Total Uterine Prolapse in a 15 Years Aged Doe
Y.V.Pridhvidhar Reddy1* , B.Sudhakara Reddy2, K.Jyothi1, S.Sivajothi3, and L.S.S.Varaprasad Reddy3
1 Assistant Professor, Dept. of Gynaecology and Obstetrics, College of Veterinary Science, Sri Venkateswara Veterinary University,Proddatur - 516360, Y.S.R.District, Andhra Pradesh, India.
2 Assistant Professor (Veterinary Medicine), Teaching Veterinary Clinical Complex, College of Veterinary Science, Sri Venkateswara Veterinary University, Proddatur - 516360, Y.S.R.District, Andhra Pradesh, India.
3. Assistant Professor, Dept. of Veterinary Parasitology, College of Veterinary Science, Sri Venkateswara Veterinary University, Proddatur - 516360, Y.S.R.District, Andhra Pradesh, India.
4 Assistant Professor, Dept. of Veterinary Physiology, College of Veterinary Science, Sri Venkateswara Veterinary University, Proddatur - 516360,Y.S.R.District, Andhra Pradesh, India.
*Corresponding Author
Y.V.Pridhvidhar Reddy,
Assistant Professor, Dept. of Gynaecology and Obstetrics,
College of Veterinary Science,
Sri Venkateswara Veterinary University,Proddatur - 516360,
Y.S.R.District,Andhra Pradesh, India.
E-mail: prithvi524@gmail.com
Article Type: Review Article
Received: August 27, 2014; Accepted: September 25, 2014; Published: October 6, 2014
Citation: Y.V.Pridhvidhar Reddy, et al. (2014). Total Uterine Prolapse in a 15 Years Aged Doe, Int J Vet Health Sci Res, 02(04), 28-30. doi: dx.doi.org/10.19070/2332-2748-140008
Copyright: Y.V.Pridhvidhar Reddy© 2014. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Present communication about the doe which was brought to clinic with the complaint of prolapse of uterus. Upon clinical examination it had soiled, necrosed debris on the uterus with abnormal vital parameters. On laboratory examination doe had anaemia, leucocytosis, low levels of total serum protein, serum albumin and calcium . Doe also had severe Haemonchus spp. infection. The everted organ was washed with KMnO4 solution and gross debris was removed. Epidural anesthesia was achieved by 2 % lignocaine hydrochloride and prolapsed mass was slowly pushed inside the pelvic cavity through vagina and replaced in its normal position. Doe was treated by administering the enrofloxacin, tolfenamic acid, oxytocin, calcium borogluconate and fenbendazole. After close monitoring for three days no incidence of prolapse was noticed.
2.Introductiont
3.Case History And Observations
4.Treatment And Discussion
5.References
Key words
Doe, Haemonchus, Hypocalcaemia, Treatment, Uterine prolapse
Introduction
Prolapse of genital organs were an important maternal abnormality in ruminants which were observed mostly after parturition. It can be recognized by the protrusion of varying parts of genital tract through vulva [1]. Protrusion of all or a part of everted organ is a common condition in pluriparous ruminants although exact cause of the disorder is not known [2]. Post partum uterine prolapse occurs most commonly in the cow and ewe less common in doe and rare in mare. Prolapse of uterus generally occurs immediately or a few hours after parturition when the cervix is open and uterus lacks tone [3]. Prolapse that occurs more than 24 hours postpartum is extremely rare and is complicated by partial closure of the cervix, making replacement difficulty even impossible [4]. The etiology of uterine prolapse is unknown, but many factors have been associated [3]. Conditions such as poor uterine tone, increased straining caused by pain and discomfort after parturition excessive traction at assisted parturition, excessive weight of retained fetal membranes and reduced calcium levels during peripartum period may also be associated. Success of treatment depends on the type of case, duration of case, degree of damage and contamination. This article describes case of total uterine prolapse in a 15 years aged doe and its successful treatment.
Case History And Observations
A 15 years old doe with poor body conditions weighing 27 kg was presented for the treatment of uterine prolapse (Figure-1). The animal was suffering from enteritis that further soiled the prolapsed mass. Kidding (two kids) happened two days back and prolapse was seen few hours after kidding. Physical examination was carried out and vital parameters were recorded according to the procedures mentioned previously [5]. Doe had rectal temperature (101.8º F), increased heart rate (112/min), rapid respiratory rate (46/min) and rumen motility (2/4 min). The conjunctival mucus membranes were pink, prolapsed uterus mass was swollen, necrotic and stained with faecal materials and debris. Whole blood with addition of Ethylenediaminetetraacetic acid, peripheral blood smears, serum, ruminal fluid and faecal samples were collected for laboratory analysis according to the previous studies [6,7].
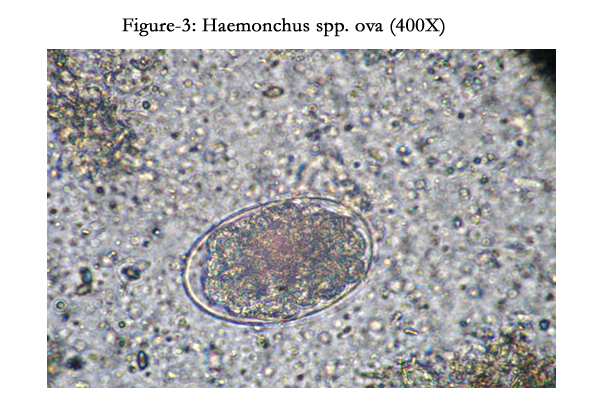
No blood parasites were detected in wet blood film, Giemsa stained smears and buffy coat smears. Presence of the Haemonchus ova (++++) was noticed in the faecal samples. Ruminal fluid revealed presence of low number of ruminal micro flora with pH of 7.0. Haematology revealed hemoglobin (8.8 g/dl), RBC (3.24 x 106/μL), PCV (28%), Higher TLC (24000/μL) with neutrophilia (11400/μL) and lymphocyte count (12250/μL). Serum analysis detected that there was decrease in total protein (6.2 g/dL), albumin (2.8 g/dL) while considerable increased values were observed in blood urea nitrogen (42 mg/dL), creatinine (1.12 mg/dL), serum glutamic oxaloacetic transaminase (SGOT) (96 U/L) levels, serum glutamic pyruvic transaminase (SGPT) (46U/L) and glucose (86 mg/dL). It had low calcium (10.2 mg/dL) level when compared with reference values.
Treatment And Discussion
Epidural anesthesia was achieved by administering with 2 % lignocaine hydrochloride into 1st inter coccygeal space to prevent straining during replacement of prolapsed organ. Debris and faecal material were gently removed and the prolapsed uterus was washed with warm diluted KMnO4 (0.005%) solution. Necrotic area was washed, cleansed and lubricated. Both hind limbs were lifted by assistant. Using both hands with moderate lubrication prolapsed uterus was pushed gently back in to the vagina. Enrofloxacin was given @ 5 mg per kg body weight IM and tolfenamic acid @ 2 mg per kg body weight IM for 5 days. 20 IU of oxytocin, Calcium borogluconate @ 100 ml, I.V., DNS @ 10ml per kg body weight, I.V., Neblon powder 30g PO, fenbendazole @ 7 mg per kg body weight, PO was given on the day of presentation. The vital parameters which were above normal when case was presented to the clinic returned to normal after completion of 3 days of therapy.
Prolapse is a hereditary peri partum problem mostly associated with hypocalcaemia. Exposed mass was vulnerable to damage and possibly infection [8]. Prolapse of the uterus occur during the 3rd stage of labour at a time when the fetus has been expelled and the fetal cotyledons has separated from the maternal caruncles[1].
The uterine prolapse can be replaced with the animal in standing or recumbent position [3]. Once the uterus is replaced, the operators hand should be inserted to the tip of both horns to be sure that no remaining invagination could incite abdominal straining and prolapse [4]. If the uterus is completely and fully replaced all the way to the tips of the uterine horns, the prolapsed is unlikely to occur [3].
It has also been reported that most animals with uterine prolapse are hypocalcaemic where signs of hypocalcaemia are noticed such animals should therefore be given calcium borogluconate [4]. An injectable broad spectrum of 3 to 5 days after replacement of the prolapsed will prevent secondary bacterial infection[9]. Animals with uterine prolapse that were properly managed can conceive again without getting problems. Complications develop when lacerations, necrosis and infections are present or when treatment is delayed; shock, hemorrhages and thrombo embolism is potential sequel of a prolonged prolapse[1]. The high vital parameters witnessed in the present case when animals were brought could be a result of metritis caused by secondary bacterial infection especially as the animal was brought for treatment after 48 hours of occurrence of Prolapse [10]. In the present case severe leucocytosis was noticed, it may be due to the presence of secondary bacterial infection and development of metritis. Observed anaemia might be due to severe associated Haemonchus infection and these parasites suck the blood in the intestines and causes diarrhea. Recorded low level of calcium levels was in association with the previous study [11].
References
- Noakes D E, Parkinson T J, England G.C.W (2009) Veterinary Reproduction and Obstetrics. (9th edtn), W.B. Saunders Co.Ltd, London. 146-153.
- Roberts S J. (1971) Veterinary Obstetrics and Genital diseases (Theriogenology). (2nd edtn) (Indian reprint). CBS publishers and distributors, New Delhi, India. 189-196.
- Hanie E A. (2006) Prolapse of the vaginal and uterus: Text book of Large Animal Clinical Procedures for Veterinary Technicians, Elsevier. 218-221.
- Fubini SL,Ducharme G N (2006) Surgical conditions of the post partum period. Text book of farm animal surgery 333-338.
- Reddy LSSVP, Reddy BS, Naik BR, Prasad CS (2014) Haematological and clinical alterations with traumatic reticuloperitonitis in cattle. Inter J Vet Sci 3(4): 203-205.
- Sivajothi S, Reddy BS, Kumari KN, Rayulu VC (2014) Haematological changes in Trypanosoma evansi infected cattle. Int J of Sci World 2(1):27– 30.
- Reddy BS, Sivajothi S, Reddy LSSVP, Raju KGS (2014) Clinical and laboratory findings of Babesia infection in dogs. J Parasit Dis.
- Jackson P C G. (2004) Postpartum problems in large animals. Hand book of Veterinary Obstetrics (2nd edtn). Elsever Saunders 209-231.
- Borabia-Belsue J. (2006). Replacement of rectal prolapse in Sows. Vet Rec 380.
- Wachida N,Kisani A I (2011) Uterine Prolapse in a Doe: A case report. Int.J. Anim. Vet. Adv 3(3): 135-137. [
- Jyothi K, Reddy BS, Reddy YVP, Rao KP, Sivajothi S, et al. (2014) Pregnancy toxemia associated with Dystocia in a Nellore Brown Ewe. Advances in Applied Science Research 5(3):325-327.