acid non-histonic auxiliary protein of the DNA polymerase-δ taking part in DNA replication. Its weight is 36 kDa and it consists of 261 amino acids. In the nucleus, protein appears at the end of the G1-phase of the cell cycle, the maximum concentration is achieved in the Sphase, and then gradually decreases in the G2-phase [8]. However, the Ki-67 antigen, similarly to PCNA, is an intranuclear, non-histonic protein, which during electrophoresis yields a double beam of 345 and 395 kDa weights. Its function has not been completely established yet. It has been supposed that during mitosis it supports the DNA structure. Increase in antigen Ki-67 expression occurs in the second half of the G1-phase, its level still increases in the S- and G2-phases, to achieve its peak in the M-phase and then decrease rapidly [9,10]. Both antigens are considered as cellular proliferation markers and studied within the prognostic aspect in many tumour types in humans and animals including mammary tumours [11-16].
The precise determination of the cell fractions located in the Sphase of the cell cycle based on the DNA content curve is possible by using flow cytometry analysis [17,18]. Its advantage is that the measurement is performed separately for each cell. Therefore, this is the most precise method and it can be considered as the reference method for studies on proliferation. In the case of malignant mammary tumours, correlation between the elevated Sphase fraction and clinical disease course has been demonstrated [19,20].
The aim of our study was to assess the intensity of cell proliferation on the basis of PCNA and Ki-67 expression in canine malignant mammary carcinomas, in which the S-phase fraction was determined earlier by the use of flow cytometry analysis and
which proved to be an independent predictor for survival [20].
Materials and Methods
The material for the study consisted of 47 mammary carcinomas surgically removed from the female dogs with different follow-up clinical status selected from the archival material and sections of normal glandular tissue. Based on the results of 24-month long observation, the animals whose carcinomas were studied were divided in three groups. The first group (I) consisted of 17 (36%) female dogs which survived the observation period without any malignant process progression, the second group (II) of 25 (53%) dogs that died or were euthanatized because of their mammary tumours, verified by necropsy, and/or died with X-ray verified lung metastases, and the third group (III) of 5 (11%) dogs that died from other diseases verified by post-mortem examination.
Samples for histological examination, were taken from each tumour, fixed in 10% formalin of pH = 7.2 for 24 hours and routinely processed and embedded in paraffin blocks. The histological sections were stained with haematoxylin and eosin and evaluated according to the classification recommended for canine mammary tumours in dogs [21]. Sections designed for immunohistochemical staining were placed on slides covered with monosilane. For immunohistochemical assessment ABComplex/HRP method was applied with ABC-Elite reagent (Vector). The optimal concentrations tested on normal mammary gland tissue for the Ki-67, clone MIB-1 (Dako) and PCNA, clone PC-10 (Dako) antibodies were titrated and used at 1:1000 and 1:30 000 respectively. The antigen retrieval method was evaluated for both of antibodies. The best results were obtained with decloaking chamber unmasking procedure and with Unmasking Solution (Vector) diluted 1:100 for Ki-67 antibody and citrate buffer pH=6.0 for the PCNA antibody. The sections with primary antibodies were applied for over night incubation at +4°C. 3,3-diaminobensidine-DAB (Vector) was applied as the chromogen. Counter stain was done with Mayer’s haematoxylin for 2-3 minutes. For each assay, a double control was made i.e. method control and negative control. In the negative controls the incubation with primary antibodies were replaced by incubation with mouse IgG serum respectively in the same conditions of time and temperature. The positive control was the healthy unchanged tissue of the canine mammary gland.
The evaluation of positive stained cells was performed with the semi-automatic computer image analysis system. The system consisted of: a light microscope coupled with a camera and computer with software (NIS-Elements BR-2.20, Laboratory Imaging). A lens with 40x magnification and a computer screen were used to count the percentage of positive cells in 1000 malignant cells.
After completing the computer analysis, a comparison was made of the clinical parameters concerning malignant disease course,
histological types, and evaluated proliferation indices, and also results on S-phase content previously obtained by the use of flow cytometry analysis [20]. The S-phase rate was estimated from DNA histograms. The fraction of cells located between G0/G1 and G2 phases was interpreted as proliferating cells. The percentage of cells within given channel numbers was calculated, and the S-phase was designated as low ≤5% and elevated >5% [22]. Obtained results were statistically analyzed using Statgraphics Plus v. 5.0 software and the following tests: variance analysis (ANOVA regression test) and t-student test. The significance in the variances was determined using the Fisher exact and Chi-square test.
Results and Discussion
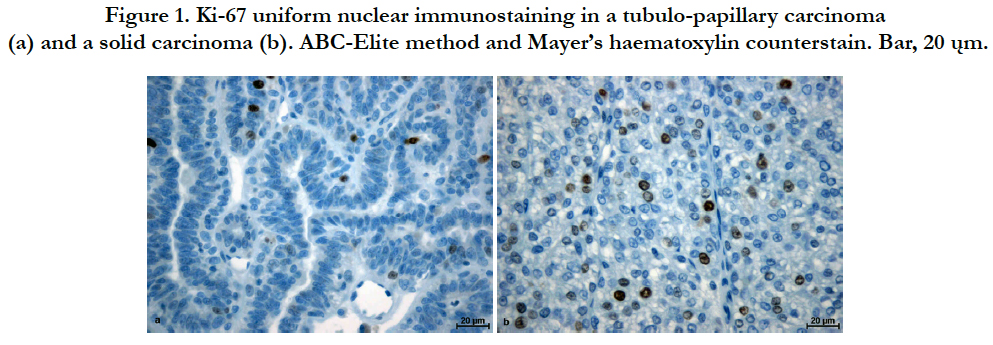
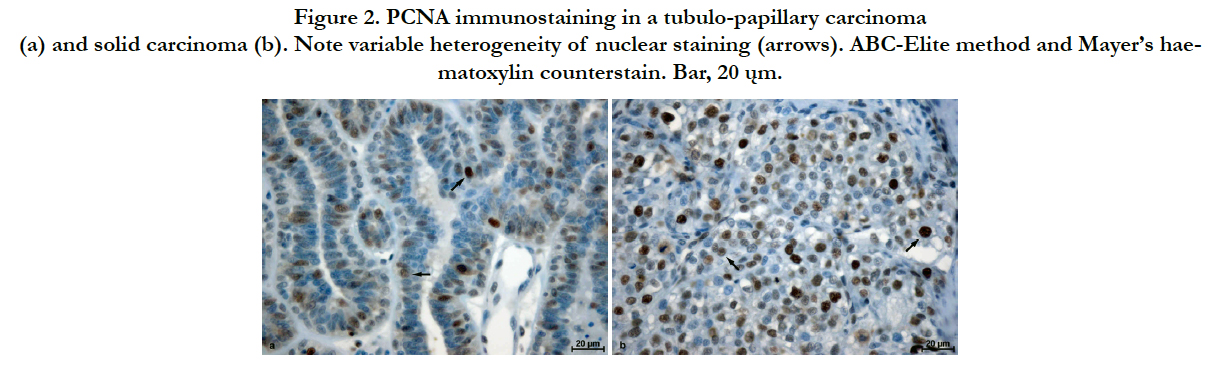
A positive reaction for PCNA and Ki-67 was observed in the cell nuclei of the carcinomas. In the case of Ki-67 the reactivity was evenly intensified for the majority of positively reacting cells (Figure 1). However, for PCNA, both staining intensity and reaction localization were characterized by great heterogeneity. Areas of small numbers of positively labelled cells were adjacent to areas of high antigen expression. Significant differences in reaction intensity in individual malignant cell nuclei were also observed. Adjacent to less numerous cells with intensively stained nucleus, cells with moderately to poorly stained nuclei were present (Figure 2). In several cases, weak cytoplasmic reactions were also observed. Regarding both Ki-67 and PCNA, only malignant cells with a clearly stained nucleus were considered as demonstrating positive reactions.
Figure 1. Ki-67 uniform nuclear immunostaining in a tubulo-papillary carcinoma (a) and a solid carcinoma (b). ABC-Elite method and Mayer’s haematoxylin counterstain. Bar, 20 ųm.
Figure 2. PCNA immunostaining in a tubulo-papillary carcinoma (a) and solid carcinoma (b). Note variable heterogeneity of nuclear staining (arrows). ABC-Elite method and Mayer’s haematoxylin counterstain. Bar, 20 ųm.
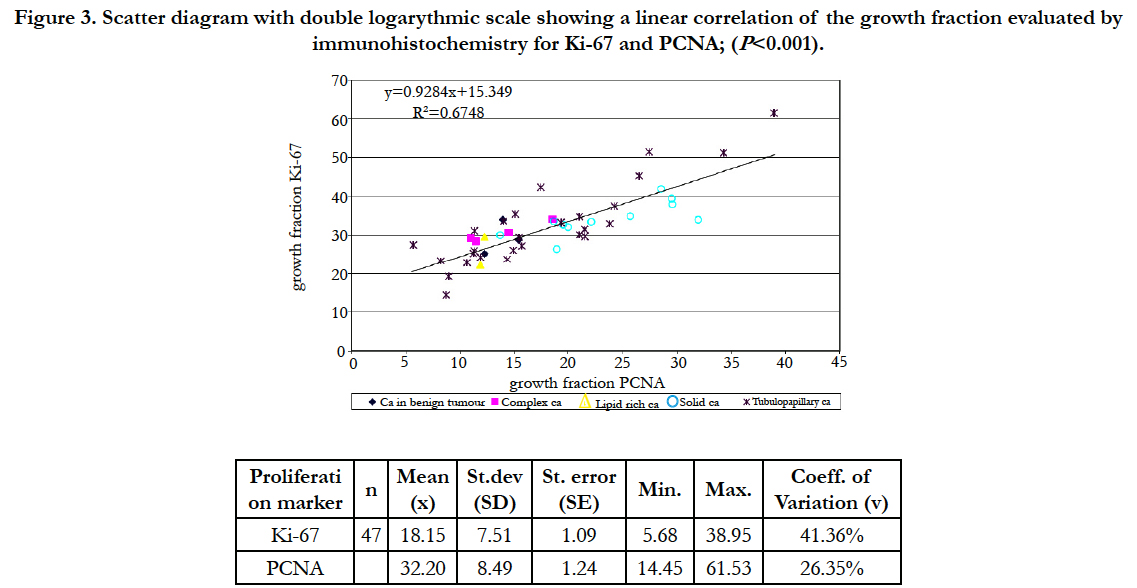
The values of the studied proliferative indices in the studied malignancy group ranged from 5.68 to 38.95 for Ki-67 and from 14.45 to 61.53 in case of PCNA. In normal tissue 4.06 to 22.28 and from 13.43 to 23.38 respectively. Statistically significant correlation between both index values (r= 0,82, P<0.001) was observed, but PCNA index value was generally two times higher than Ki-67. The relation between index values in a studied malignancy group was shown in Figure 3.
Figure 3. Scatter diagram with double logarythmic scale showing a linear correlation of the growth fraction evaluated by immunohistochemistry for Ki-67 and PCNA; (P<0.001).
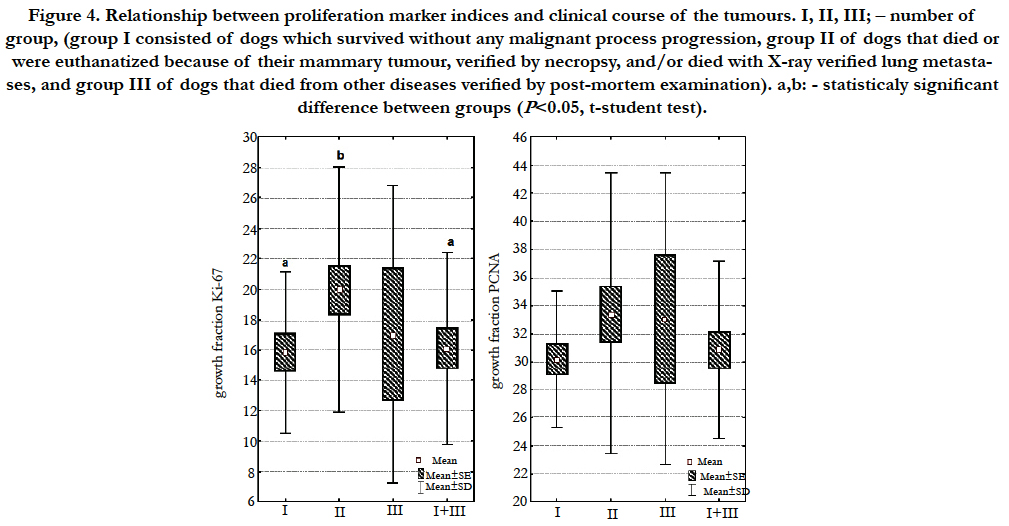
Statistically significant difference (P<0.05) between Ki-67 index value and the clinical course of the malignant disease in our study was stated using the t-student test (Figure 4). In the group of female dogs which died or were euthanatized due to the metastases (group II), the mean Ki-67 index value was significantly higher in comparison to the group of animals which survived the observation period without malignant process progression (group I). Statistically significant difference occurred also between group II, and group I and III together. Such a relation was not observed for PCNA. Similarly, a statistically significant difference was not determined between the studied indices and disease clinical course using a variance analysis test.
Figure 4. Relationship between proliferation marker indices and clinical course of the tumours. I, II, III; – number of group, (group I consisted of dogs which survived without any malignant process progression, group II of dogs that died or were euthanatized because of their mammary tumour, verified by necropsy, and/or died with X-ray verified lung metastases, and group III of dogs that died from other diseases verified by post-mortem examination). a,b: - statisticaly significant difference between groups (P<0.05, t-student test).
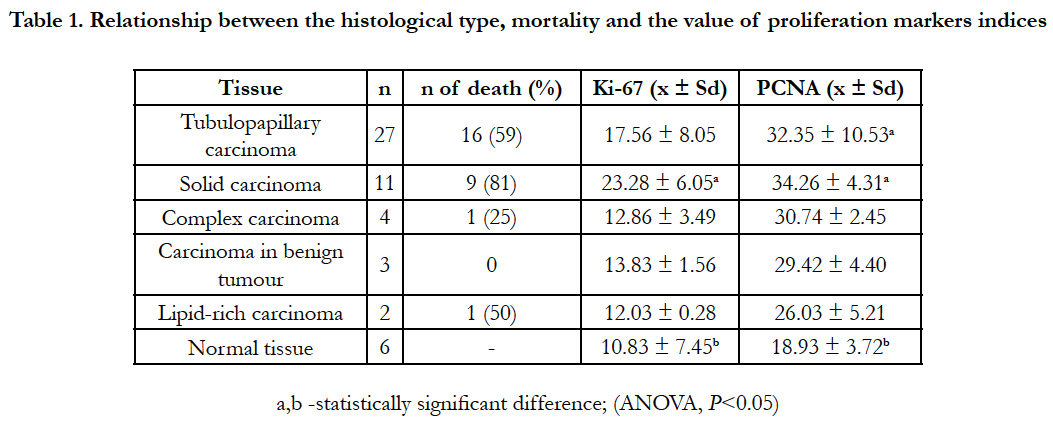
The results of histopathological examination showed that the most frequently diagnosed type was tubulo-papillary carcinoma (27 cases), and in further order, 11 solid carcinomas, 4 complex carcinomas, 3 carcinomas in benign tumours and 2 cases of lipidrich carcinomas.
Differences determined between both index values and the histopathological type of tumours under the variance analysis test was not statistically significant. Table 1 shows data concerning proliferative index values in the studied histological types and data concerning animal survival during the observation period. The highest mean values for both studied indices observed in the group of solid carcinomas were 23.28% for Ki- 67 and 34.26% for PCNA. Simultaneously, the highest percentage of deaths (81%) was also observed in the case of solid carcinoma. Out of 11 female dogs diagnosed with solid carcinoma, only 2 survived the observation period. The second highest percentage of carcinoma causing deaths (59%) was tubulo-papillary carcinoma. The proliferative index values for that histopathological type were slightly lower than those of the solid carcinoma. For lipid-rich carcinoma, the percentage of death was 50%, and 25% for complex carcinoma. Death was not observed for the carcinoma in benign tumours.
Table 1. Relationship between the histological type, mortality and the value of proliferation markers indices
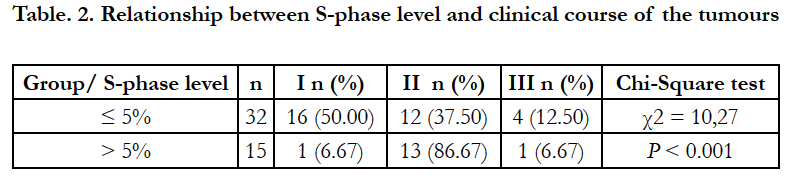
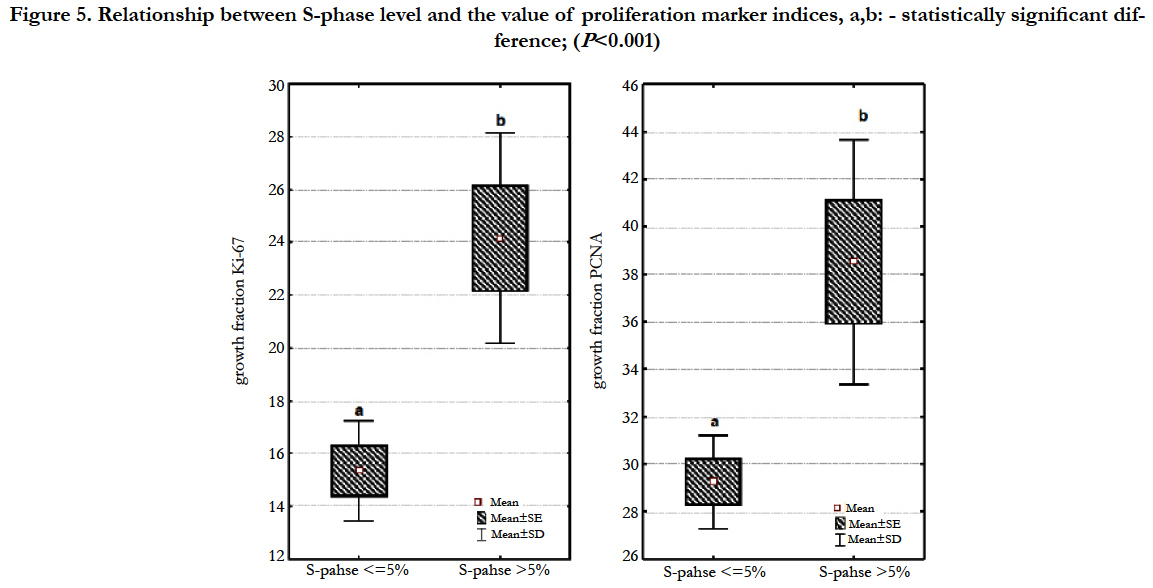
However, in the conducted studies a high correlation (P<0.001) was confirmed between cell fraction in the S-phase of the cell cycle and disease clinical course (Table 2). Among 32 female dogs with tumours of low level of cells in the S-phase, 16 (50.00%) dogs survived the observation period without any sings of neoplastic disease, 12 (37.5%) animals died or were euthanatized due to malignant disease progression during the observation period and 4 (12.50%) female dogs died from other diseases. However, of 15 female dogs with tumours of elevated level of cells in the S-phase, 13 (86.7%) animals died or were euthanatized due to malignant progression, and only 1 dog (6.67%) survived the observation and 1 dog died from other diseases. The index values for both antigens in our study were statistically significantly higher (P<0.001) in the group of neoplasms with increased levels of cells in the S-phase of the cell cycle determined by flow cytometry analysis when compared to the group with low levels of cells in the S-phase (Figure 5).
Figure 5. Relationship between S-phase level and the value of proliferation marker indices, a,b: - statistically significant difference; (P<0.001)
Discussion
The higher PCNA index values in comparison to the Ki-67 index values, as well as the differences in reaction intensity in the cell nuclei in the case of PCNA, are consistent with the observations of other authors [23-27]. The differences can be explained by the relatively long half-life of PCNA protein in cells. It is assumed to last about 8 hours for dividing cells, and about 20 hours for cells which have passed the resting phase [28]. This may yield false positive results because the protein does not degrade directly after the cell division and may last in cells, which have completed their cycle. Another cause of high PCNA index value may be the fact that this protein takes part in many cell metabolic processes [29]. As an auxiliary protein of the DNA polymerase-δ, it is active among others in DNA repair processes taking place with an increased activity in malignant tumours cells. For this reason, when some authors present the results of PCNA expression analysis they do so using both indices: SP-PCNA (strong positive labelled cells) and TP-PCNA (total positive labelled cells) [27,30]. SPPCNA index values are then much lower and closer to the Ki-67 index values, at the same time more honestly reflecting the real number of proliferating cells. However, determination of the SP-PCNA index is linked to a drawback caused by the subjective assessment of the observer and, in the case of using computerized systems of picture analysis, by the differences in parameters assumed in the morphological analysis. This is the reason why the TP-PCNA index seems to be more objective and have wider use for comparing the results. The time of fixing the material and procedure for the immunohistochemical reaction, and especially the concentration of the primary antibody and the process of antigen unmasking, have all an important influence on the expression of the above antigens [31]. When compared to those described in the literature, very low concentrations of the primary antibodies and the antigen unmasking process, using a decloaking chamber and various buffer solutions, were used in the conducted study, after a series of introductory experiments. One of the reasons for using such low concentrations of primary antibodies were the observations made by McCormick et al. [32] indicating that an increasing PC-10 antibody concentration results in an increase of antigen expression in cells, and therefore leads to false positive results. Despite using a very low concentration of PC-10 antibody, heterogeneity in staining of the cell nuclei could not be eliminated. McDermott et al. [33] stressed the fact that keeping the archival material in paraffin blocks for long periods may reduce the expression of proliferation antigens and therefore, influence the results.
Similar level of correlation to ours, between the Ki-67 and PCNA indices, was noted [34], although others observed lower values [24]. A positive correlation between the above indices for the dysplasias and benign tumours, but not for malignant tumours was observed [26]. Absolute values of the measured indices are different in studies by various authors [23,24,26,34]. The observed differences are probably caused by the lack of standardization of the method concerning proliferation indices, including the use of different concentrations of primary antibodies, the methods of unmasking the antigen, as well as the methods of expression assessment. Another reason may be the heterogeneous histological structure of canine mammary carcinomas. Pena et al., [26] suggested that fields of maximal proliferation antigen expression in areas of the lowest morphological differentiation should be chosen for morphometrical analysis. A correlation between the increase in Ki-67 or PCNA indices and the shorter time of remission, the existence of metastasis and the shorter survival time of patients operated on or treated because of malignant tumours, has been observed in human patients [35]. Similar observations were noted in some canine tumours, including mast cell tumour, soft tissue sarcoma and malignant melanoma [36-39]. In the case of canine mammary tumours in female dogs, many studies involved the demonstration of the presence of proliferation processes or confirmation of the expression of the above mentioned antigens, as well as correlation between the intensity of their expression and clinical indices such as tumour size, the presence of lymph nodes metastasis, hormonal status, histological type and histological degree of malignancy and other assessed parameters [27,30,40-42]. In most of the conducted studies of canine mammary tumours, a statistically important difference between the values of proliferation indices was noted between the groups of non-malignant and malignant tumours, whereas the differences between different histological types were less important or did not exist [24,25,41,43,44]. Studies with the prognostic aspect, in which the expression of proliferation antigens were compared to the disease-free time and the survival time of patients, are not numerous.
Ki-67 antigen values assessed in cytological smears correlated with the occurrence of metastasis and a shorter survival time after the surgery [23]. Statistically important correlation between Ki-67 index value, mitosis index and the survival time of animals operated on were also observed [45]. Similarly, Pena et al. [26] showed, in a multivariate analysis, the correlation between increased Ki-67 expression and the occurrence of metastasis, time of remission and patients survival time. However, in the same study, PCNA index values were shown to correlate only with the histological degree of malignancy of tumours and with nuclear grade. In the other study only the correlation between PCNA index and the occurrence of local remission after the operation was shown [34].
In conducted studies a correlation between values of both proliferation indices and the S-phase fraction was noted, although the absolute values of proliferation indices were much higher than the value of S-phase fraction. The obtained results are similar to those obtained in studies of some human carcinomas [46]. We did not find any literature concerning comparison of S-phase fraction value, assessed using the flow cytometry method, with Ki-67 or PCNA in canine mammary carcinomas in female dogs. However, our observations are close to the results of the studies in which Ki-67 and PCNA index values were compared to another marker of proliferation – the incorporating index of bromodeoxyuridine (BrdU). These studies showed that Ki-67 and/or PCNA proliferation index in human and canine mammary tumours were a few times higher than the BrdU index [16,24,47]. The results of our current study confirm earlier observations showing that the determination of the S-phase fraction using flow cytometry is the most accurate method of assessment of cellular proliferation increase, and at the same time, a valuable prognostic factor [20]. However, its use in the clinical setting is limited because of the expensive equipment needed and the overlap in cellcycle phases compromise the distinction between the G2 and M-phases of the cell cycle [48]. Based on the research, it can be stated that assessment of Ki-67 antigen proliferation index, using immunohistochemical methods, presents more advantages when compared to flow cytometry: including that it is less expensive, easier to use and can be done in a standard histological laboratory. The presence of a significant difference between the Ki-67 antigen index and the clinical course of the disease, calculated using the t-student test, with the lack of such a difference in variance analysis test in case of canine mammary carcinomas, suggests that it should be treated only as a prognostic marker helper. In the case of PCNA, difficulties in determining the suitable concentration of the primary antibody, the heterogeneity of staining the cell nuclei and the lack of significant correlations with the clinical course of the tumour disease, seems to disqualify it as a prognostic factor in canine mammary carcinomas.
References
- Vascellari M, Baioni E, Ru G, Carminato A, Mutinelli F (2009) Animal tumour registry of two provinces in northern Italy: incidence of spontaneous tumours in dogs and cats. BMC Vet Res 5: 39.
- Egenvall A, Bonnett BN, Ohagen P, Olson P, Hedhammar A, et al. (2005) Incidence of and survival after mammary tumors in a population of over 80,000 insured female dogs in Sweden from 1995 to 2002. Prev Vet Med 69: 109-127.
- Misdorp, W. (2002) Tumors of the Mammary Gland, in Tumors in Domestic Animals (4rth edtn) (Ed D. J. Meuten), Iowa State Press, Ames, Iowa, USA. 575-606.
- Stratmann N, Failing K, Richter A, Wehrend A (2008) Mammary tumor recurrence in bitches after regional mastectomy. Vet Surg 37: 82-86.
- Santos AA, Lopes CC, Ribeiro JR, Martins LR, Santos JC, et al. (2013) Identification of prognostic factors in canine mammary malignant tumours:a multivariable survival study. BMC Vet Res 9: 1.
- Matos AJ, Baptista CS, Gartner MF, Rutteman GR (2012) Prognostic studies of canine and feline mammary tumours: The need for standardized procedures. Vet J 193(1):24-31.
- Chang SC, Chang CC, Chang TJ, Wong ML (2005) Prognostic factors associated with survival two years after surgery in dogs with malignant mammary tumors: 79 cases (1998-2002). J Am Vet Med Assoc 227: 1625-1629.
- Naryzhny SN (2008) Proliferating cell nuclear antigen: a proteomics view. Cell Mol Life Sci 65: 3789-3808.
- Brown DC, Gatter KC (2002) Ki67 protein: the immaculate deception?. Histopathology 40: 2-11.
- Scholzen T, Gerdes J (2000) The Ki-67 protein: from the known and the unknown. J Cell Physiol 182: 311-322.
- Ekholm M, Beglerbegovic S, Grabau D, Lovgren K, Malmstrom P, et al. (2014) Immunohistochemical assessment of Ki67 with antibodies SP6 and MIB1 in primary breast cancer: a comparison of prognostic value and reproducibility. Histopathology 65: 252-260.
- Pathmanathan N, Balleine RL (2013) Ki67 and proliferation in breast cancer. J Clin Pathol 66: 512-516.
- Vinothini G, Murugan RS, Nagini S (2009) Evaluation of molecular markers in a rat model of mammary carcinogenesis. Oncol Res 17: 483-493.
- Kumaraguruparan R, Prathiba D, Nagini S (2006) Of humans and canines: Immunohistochemical analysis of PCNA, Bcl-2, p53, cytokeratin and ER in mammary tumours. Res Vet Sci 81: 218-224.
- Urruticoechea A, Smith IE, Dowsett M (2005) Proliferation marker Ki-67 in early breast cancer. J Clin Oncol 23: 7212-7220.
- Goodson WH 3rd, Moore DH 2nd, Ljung BM, Chew K, Mayall B, et al. (2000) The prognostic value of proliferation indices: a study with in vivo bromodeoxyuridine and Ki-67. Breast Cancer Res Treat 59: 113-123.
- Woo J, Baumann A, Arguello V (2014) Recent advancements of flow cytometry: new applications in hematology and oncology. Expert Rev Mol Diagn 14: 67-81.
- Reggeti F, Bienzle D (2011) Flow cytometry in veterinary oncology. Vet Pathol 48:223-235.
- Perez Alenza MD, Rutteman GR, Kuipers-Dijkshoorn NJ, Pena L, Montoya A, et al. (1995) DNA flow cytometry of canine mammary tumours: the relationship of DNA ploidy and S-phase fraction to clinical and histological features. Res Vet Sci 58: 238-243.
- Hellmen E, Bergstrom R, Holmberg L, Spangberg IB, Hansson K, et al. (1993) Prognostic factors in canine mammary tumors: a multivariate study of 202 consecutive cases. Vet Pathol 30: 20-27.
- Misdorp W, Else RW, Hellmen E, Limpscomb TP (1999) Histological Classification of Mammary Tumors of the Dog and the Cat. Washington D.C.: Armed Forces Institute of Pathology in cooperation with the American registry of Pathology and The World Health Organization Collaborating Center for Worldwide Reference on Comparative Oncology 5-58.
- Hellmen E, Lindgren A, Linell F, Matsson P, Nilsson A (1988) Comparison of histology and clinical variables to DNA ploidy in canine mammary tumors. Vet Pathol 25: 219-226.
- Zuccari DA, Santana AE, Cury PM, Cordeiro JA (2004) Immunocytochemical study of Ki-67 as a prognostic marker in canine mammary neoplasia. Vet Clin Pathol 33: 23-28.
- Zacchetti A, van Garderen E, Teske E, Nederbragt H, Dierendonck JH, et al. (2003) Validation of the use of proliferation markers in canine neoplastic and nonneoplastic tissues: comparison of KI-67 and proliferating cell nuclear antigen (PCNA) expression versus in vivo bromodeoxyuridine labelling by immunohistochemistry. APMIS 111: 430-438.
- Lopuszynski W, Szczubial M, Nozdryn-Plotnicki Z, Smiech A (2002) Assessment of the proliferating activity of mammary gland neoplasms in bitches based on the expression of proliferating cell nuclear antigens (PCNA). Medycyna Weterynaryjna 58: 275-280.
- Pena LL, Nieto AI, Perez-Alenza D, Cuesta P, Castano M (1998) Immunohistochemical detection of Ki-67 and PCNA in canine mammary tumors: relationship to clinical and pathologic variables. J Vet Diagn Invest 10: 237-246.
- Sarli G, Benazzi C, Preziosi R, Marcato PS (1995) Assessment of proliferative activity by anti-PCNA monoclonal antibodies in formalin-fixed, paraffinembedded samples and correlation with mitotic index. Vet Pathol 32: 93-96.
- Morris GF, Mathews MB (1989) Regulation of proliferating cell nuclear antigen during the cell cycle. J Biol Chem 264: 13856-13864.
- De Biasio A, Blanco FJ (2013) Proliferating cell nuclear antigen structure and interactions: too many partners for one dancer? Adv Protein Chem Struct Biol 91: 1-36.
- Preziosi R, Sarli G, Benazzi C, Marcato PS (1995) Detection of proliferating cell nuclear antigen (PCNA) in canine and feline mammary tumours. J Comp Pathol 113: 301-313.
- Ramos-Vara JA (2005) Technical aspects of immunohistochemistry. Vet Pathol 42: 405-426.
- McCormick D, Yu C, Hobbs C, Hall PA (1993) The relevance of antibody concentration to the immunohistological quantification of cell proliferatio associated antigens. Histopathology 22: 543-547.
- McDermott NC, Farch N, Butler D, Milburn C, Kay EW, et al. (1997) MIB1 staining in archival material - Unanticipated problems with immunostaining in paraffin embedded tissue. Journal of Pathology 181: A21-A21.
- Lohr CV, Teifke JP, Failing K, Weiss E (1997) Characterization of the proliferation state in canine mammary tumors by the standardized AgNOR method with postfixation and immunohistologic detection of Ki-67 and PCNA. Vet Pathol 34: 212-221.
- Stuart-Harris R, Caldas C, Pinder SE, Pharoah P (2008) Proliferation markers and survival in early breast cancer: a systematic review and meta-analysis of 85 studies in 32,825 patients. Breast 17: 323-334.
- Thompson JJ, Yager JA, Best SJ, Pearl DL, Coomber BL, et al. (2011) Canine subcutaneous mast cell tumors: cellular proliferation and KIT expression as prognostic indices. Vet Pathol 48: 169-181.
- Webster JD, Yuzbasiyan-Gurkan V, Miller RA, Kaneene JB, Kiupel M (2007) Cellular proliferation in canine cutaneous mast cell tumors: associations with c- KIT and its role in prognostication. Vet Pathol 44: 298-308.
- Ettinger SN, Scase TJ, Oberthaler KT, Craft DM, McKnight JA, et al. (2006) Association of argyrophilic nucleolar organizing regions, Ki-67, and proliferating cell nuclear antigen scores with histologic grade and survival in dogs with soft tissue sarcomas: 60 cases (1996-2002). J Am Vet Med Assoc 228: 1053-1062.
- Millanta F, Fratini F, Corazza M, Castagnaro M, Zappulli V, et al. (2002) Proliferation activity in oral and cutaneous canine melanocytic tumours: correlation with histological parameters, location, and clinical behaviour. Res Vet Sci 73: 45-51.
- De Matos AJ, Lopes CC, Faustino AM, Carvalheira JG, Dos Santos MS, et al. (2006) MIB-1 labelling indices according to clinico-pathological variables in canine mammary tumours: a multivariate study. Anticancer Res 26: 1821-1826.
- Yang WY, Liu CH, Chang CJ, Lee CC, Chang KJ, et al. (2006) Proliferative activity, apoptosis and expression of oestrogen receptor and Bcl-2 oncoprotein in canine mammary gland tumours. J Comp Pathol 134: 70-79.
- Funakoshi Y, Nakayama H, Uetsuka K, Nishimura R, Sasaki N, et al. (2000) Cellular proliferative and telomerase activity in canine mammary gland tumors. Vet Pathol 37: 177-183.
- Torres LN, Matera JM, Vasconcellos CH, Avanzo JL, Hernandez-Blazquez FJ, et al. (2005) Expression of connexins 26 and 43 in canine hyperplastic and neoplastic mammary glands. Vet Pathol 42: 633-641.
- Funakoshi Y, Nakayama H, Uetsuka K, Nishimura R, Sasaki N, et al. (2000) Cellular proliferative and telomerase activity in canine mammary gland tumors. Veterinary Pathology Online 37: 177-183.
- Sarli G, Preziosi R, Benazzi C, Castellani G, Marcato PS (2002) Prognostic value of histologic stage and proliferative activity in canine malignant mammary tumors. J Vet Diagn Invest 14: 25-34.
- Martinez-Arribas F, Nunez MJ, Piqueras V, Lucas AR, Sanchez J, et al. (2002) Flow cytometry vs. Ki67 labelling index in breast cancer: a prospective evaluation of 181 cases. Anticancer Res 22: 295-298.
- Nowak M, Madej JA, Dziegiel P, Kanzawa H (2006) Immunohistochemical identification method of tumour cells in the S phase of mitotic cycle and its usefulness in diagnostics of mammary gland adenocarcinomas in bitches. Pol J Vet Sci 9: 57-62.
- Heiden T, Auer G, Tribukait B (2000) Reliability of DNA cytometric Sphase analysis in surgical biopsies: assessment of systematic and sampling errors and comparison between results obtained by image and flow cytometry. Cytometry 42: 196-208.